Introduction
Infertility has been defined as the failure of a
couple to conceive and reproduce after 12 months of regular
intercourse (1). Approximately
13–15% of couples of reproductive age worldwide are affected by
this common clinical problem, and half of these cases are related
to male dysfunction (2). Among all
males with infertility, the cause of more than half is unknown
(idiopathic). Irregularities in sperm function or spermatogenesis
are believed to be associated with idiopathic male infertility
(3).
Spermatogenesis and spermatozoa maturation is an
androgen-dependent process (4).
Dihydrotestosterone (DHT) is the main androgen responsible for
spermatozoa maturation (5), as
well as for the maintenance of normal spermatogenesis (6). The enzyme steroid 5α-reductase
(SRD5A) is a key enzyme involved in converting testosterone into a
more potent androgen, DHT, in male reproductive tissues. Human
5α-reductase mainly consists of type 1 (SRD5A1) and type 2
(SRD5A2) isozymes, but type 2 is more essential for
development of the male reproductive organs (7) and thus may be associated with semen
quality compared with type 1.
The SRD5A2 gene is on chromosome 2p23 with 5
exons, encoding a 254 amino acid protein (7). Several missense polymorphisms affect
enzyme activity (8): some normal
variations in the SRD5A2 gene may be associated with
prostate cancer (9), while other
serious mutations of SRD5A2 reduce or eliminate enzymatic
activity and cause deficient virilization of the male external
genitalia or even pseudohermaphrodism during development (10–12).
Of these, three single nucleotide polymorphisms (SNPs) alter other
amino acids of SRD5A2 that are more noteworthy. The most common
variation is a valine to leucine substitution at codon 89
(V89L, rs523349), which has been associated with prostate
cancer. Due to this variation, there is an approximately 30%
reduced activity of SRD5A2 in Asian men (13), which is not found in European
populations (14–15). The other missense polymorphism is
at codon 49, substituting an alanine with a threonine at codon 49
(A49T). The T-variant has mainly been associated with
prostate cancer and was shown to increase the enzymatic activity to
a great extent (16–17). The R227Q mutation substitutes
arginine with glutamine, leading to SRD5A2 enzymatic activity being
almost completely absent. With the exception of the above three
mutations, other mutations are detected at a very low frequency
(8). Nevertheless, all of the
above shows that variations of SRD5A2 are associated with an
increased risk of developing diseases of the male reproductive
organs and functions. Thus, we speculate whether the polymorphisms
in the SRD5A2 gene are associated with semen quality in the
Han-Chinese population.
In the present study, we investigated the frequency
distribution of five tagSNPs representing genetic variation across
the entire SRD5A2 gene in 708 patients with idiopathic
infertility. This study may add to our knowledge regarding the
impact of SRD5A2 polymorphisms on male infertility. The SNP
tagging method is used to select tagSNPs. Five tagSNPs were
captured to present 648 SNPs of human SRD5A2. Therefore, the
purpose of this study was to investigate the possible association
between the genetic variants of SRD5A2 and semen quality in
708 males with definite idiopathic infertility in a Han-Chinese
population.
Materials and methods
Subjects
A total of 708 ethnic Han-Chinese males with
definite idiopathic infertility (without diagnosed infertile wives)
visiting the First Affiliated Hospitals of Nanjing Medical
University were consecutively recruited between March 2010 and
March 2011. All the subjects had been unable to conceive for at
least 12 months. Those with a history of orchitis, obstruction,
cryptorchidism, congenital bilateral absence of vas deferens,
cytogenetic abnormalities and Y chromosome microdeletions were
excluded from the study after a complete historical and physical
examination (18–19). All participants provided informed
consent and completed a questionnaire including information about
age, smoking and drinking habits and other lifestyle factors. Each
subject donated 5 ml of peripheral blood for genomic DNA extraction
and an ejaculate for semen analysis. This study was approved by the
ethics review board of Nanjing Medical University.
Semen analysis
The computer-assisted semen analysis system
(WLJY-9000; Weili New Century Science and Tech Dev., Beijing,
China) was used to perform the semen analysis according to the
World Health Organization guidelines (World Health Organization,
1999). Four parameters of semen quality were selected for the
statistical analysis: Semen volume, sperm concentration
(106/ml), sperm number per ejaculate
(106/ejaculate) and sperm motility. The sperm numbers
and sperm motility characteristics provided a reliable estimation
of the fertilizing ability of human spermatozoa (20). Strict quality control measures were
enforced throughout the study. Each sample was assessed twice.
SNP selection
Genotype data obtained from unrelated Han Chinese
individuals from Beijing in the HapMap (HapMap Data Rel 24/phase II
nov08, on NCBI B36 assembly, dbSNP b126; http://hapmap.ncbi.nlm.nih.gov) were used to select
the SNPs. SNPs with a minor allele frequency >0.05 in Han
Chinese in Beijing were selected within the 56318 bp human
SRD5A2 gene, which was pinpointed to chromosome 2 from
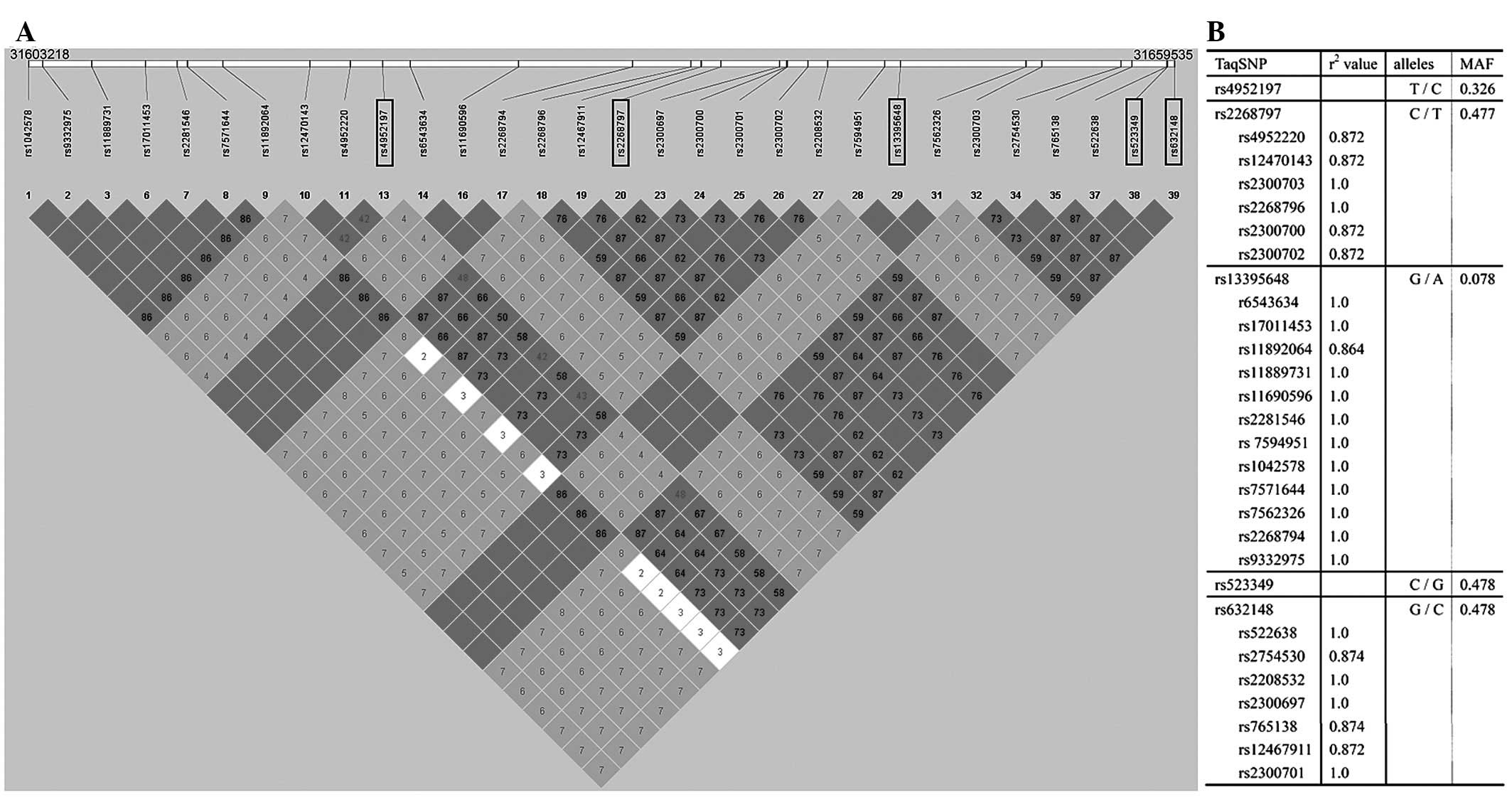
31603218 to 31659535. Thirty SNPs were captured in this region. A
linkage disequilibrium (LD) plot of this region was made using
Haploview 4.0 software (21),
based on the r2 values, which indicate the ability of a
certain SNP to predict another SNP (Fig. 1). Using Tagger and a tagging
threshold of r2>0.80, five tagSNPs were selected
(rs4952197, rs2268797, rs13395648, rs523349 and rs632148) that
could represent other SNPs of this region with a mean r2
of 0.959 (Fig. 1).
Genotyping
Genomic DNA was extracted from peripheral blood
leukocytes of 708 Han-Chinese males suffering from infertility
according to standard protocols (Genomic DNA kit; Tiangen, Beijing,
China). TaqMan SNP genotyping assays were performed using the
Taq amplification method in a 7900 HT Fast Real-Time PCR
system (Applied Biosystems, Foster City, CA, USA).
PCR amplification of rs4952197, rs2268797 and
rs13395648 was performed at 95°C for 10 min, followed by 40 cycles
at 95°C for 15 sec, 56°C for 10 sec and 60°C for 1 min, with one
additional cycle at 60°C for 10 min. The amplification conditions
of rs523349 and rs632148 were similar to the above except that 55
cycles were used due to the higher relative GC content. Ten percent
of study participants were randomly chosen and genotyped in
duplicate to confirm the concordance of the genotyping results. In
our study, the call rates for these SNP genotyping were >97% and
the concordance of duplicates was 100%.
Statistical analysis
Logarithmic transformation of the sperm
concentration and sperm number per ejaculate was undertaken to
achieve homogeneity of variance and normal distribution of
residuals. No transformation was performed for the remaining
parameters. Differences in selected demographic variables, smoking
and alcohol status were evaluated by the χ2 test. The
Student’s t-test was used to evaluate continuous variables,
including age, BMI and pack-years of cigarette smoking. For
statistical analysis, a multiple linear regression analysis was
applied for the comparison of semen parameters as considered for
the genotypes of each SNP. Statistical analyses were carried out
using Stata (Version 9.0, StataCorp, LP). P<0.05 was considered
to indicate a statistically significant difference.
Results
Characteristics of the study
populations
The study population comprised 708 ethnic
Han-Chinese males with definite idiopathic infertility at
30.57±5.14 years of age. The mean duration of sexual abstinence
prior to semen collection was 5.23±3.52 days. The sperm parameters
between the stratification of selected characteristics were
analyzed (Table I). No significant
differences were found in the stratification of age, smoking and
BMI (body mass index) for all four parameters. However, the sperm
number per ejaculate and the sperm motility were significantly
higher in the never drinking group when compared with the ever
drinking group (P=0.015 and 0.0425, respectively). No significant
difference was observed in the remaining parameters (semen volume
and concentration) between the ever drinking and never drinking
groups. For the duration of sexual abstinence (Abs), the 4–7 days
group and ≥7 days group were associated with a significantly higher
semen volume (P<0.001 and P<0.001, respectively) and sperm
number per ejaculate (P=0.025 and P<0.001, respectively)
compared with the <4 days group. The sperm concentration between
the ≥7 days group and the <4 days group was also significantly
different (P=0.013). The sperm motility parameter among the three
stratifications of the duration of sexual abstinence was
similar.
 | Table IAssociation between the selected
individual characteristics and sperm parameters in 708 males with
definite idiopathic infertility. |
Table I
Association between the selected
individual characteristics and sperm parameters in 708 males with
definite idiopathic infertility.
| N (%) | Semen volume
(ml) |
Concentrationa | Sperm number per
ejaculatea | Motility (%) |
|---|
| Age (years) |
| <28 | 224 (31.64) | 3.76±1.53 | 3.50±1.23 | 4.62±1.31 | 52.32±24.50 |
| 28–32 | 216 (30.51) | 3.41±1.42 | 3.65±1.16 | 4.79±1.28 | 53.52±22.94 |
| ≥32 | 268 (37.85) | 3.45±1.33 | 3.72±1.13 | 4.89±1.24 | 49.23±25.21 |
| Smoking |
| Yes (ever) | 364 (51.41) | 3.35±1.36 | 3.57±1.22 | 4.71±1.33 | 51.99±24.00 |
| No (never) | 344 (48.59) | 3.49±1.49 | 3.68±1.13 | 4.85±1.23 | 51.01±24.74 |
| Drinking |
| Yes (ever) | 324 (45.76) | 3.38±1.43 | 3.55±1.23 | 4.69±1.36b | 49.49±24.88b |
| No (never) | 384 (54.24) | 3.45±1.42 | 3.69±1.13 | 4.85±1.20 | 53.23±23.78 |
| BMI |
| <20 | 82 (11.58) | 3.23±1.40 | 3.46±1.25 | 4.57±1.32 | 51.78±24.56 |
| 20–25 | 396 (55.93) | 3.39±1.36 | 3.60±1.20 | 4.74±1.31 | 50.72±24.86 |
| ≥25 | 230 (32.49) | 3.52±1.53 | 3.73±1.10 | 4.91±1.19 | 52.79±23.41 |
| Abs (days) |
| <4 | 176 (24.86) | 2.91±1.09 | 3.50±1.11 | 4.51±1.17 | 53.69±22.69 |
| 4–7 | 357 (50.42) | 3.52±1.51c | 3.60±1.15 | 4.76±1.28c | 51.20±24.44 |
| ≥7 | 175 (24.72) | 3.72±1.41c | 3.82±1.29c | 5.09±1.33c | 49.97±25.71 |
Associations between SRD5A2 SNPs and
semen quality
Table II shows the
association of the genotype frequency and sperm parameters of each
variant. The sperm concentration and sperm number per ejaculate
were not significantly different between the genotypes of each
genetic variant. The semen volume was also similar among the
genotypes of three variants (rs4952197, rs2268797 and rs523349).
For rs13395648, the subjects with the TC genotype presented a
significantly lower semen volume compared with the TT genotype
(P=0.016). The same trend was found between the combination of the
TC and CC genotypes and the TT genotype (P=0.020) (Table II).
 | Table IISperm parameters according to the
genetic variants of the SRD5A2 gene in 708 males with
definite idiopathic infertility. |
Table II
Sperm parameters according to the
genetic variants of the SRD5A2 gene in 708 males with
definite idiopathic infertility.
| Variant | Genotype | N (%) | Semen volume
(ml) |
Concentrationa | Sperm number per
ejaculatea | Motility (%) |
|---|
| rs4952197 |
| GG | 228 (32.20) | 3.40±1.45 | 3.63±1.11 | 4.78±1.23 | 52.75±25.27 |
| GA | 340 (48.02) | 3.40±1.41 | 3.66±1.20 | 4.80±1.29 | 51.57±24.38 |
| AA | 140 (19.77) | 3.47±1.42 | 3.54±1.23 | 4.70±1.33 | 49.38±22.71 |
| GA+AA | 480 (67.80) | 3.42±1.41 | 3.63±1.21 | 4.77±1.30 | 50.93±23.90 |
| rs2268797 |
| CC | 299 (42.65) | 3.37±1.37 | 3.55±1.21 | 4.69±1.30 | 50.86±23.58 |
| CT | 301 (42.94) | 3.41±1.38 | 3.69±1.14 | 4.85±1.24 | 51.15±24.85 |
| TT | 101 (14.41) | 3.51±1.66 | 3.65±1.18 | 4.79±1.33 | 54.15±25.25 |
| CT+TT | 402 (57.35) | 3.44±1.45 | 3.68±1.15 | 4.83±1.26 | 51.91±24.95 |
| rs13395648 |
| TT | 583 (84.01) | 3.46±1.44 | 3.62±1.19 | 4.78±1.30 | 51.88±24.40 |
| TC | 97 (13.98) | 3.07±1.32b | 3.62±1.10 | 4.68±1.16 | 49.78±25.36 |
| CC | 14 (2.20) | 3.34±1.09 | 3.38±1.26 | 4.54±1.29 | 56.22±16.00 |
| TC+CC | 111 (15.99) | 3.10±1.29c | 3.60±1.12 | 4.66±1.17 | 50.60±24.42 |
| rs523349 |
| CC | 133 (19.44) | 3.29±1.52 | 3.68±1.13 | 4.77±1.24 | 53.17±25.27 |
| CG | 275 (40.20) | 3.47±1.36 | 3.58±1.14 | 4.75±1.26 | 49.45±24.51 |
| GG | 276 (40.35) | 3.42±1.38 | 3.65±1.22 | 4.80±1.32 | 51.81±23.79 |
| CG+GG | 551 (80.56) | 3.44±1.37 | 3.61±1.19 | 4.78±1.29 | 50.63±24.16 |
| rs632148 |
| GG | 132 (18.88) | 3.27±1.34 | 3.70±1.10 | 4.80±1.20 | 55.60±24.29 |
| GC | 307 (43.92) | 3.50±1.49 | 3.61±1.16 | 4.79±1.26 | 49.47±24.82d |
| CC | 260 (37.20) | 3.39±1.39 | 3.62±1.23 | 4.77±1.33 | 52.32±23.73e |
| GC+CC | 567 (81.12) | 3.45±1.44 | 3.62±1.19 | 4.78±1.29 | 50.78±24.35f |
For rs632148, the GC genotype carriers had a
significantly lower motility compared with the GG genotype
(P=0.029). However, the difference between the CC and GG genotypes
was not statistically significant (P=0.078), whereas the
combination of GC and CC genotype carriers had a significantly
lower motility compared with the GG genotype (P=0.033) (Table II). The sperm motility was not
significantly different between the genotypes of rs4952197,
rs2268797, rs13395648 and rs523349 (Table II).
Discussion
In the present study, five tagSNPs (rs4952197,
rs2268797, rs13395648, rs523349 and rs632148) in the SRD5A2
gene were selected using the tagSNP method. In total, 708
Han-Chinese males with idiopathic infertility were genotyped for
these SNPs using TaqMan-based genotyping. Significant associations
were detected between the genetic variant rs13395648 and semen
volume, and between the genetic variant rs632148 and sperm
motility. It was demonstrated that subjects with the rs13395648 TC
genotype had significantly lower semen volume compared with those
with the TT variant. The same trend was also found in subjects
carrying a C allele at rs13395648 (TC and CC). Regarding rs632148,
the sperm motility was significantly lower in subjects with the GC
genotype compared with those with the GG genotype. The combination
of GC and CC genotype carriers also had a significantly lower
motility compared with the GG genotype. No association was found
between any of the remaining three polymorphisms and semen
quality.
The sperm number and motility characteristics are
known to be the most informative parameters in semen quality
analysis and may provide a reliable estimation of the fertilizing
ability of human spermatozoa (20), which are dependent on normal
spermatogenesis and spermatozoa maturation (2,22).
Spermatogenesis and spermatozoa maturation are androgen-dependent
processes (4–5,23).
SRD5A is a key enzyme converting testosterone into a more potent
androgen, DHT. Therefore, the SRD5A enzyme is crucial in these
processes. Furthermore, it is more likely that milder polymorphic
variations of SRD5A2 are associated with semen quality. In
this study, we examined the association of the SRD5A2 gene
with semen parameters in 708 Han-Chinese males with definite
idiopathic infertility. Results showed that the heterozygote at
rs13395648 and rs632148 in SRD5A2 was significantly
associated with semen volume and sperm motility, respectively.
As mentioned previously, V89L, A49T
and R227Q are three special mutations that alter other amino
acids of SRD5A2 among the 648 SNPs detected in the SRD5A2
gene according to public genome databases. The V89L
(rs523349) polymorphism has been extensively examined in relation
to prostate cancer (24). Although
certain studies have shown an association with prostate disease,
the results are conflicting (25–27).
As yet, there are no conclusions on whether V89L mutation is
associated with the risk of prostate cancer (15,28),
and there is no conclusive evidence of V89L polymorphism
affecting serum hormone levels (25). Our study suggests that there is no
association between rs523349 and semen quality. Concerning the
A49T and R227Q mutations, they are not captured by
tagSNP selection due to their very low frequency.
In their study, Elzanaty et al investigated
the effect of SRD5A2 polymorphisms on sperm parameters in
Swedish military conscripts. Their results showed that variants of
A49T and V89L are associated with sperm concentration
and motility, respectively (29).
Our results are not in agreement with those authors in that the
mutation at rs632148 demonstrated an association with sperm
motility, but not the rs523349 (V89L) mutation. This
discrepancy may be due to ethnic differences. Another study
investigating the effect of SRD5A2 polymorphisms on male
infertility in an Estonian population has shown that the
SRD5A2 polymorphism exhibited a significant association with
sperm motility in normozoospermic men, while no correlation was
found between any tagSNP in SRD5A2 and the sperm number
(30). Our results are generally
consistent with that finding.
SRD5A2 is expressed in the adult epididymis
(31), the prostate (32), the male external genitalia, seminal
vesicles (33) and intact
seminiferous tubules (23).
Spermatogenesis and spermatozoa maturation are androgen-dependent
processes (4–5,23).
DHT is the main androgen responsible for spermatozoan maturation in
the epididymis (34) and
maintenance of normal spermatogenesis in the testis (23), as well as maintenance of normal
function of the prostate and seminal vesicles (35). SRD5A is a key enzyme converting
testosterone into DHT. Thus the activity of SRD5A2 may be
associated with sperm motility, seminal volume and even sperm
count.
In our study, the heterozygote at rs13395648 was
significantly associated with semen volume. A possible explanation
for this finding is that SRD5A2 activity affected by mutation leads
to a reduction of DHT in the microenvironment of the sexual gland
and accessory sexual gland, and causes seminal plasma secretion to
be reduced.
Additionally, the heterozygote at rs632148 was
significantly associated with sperm motility. Sperm progressive
motility is necessary for the transit of spermatozoa through the
female genital tract and fertilizing ova after ejaculation in the
natural process of pregnancy. The epididymis performs is important
in the maturation of spermatozoa, including their acquisition of
progressive motility and fertilizing ability (36). DHT is the main androgen responsible
for spermatozoan maturation in the epididymis (34). Seminal DHT is of primarily
epididymal origin (37). Thus, the
activity of SRD5A2 may be associated with sperm motility. A study
in rats on epididymal sperm maturation has shown that the number of
motile sperm is reduced following treatment with dual 5α-reductase
inhibitor (38). This indicates
the association between SRD5A2 activity and sperm motility.
The majority of the male pseudohermaphrodites with
inherited SRD5A2 enzyme deficiency, and thereby decreased DHT
production, have shown a low total sperm count, although a few of
them maintain normal sperm concentration and motility (39). The A49T mutation was
associated with a significantly higher sperm concentration
(29). Both of these findings
indicate the role of SRD5A2 on spermatogenesis. However, we found
no association between any studied polymorphism and sperm number or
concentration. This observation may be explained by the fact that
lower enzyme activities moderately altered by the SRD5A2
polymorphisms studied may be sufficient for normal spermatogenesis
(30), although they affected
semen parameters dependent on prostate or epididymis functions,
such as semen volume or sperm motility.
A previous study on finasteride and dutasteride
revealed a small but significant reduction in semen volume and
sperm motility in normal men during treatment and follow-up
(40), indicating a role of SRD5A2
activity in producing seminal plasma and gaining sperm motility.
Nevertheless, the SRD5A2 polymorphisms have shown an
association with seminal volume and sperm motility in Han-Chinese
populations with definite idiopathic infertility in our study.
However, our results should be interpreted with caution since there
is no clear mechanism explaining the differentiation of
SRD5A2 expression in the testis, epididymis, prostate and
other reproductive tissue, as well as how genetic variants
associated with moderately altered enzyme activity or intronic
polymorphisms with unknown function would affect semen quality.
We did not collect data to analyze reproductive
hormones, but results of the studies by Elzanaty et al and
Peters et al did not show any correlation between
SRD5A2 allelic variants and the serum levels of reproductive
hormones (29,30). Moreover, the treatment of healthy
men with 5α-reductase inhibitors did not cause significant changes
in their serum gonadotropin levels (40). Thus, it is supposed that the
studied polymorphisms or 5α-reductase inhibitors affect the
concentrations of DHT in the microenvironment but not in the serum,
and are unlikely to affect the serum levels of other reproductive
hormones.
In conclusion, this study, to the best of our
knowledge, is the first to examine the association of SRD5A2
genetic variants with semen quality in a Han-Chinese population
with idiopathic male infertility. Our data suggest that the
SRD5A2 genetic variants rs13395648 and rs642138 are
associated with seminal volume and sperm motility, respectively.
The discrepancies in semen quality characteristics between the
genotypes of some variants remain unclear. Thus, larger sample size
studies and in vivo or in vitro functional studies
are required to confirm the findings of this study.
Acknowledgements
This work was supported by grants from the National
Natural Science Foundation of China (grant no. 30973199) and the
National Eleventh-Five Science and Technology Support Program of
China (grant no. 2006BAI03B12).
References
|
1
|
Krausz C and Forti G: Clinical aspects of
male infertility. Results Probl Cell Differ. 28:1–21. 2000.
View Article : Google Scholar
|
|
2
|
Huynh T, Mollard R and Trounson A:
Selected genetic factors associated with male infertility. Hum
Reprod Update. 8:183–198. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Jamsai D, Reilly A, Smith SJ, et al:
Polymorphisms in the human cysteine-rich secretory protein 2
(CRISP2) gene in Australian men. Hum Reprod. 23:2151–2159. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Moore HD and Akhondi MA: In vitro
maturation of mammalian spermatozoa. Rev Reprod. 1:54–60. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Viger RS and Robaire B: Steady state
steroid 5 alpha-reductase messenger ribonucleic acid levels and
immunocytochemical localization of the type 1 protein in the rat
testis during postnatal development. Endocrinology. 136:5409–5415.
1995.
|
|
6
|
Steinberger E: Hormonal control of
mammalian spermatogenesis. Physiol Rev. 51:1–22. 1971.
|
|
7
|
Sultan C, Lumbroso S, Paris F, et al:
Disorders of androgen action. Semin Reprod Med. 20:217–228. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Makridakis NM, di Salle E and Reichardt
JK: Biochemical and pharmacogenetic dissection of human steroid 5
alpha-reductase type II. Pharmacogenetics. 10:407–413. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Debes JD and Tindall DJ: Mechanisms of
androgen-refractory prostate cancer. N Engl J Med. 351:1488–1490.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Can S, Zhu YS, Cai LQ, et al: The
identification of 5 alpha-reductase-2 and 17 beta-hydroxysteroid
dehydrogenase-3 gene defects in male pseudohermaphrodites from a
Turkish kindred. J Clin Endocrinol Metab. 83:560–569.
1998.PubMed/NCBI
|
|
11
|
Fernandez-Cancio M, Nistal M, Gracia R, et
al: Compound heterozygous mutations in the SRD5A2 gene exon 4 in a
male pseudohermaphrodite patient of Chinese origin. J Androl.
25:412–416. 2004.PubMed/NCBI
|
|
12
|
Chavez B, Valdez E and Vilchis F:
Uniparental disomy in steroid 5alpha-reductase 2 deficiency. J Clin
Endocrinol Metab. 85:3147–3150. 2000.PubMed/NCBI
|
|
13
|
Makridakis N, Ross RK, Pike MC, et al: A
prevalent missense substitution that modulates activity of
prostatic steroid 5alpha-reductase. Cancer Res. 57:1020–1022.
1997.PubMed/NCBI
|
|
14
|
Allen NE, Forrest MS and Key TJ: The
association between polymorphisms in the CYP17 and 5alpha-reductase
(SRD5A2) genes and serum androgen concentrations in men. Cancer
Epidemiol Biomarkers Prev. 10:185–189. 2001.PubMed/NCBI
|
|
15
|
Hayes VM, Severi G, Padilla EJ, et al:
5alpha-reductase type 2 gene variant associations with prostate
cancer risk, circulating hormone levels and androgenetic alopecia.
Int J Cancer. 120:776–780. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Makridakis NM, Ross RK, Pike MC, et al:
Association of mis-sense substitution in SRD5A2 gene with prostate
cancer in African-American and Hispanic men in Los Angeles, USA.
Lancet. 354:975–978. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ross RK, Pike MC, Coetzee GA, et al:
Androgen metabolism and prostate cancer: establishing a model of
genetic susceptibility. Cancer Res. 58:4497–4504. 1998.PubMed/NCBI
|
|
18
|
Ji G, Gu A, Xia Y, et al: ERCC1 and ERCC2
polymorphisms and risk of idiopathic azoospermia in a Chinese
population. Reprod Biomed Online. 17:36–41. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Osborne EC, Lynch M, McLachlan R, Trounson
AO and Cram DS: Microarray detection of Y chromosome deletions
associated with male infertility. Reprod Biomed Online. 15:673–680.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Larsen L, Scheike T, Jensen TK, et al:
Computer-assisted semen analysis parameters as predictors for
fertility of men from the general population. The Danish First
Pregnancy Planner Study Team. Hum Reprod. 15:1562–1567. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Barrett JC, Fry B, Maller J and Daly MJ:
Haploview: analysis and visualization of LD and haplotype maps.
Bioinformatics. 21:263–265. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Menkveld R, Wong WY, Lombard CJ, et al:
Semen parameters, including WHO and strict criteria morphology, in
a fertile and subfertile population: an effort towards
standardization of in-vivo thresholds. Hum Reprod. 16:1165–1171.
2001. View Article : Google Scholar
|
|
23
|
Payne AH, Kawano A and Jaffe RB: Formation
of dihydrotestosterone and other 5 alpha-reduced metabolites by
isolated seminiferous tubules and suspension of interstitial cells
in a human testis. J Clin Endocrinol Metab. 37:448–453. 1973.
View Article : Google Scholar
|
|
24
|
Lindstrom S, Adami HO, Balter KA, et al:
Inherited variation in hormone-regulating genes and prostate cancer
survival. Clin Cancer Res. 13:5156–5161. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ntais C, Polycarpou A and Ioannidis JP:
SRD5A2 gene polymorphisms and the risk of prostate cancer: a
meta-analysis. Cancer Epidemiol Biomarkers Prev. 12:618–624.
2003.PubMed/NCBI
|
|
26
|
Cicek MS, Conti DV, Curran A, et al:
Association of prostate cancer risk and aggressiveness to androgen
pathway genes: SRD5A2, CYP17, and the AR. Prostate. 59:69–76. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Salam MT, Ursin G, Skinner EC, Dessissa T
and Reichardt JK: Associations between polymorphisms in the steroid
5-alpha reductase type II (SRD5A2) gene and benign prostatic
hyperplasia and prostate cancer. Urol Oncol. 23:246–253. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Pearce CL, Makridakis NM, Ross RK, et al:
Steroid 5-alpha reductase type II V89L substitution is not
associated with risk of prostate cancer in a multiethnic population
study. Cancer Epidemiol Biomarkers Prev. 11:417–418.
2002.PubMed/NCBI
|
|
29
|
Elzanaty S, Giwercman YL and Giwercman A:
Significant impact of 5alpha-reductase type 2 polymorphisms on
sperm concentration and motility. Int J Androl. 29:414–420. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Peters M, Saare M, Kaart T, et al:
Analysis of polymorphisms in the SRD5A2 gene and semen parameters
in Estonian men. J Androl. 31:372–378. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Mahony MC, Swanlund DJ, Billeter M,
Roberts KP and Pryor JL: Regional distribution of 5alpha-reductase
type 1 and type 2 mRNA along the human epididymis. Fertil Steril.
69:1116–1121. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Berthaut I, Mestayer C, Portois MC,
Cussenot O and Mowszowicz I: Pharmacological and molecular evidence
for the expression of the two steroid 5 alpha-reductase isozymes in
normal and hyperplastic human prostatic cells in culture. Prostate.
32:155–163. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Thigpen AE, Silver RI, Guileyardo JM,
Casey ML, McConnell JD and Russell DW: Tissue distribution and
ontogeny of steroid 5 alpha-reductase isozyme expression. J Clin
Invest. 92:903–910. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Robaire B and Viger RS: Regulation of
epididymal epithelial cell functions. Biol Reprod. 52:226–236.
1995. View Article : Google Scholar
|
|
35
|
Mahendroo MS, Cala KM, Hess DL and Russell
DW: Unexpected virilization in male mice lacking steroid 5
alpha-reductase enzymes. Endocrinology. 142:4652–4662.
2001.PubMed/NCBI
|
|
36
|
Cornwall GA: New insights into epididymal
biology and function. Hum Reprod Update. 15:213–227. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Anderson RA, Kelly RW and Wu FC:
Comparison between testosterone enanthate-induced azoospermia and
oligozoospermia in a male contraceptive study. V Localization of
higher 5 alpha-reductase activity to the reproductive tract in
oligozoospermic men administered supraphysiological doses of
testosterone. J Androl. 18:366–371. 1997.
|
|
38
|
Henderson NA and Robaire B: Effects of
PNU157706, a dual 5alpha-reductase inhibitor, on rat epididymal
sperm maturation and fertility. Biol Reprod. 72:436–443. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Cai LQ, Fratianni CM, Gautier T and
Imperato-McGinley J: Dihydrotestosterone regulation of semen in
male pseudohermaphrodites with 5 alpha-reductase-2 deficiency. J
Clin Endocrinol Metab. 79:409–414. 1994.PubMed/NCBI
|
|
40
|
Amory JK, Wang C, Swerdloff RS, et al: The
effect of 5alpha-reductase inhibition with dutasteride and
finasteride on semen parameters and serum hormones in healthy men.
J Clin Endocrinol Metab. 92:1659–1665. 2007. View Article : Google Scholar : PubMed/NCBI
|