Introduction
In recent years, biochip technology has developed
extremely rapidly and may soon be utilized in routine clinical
analysis. Cytokines are known to be important substances in the
human body, possessing multiple biological activities, promoting
target cell proliferation, enhancing anti-inflammation effects and
NK cell activity, modulating cell metabolism and mediating
inflammation. Variations in the concentration of cytokines in
vivo are associated with the pathological processes of a number
of diseases (1).
The study of cytokines has developed from four
independent study areas. The first and most significant area is
immunology, specifically the study of lymphokines. The second
source of cytokine research is associated with interferons (IFNs).
Hematopoietic growth or colony-stimulating factors is the third
area and the study of nonhematopoietic growth factors is the fourth
source. The role of cytokines in the regulation of immune and
inflammatory responses is now clearly recognized and studies on
cytokines have led to their association with numerous pathological
conditions.
Cytokines are markedly associated with roles in
diseases, particularly chronic diseases during which cytokines are
released in response to infection or inflammation. In a mouse
model, blockage of interleukin (IL)-10 production was identified as
a potential therapeutic for pulmonary tuberculosis (2). In an additional study, IL-10 was
found to be critical for protecting the cerebral microcirculation
from spirochaetal injury by inhibiting the effects of tumor
necrosis factor (TNF) (3). IL-10
levels are associated with early treatment failure of advanced
stage Hodgkin's lymphoma and are independent of current known
clinical factors (4). Serum TNFα,
IL-6 and nitric oxide (NO) levels have also been identified as
biomarkers during systemic inflammatory response in chronic
obstructive pulmonary disease (COPD) patients and, to a lesser
degree, in COPD exacerbation or monitoring recovery following
exacerbation (5). Elevated levels
of TNFα and IL-6 have also been reported in coronary artery disease
(CAD) and systolic heart failure (6) and are associated with the
immunoinflammatory activation process. Finally, overproduction of
IL-6 accelerates pathogenesis of CAD in Chlamydia
pneumonia(7). A previous study
demonstrated that inhaling NO may reduce primary graft dysfunction
following lung transplantation by downregulation of the
inflammatory response through reduction of IL concentrations,
including IL-6 and −8 (8).
Alterations in cytokine levels regulate gene expression. For
example, TNFα and IL-1β induce NO production which regulates MMP-9
and TIMP-1 expression in the human mesangial cell line (9). Monocyte chemoattractant protein-1
(MCP-1) is induced by NF-κB activation in homocysteine which is an
independent risk factor for cardiovascular disorders when
overaccumulated in the blood (10). In addition, serum cytokine levels,
including IL-6, −1β, −2, −4, −5, −7, −8, −10, −12, −13 and −17,
TNFα, granulocyte colony-stimulating factor (G-CSF), granulocyte
macrophage colony-stimulating factor (GM-CSF), macrophage
inflammatory protein (MIP)-1β and MCP-1, have been found to vary in
middle-aged females and may be associated with the progression of
various diseases in pre- and postmenopausal females (11). Vascular endothelial growth factor
(VEGF) levels in peripheral blood are used to determine asthma
progression by analyzing elevation in airway inflammation and
remodeling in asthma (12).
Therefore, cytokines are important in pathogenesis and process of
diseases. At present, a significant issue remains unsolved
concerning the storage and evaluation of cytokine samples.
The EVIDENCE 180 analyzer is a fully automated
Biochip Array system and a number of immunoassay-based
multi-analyte arrays have been developed for this system. The
Cytokine Array biochip quantitatively and simultaneously analyzes
levels of IL-1α, −1β, −2, −4, −6, −8 and −10, VEGF, IFNγ, epidermal
growth factor (EGF), MCP-1 and TNFα.
In the present study, the stability of cytokines in
various sample types and the effect of different storage conditions
on the samples was analyzed. In addition, the within- and
between-run precision of the EVIDENCE 180 system was evaluated.
Materials and methods
Study population
Nine individual volunteers (6 males, 18–46 years
old; 3 females, 19–36 years old) provided blood samples for the
current study. Informed consent was obtained from each volunteer
and all individuals passed a physical examination. This study was
conducted in accordance with the Helsinki Declaration and approved
by the Medical Ethics Committee of the Chinese PLA General Hospital
(Beijing, China).
Comparison of cytokine levels in various
samples
Nine volunteers provided 9 ml of whole blood
collected into gel, glass and lithium heparin (LH) tubes (all
sample tubes purchased from BD Biosciences, Franklin Lakes, NJ,
USA). Samples were obtained in the morning following a 12-h fast.
Each type of sample tube held 3 ml whole blood. Blood samples were
kept at room temperature for 30 min and then centrifuged for 10 min
at 3,000 rpm. Following centrifugation, the serum and plasma
samples of each volunteer were immediately separated and analyzed
to compare cytokine levels.
Freeze/thaw cycles study
Briefly, each volunteer provided 30 ml whole blood
in total into gel, glass and LH tubes in the morning following a
12-h fast. Each sample tube held 10 ml whole blood. Blood samples
were kept at room temperature for 30 min and then centrifuged for
10 min at 3,000 rpm. Following centrifugation, samples (serum or
plasma) were divided into one 0.5-ml and thirty 0.3-ml aliquots.
The 3 types of 0.5-ml aliquot from each volunteer were analyzed
immediately and the results were used as baseline values for the 12
cytokines. The additional thirty 0.3-ml aliquots, stored by 3
methods, from each volunteer (total, 270 aliquots) were stored at
−80°C and thawed 1, 2, 3, 4, 5, 6, 7, 8, 9 and 10 times at room
temperature for 2 h and then stored at −80°C until analysis.
Concentration of the 12 cytokines in each sample was compared with
baseline values and plotted against the number of freeze/thaw
cycles (13).
Refrigeration study
Each volunteer provided 30 ml whole blood in total
into 2 gel, 2 glass and 2 LH tubes in the morning following a 12-h
fast. Each tube held 5 ml whole blood. Blood samples were kept at
room temperature for 30 min and then centrifuged for 10 min at
3,000 rpm. Following centrifugation, samples were subjected to
various procedures to produce 6 samples from each volunteer,
including separated and unseparated serum in gel tubes, separated
and unseparated plasma in LH tubes and separated and unseparated
serum in glass tubes. Samples were analyzed immediately and the
results used as the baseline values for the cytokines. Additional
samples were stored at 4°C for 6 and 12 h and 1, 2, 3, 4, 5 and 6
days prior to analysis. Cytokine concentrations in each sample type
were compared with baseline values.
Within- and between-run precision
To evaluate within-run precision of the 12
cytokines, high, mid and low concentration control sera (no. 2279)
were tested 10 times simultaneously. To evaluate between-run
precision of the 12 cytokines, high, mid and low concentration
control sera (no. 2295) were tested over 10 days, 1 time/day.
Precision was expressed as coefficient of variation (CV). All
controls were obtained from Randox Laboratories (Crumlin, UK).
Analyzer and reagent
The EVIDENCE 180 (14) Biochip Analyzer system and Cytokine
Array I kits (nos. 0857 and 0658, both Randox Laboratories) were
used to detect serum levels of 12 cytokines.
Statistical analysis
SPSS 13.0 software was used to perform statistical
analyses. ANOVA test was used and P<0.05 was considered to
indicate a statistically significant result.
Results
Cytokine levels in various samples
Cytokine concentrations were not found to be
significantly different between serum samples in the gel and glass
tubes (P>0.05). However, cytokine levels were identified to be
significantly different between serum and plasma samples. IL-8,
VEGF and EGF levels in plasma were significantly lower compared
with serum (P<0.01; Table I).
Cytokine levels in plasma were identified to be more stable than in
serum samples (Fig. 1).
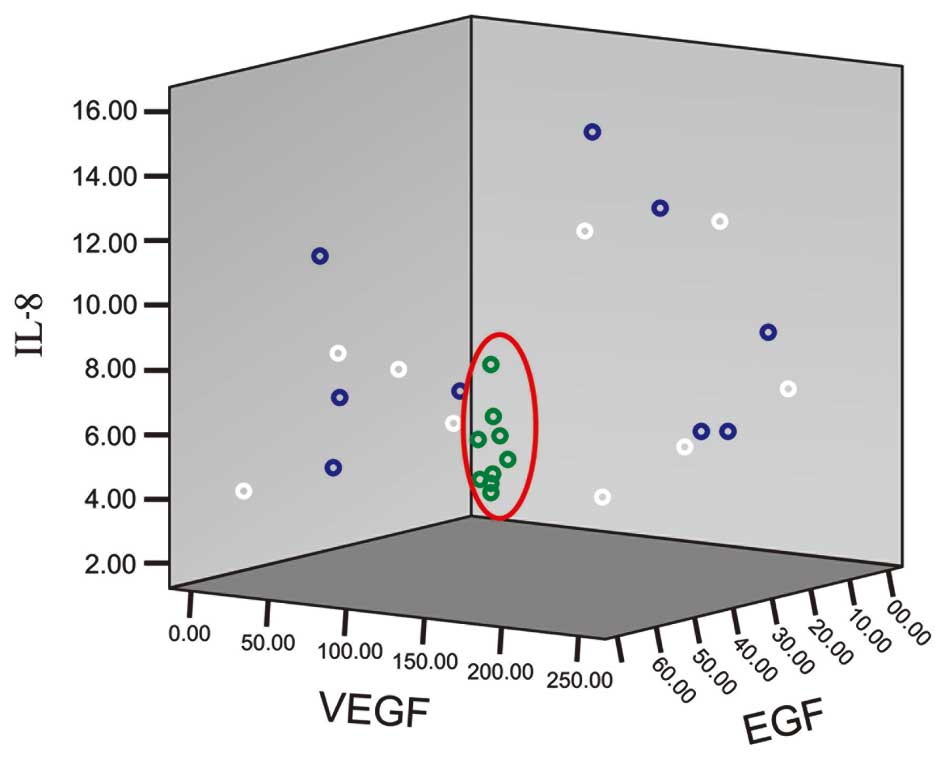 | Figure 1Levels of IL-8, VEGF and EGF in
various samples. x-, y- and z-axes show the concentration of IL-8,
VEGF and EGF (ng/l), respectively. Blue, white and green rings
represent serum in gel and glass tubes and plasma in LH tubes,
respectively. The red ring shows the plasma results. IL,
interleukin; VEGF, vascular endothelial growth factor; EGF,
epidermal growth factor; LH, lithium heparin. |
 | Table ICytokine level in various samples
(ng/l). |
Table I
Cytokine level in various samples
(ng/l).
| Cytokines | Serum in gel
tube | Serum in glass
tube | Plasma in LH
tube |
|---|
| IL-1α | 0.029±0.086 | 0.088±0.263 | 0 |
| IL-1β | 0.128±0.254 | 0.120±0.237 | 0.068±0.204 |
| IL-2 | 0 | 0.297±0.890 | 0 |
| IL-4 | 0.483±1.448 | 0.539±1.618 | 0.922±1.837 |
| IL-6 | 1.233±2.245 | 1.328±2.403 | 1.331±2.296 |
| IL-8 | 8.621±3.433 | 7.526±2.777 | 3.529±1.296a,b |
| IL-10 | 0.064±0.192 | 0.068±0.204 | 0.053±0.160 |
| VEGF | 128.09±75.470 | 134.225±78.263 | 9.639±5.039a,b |
| IFNγ | 1.141±1.441 | 1.212±1.507 | 1.205±1.155 |
| EGF | 33.967±22.337 | 38.936±21.629 | 0.510±1.039a,b |
| MCP-1 | 263.973±49.372 | 286.272±59.695 | 256.907±45.370 |
| TNFα | 8.126±9.858 | 4.009±0.938 | 2.896±0.834 |
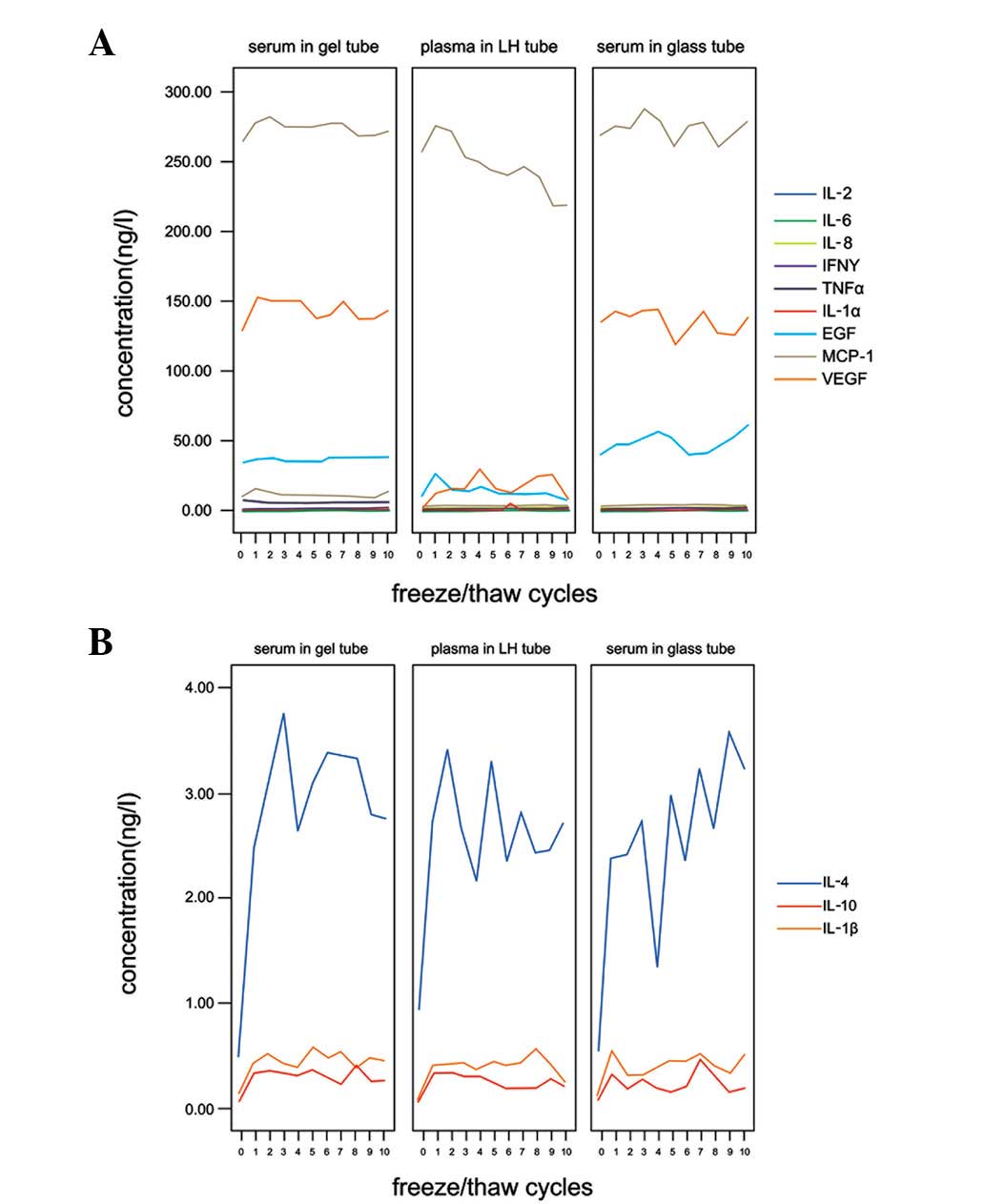
Freeze/thaw cycles
No significant difference in concentration following
10 freeze/thaw cycles was found for 9 cytokines in the 3 sample
types (P>0.05); gel, glass and LH tubes. These cytokines were
IL-1α, −2, −6 and −8, TNFα, EGF, VEGF, IFNγ and MCP-1 (Fig. 2A). IL-1β, −4 and −10 levels in the
3 sample types increased at least 3–5-fold following 1 freeze/thaw
cycle, stabilizing at these levels during the additional 9
freeze/thaw cycles (Fig. 2B).
Refrigeration study
Following storage at 4°C for 6 days, levels of 8
cytokines were unchanged in the 6 sample types; separated and
unseparated serum in gel tubes, separated and unseparated plasma in
LH tubes and separated and unseparated serum in glass tubes. These
cytokines were IL-1α, −1β, −2, −4, −6 and −10, MCP-1 and IFNγ
(Fig. 3A and B). By contrast,
IL-8, VEGF, TNFα and EGF concentrations in the 6 sample types were
identified at different levels in different samples (Fig. 3C).
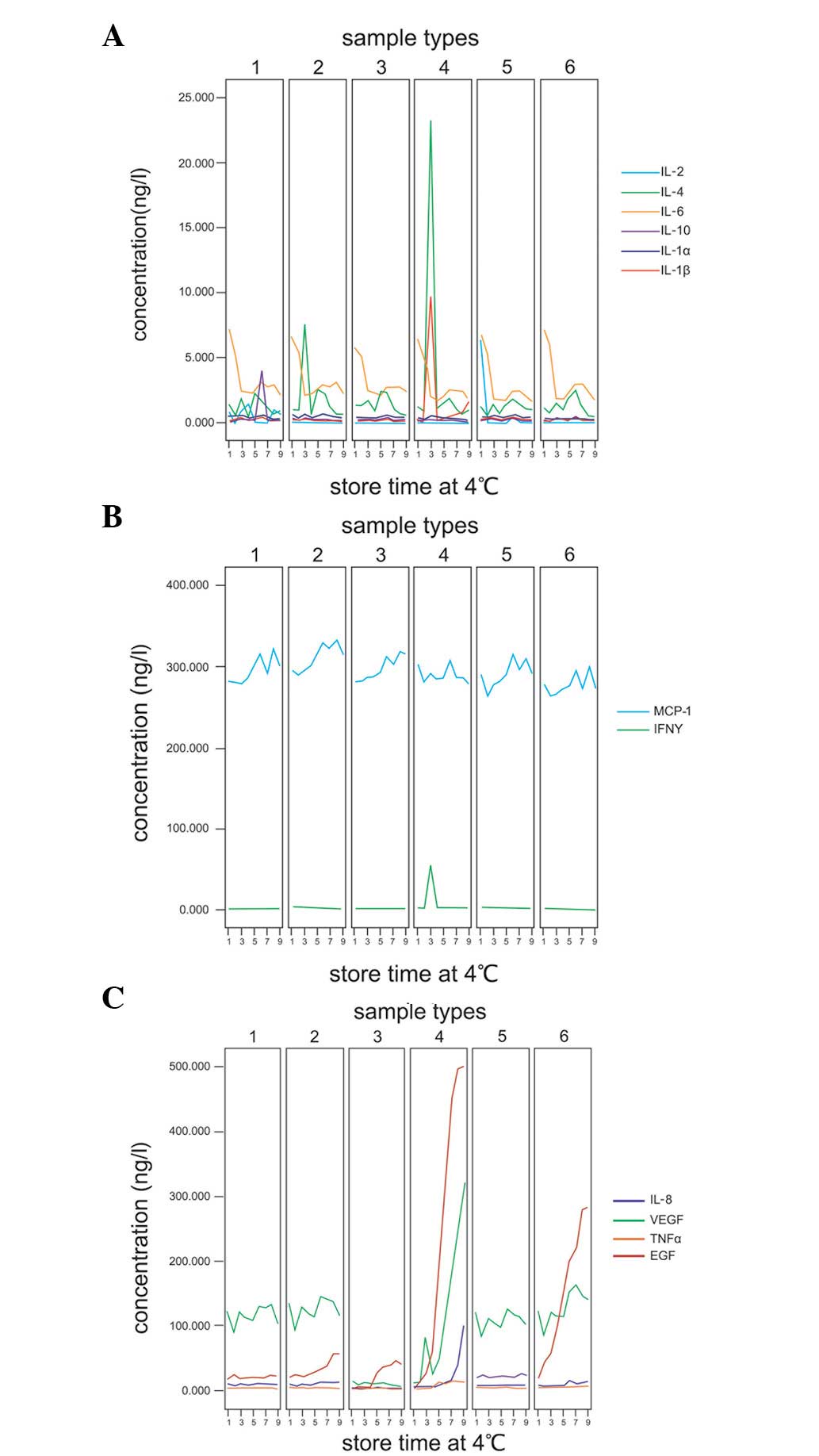 | Figure 3Cytokine concentrations following
refrigeration. (A and B) Eight cytokines of 6 sample types were
unchanged following storage at 4°C for 6 days. (C) Effect of
storage at 4°C on IL-8, VEGF, TNFα and EGF concentrations in
various sample types. Sample types 1–6 reveal separated serum in
gel tube, separated plasma in LH tube, unseparated serum in gel
tube, unseparated plasma in LH tube, separated serum in glass tube
and unseparated serum in glass tube, respectively. Storage times
1–9 include 6 sample types stored at 4°C for 0, 6 and 12 h and 1,
2, 3, 4, 5 and 6 days, respectively. IL, interleukin; VEGF,
vascular endothelial growth factor; IFN, interferon; EGF, epidermal
growth factor; MCP, monocyte chemotactic protein-1; TNF, tumor
necrosis factor; LH, lithium heparin. |
In separated serum in gel tube samples, no
significant difference in levels of the 4 cytokines were identified
when stored at 4°C for 6 days. In the unseparated serum in gel tube
samples, levels of 3 cytokines were observed to be significantly
different. IL-8 levels increased following storage at 4°C for 3
days. EGF increased by >2-fold on day 4 from 18.98 to 38.30 ng/l
and then increased to 56.33 ng/l at day 6. TNFα levels decreased on
day 6.
In separated plasma in LH tube, EGF levels were
found to be significantly increased on day 2 following storage at
4°C. In unseparated plasma in LH tube, 4 cytokines were noted at
significantly increased concentrations. EGF levels increased
extremely rapidly, >6-fold following storage at 4°C for 6 h. EGF
concentration was >500 ng/l at day 6. VEGF levels increased at
day 1, exceeding 25 times the baseline concentration at day 6. IL-8
levels increased from day 2 and were also found to exceed 25 times
the baseline levels by day 6. TNFα increased from day 2.
In separated serum samples in glass tubes, no
significant difference in levels of IL-8, VEGF, TNFα and EGF was
identified when samples were stored at 4°C for 6 days. In
unseparated serum samples in glass tubes, EGF levels were detected
at >2 times baseline levels following storage at 4°C for 6 h. At
day 6, EGF concentration was 281.92 ng/l. IL-8 and TNFα levels were
observed to be significantly increased from day 3.
Within- and between-run precision
Results for within- and between-run precision
revealed CV values <10% for 3 control levels of the 12 cytokines
tested (Tables II and III).
 | Table IIWithin and between run precision of 12
cytokines (n=10). |
Table II
Within and between run precision of 12
cytokines (n=10).
| Within run precision,
CV (%) | Between run
precision, CV (%) |
|---|
|
|
|
|---|
| Cytokines | Low | Mid | High | Low | Mid | High |
|---|
| IL-1α | 2.72 | 3.67 | 5.47 | 4.40 | 3.57 | 4.15 |
| IL-1β | 7.15 | 6.05 | 6.24 | 4.44 | 4.05 | 3.86 |
| IL-2 | 8.49 | 5.42 | 2.53 | 3.90 | 2.41 | 2.14 |
| IL-4 | 6.51 | 7.95 | 8.78 | 5.43 | 6.32 | 4.04 |
| IL-6 | 4.75 | 5.80 | 8.77 | 9.90 | 5.58 | 6.49 |
| IL-8 | 7.91 | 3.85 | 8.39 | 6.63 | 6.45 | 6.34 |
| IL-10 | 3.95 | 5.42 | 4.20 | 2.20 | 3.27 | 3.58 |
| VEGF | 8.22 | 5.77 | 8.49 | 8.77 | 6.98 | 7.43 |
| IFNγ | 5.14 | 8.42 | 5.50 | 6.20 | 6.50 | 9.40 |
| EGF | 4.34 | 4.19 | 5.40 | 3.80 | 1.77 | 3.86 |
| MCP-1 | 4.47 | 3.00 | 3.98 | 5.07 | 2.42 | 3.36 |
| TNFα | 6.81 | 6.20 | 7.84 | 6.25 | 4.03 | 5.44 |
 | Table IIIWithin and between run control levels
of 12 cytokines (n=10). |
Table III
Within and between run control levels
of 12 cytokines (n=10).
| Within run control
levels (ng/l) | Between run control
levels (ng/l) |
|---|
|
|
|
|---|
| Cytokines | Low | Mid | High | Low | Mid | High |
|---|
| IL-1α | 15.45±0.42 | 58.47±2.14 | 245.08±13.41 | 62.3±2.74 | 126.4±4.52 | 258.7±10.74 |
| IL-1β | 7.72±0.55 | 32.27±1.95 | 123.86±7.73 | 31.3±1.39 | 62.9±2.55 | 128.2±4.95 |
| IL-2 | 9.68±0.82 | 51.3±2.78 | 492.27±12.47 | 121.3±4.72 | 245.5±5.93 | 505.5±10.81 |
| IL-4 | 32.82±2.14 | 227.3±18.06 | 906.7±79.62 | 246.5±13.4 | 492.6±31.11 | 978.7±39.5 |
| IL-6 | 4.04±0.19 | 17.63±1.02 | 135.69±11.99 | 32.7±3.6 | 75.8±4.23 | 154.9±10.04 |
| IL-8 | 9.64±0.76 | 121.87±4.7 | 991.97±83.23 | 259.5±17.22 | 512.8±33.1 | 1047.9±66.49 |
| IL-10 | 13.35±0.53 | 59.19±3.21 | 229.94±9.67 | 61.3±1.35 | 122.0±3.99 | 244.2±3.27 |
| VEGF | 32.18±2.64 | 104.15±6.01 | 712.76±60.48 | 112.5±9.87 | 253.8±17.72 | 801.4±59.54 |
| IFNγ | 87.74±4.51 | 260.3±21.92 | 433.11±23.84 | 125.9±7.8 | 229.3±14.9 | 523.7±49.21 |
| EGF | 14.58±0.63 | 64.3±2.7 | 245.84±13.27 | 60.3±2.29 | 124.4±2.2 | 243.7±9.42 |
| MCP-1 | 25.33±1.13 | 123.13±3.69 | 517.74±20.61 | 120.8±6.12 | 250.4±6.07 | 485.4±16.3 |
| TNFα | 12.97±0.88 | 55.97±3.47 | 480.65±37.69 | 124.7±7.8 | 251.2±10.11 | 511.2±27.81 |
Discussion
In the present study, 12 cytokines/sample were
measured simultaneously on a biochip platform. Results of various
sample types obtained from 9 human volunteers demonstrated that
concentrations of the 12 analyzed cytokines did not vary between
serum in gel or glass tubes. However, levels were found to be
significantly different between serum and plasma. IL-8, VEGF and
EGF levels were lower in plasma compared with serum. VEGF in plasma
was >10 times lower than in serum and EGF was >60 times
lower. These observations may be associated with the blood
agglutination process, since blood cells release a number of
cytokines, including IL-8, VEGF and EGF, during the blood
agglutination process. Therefore, the concentration of cytokines in
plasma may reflect levels of various cytokines in the human body.
By contrast, the standard deviation of the 12 cytokines was lower
in plasma than in serum (Table I)
and the levels of IL-8, VEGF and EGF were concentrated in a small
area (Fig. 1). These results
indicate that cytokine levels in plasma are more stable compared
with serum. Therefore, plasma is a better matrix than serum for the
evaluation of cytokines in clinical or research analyses.
Cytokines in serum and plasma were observed to be
affected by multiple freeze/thaw cycles. In the present study 9
cytokines (IL-1α, −2, −6 and −8, TNFα, EGF, VEGF, IFNγ and MCP-1)
of 3 sample types presented no significant changes in concentration
following 10 freeze/thaw cycles, however, IL-1β, −4 and −10 were
found to increase significantly following 1 freeze/thaw cycle in
serum and plasma and remained stable at increased levels for an
additional 9 freeze/thaw cycles. These results indicate the
importance of avoiding freeze/thaw cycles to minimize the risk of
false values of cytokines. Where possible, determination of
cytokine levels must be performed immediately following sample
collection and if samples are stored frozen, levels must be
determined at the same time to establish simultaneous
comparison.
Following storage at 4°C for 6 days, levels of
IL-1α, −1β, −2, −4, −6 and −10, MCP-1 and IFNγ in 6 sample types
were unchanged. By contrast, IL-8, VEGF, TNFα and EGF
concentrations were different. In separated serum samples in gel
and glass tubes stored at 4°C for 6 days, no difference in levels
of the cytokines was found. However, in unseparated serum in gel
tubes, significant changes in the levels of 3 cytokines were
observed. IL-8 increased following storage at 4°C for 3 days, EGF
increased on day 4 and its concentration more than doubled from
18.98 to 38.30 ng/l, increasing to 56.33 ng/l by day 6, however
TNFα decreased at day 6. In separated plasma samples in LH tubes,
EGF concentration increased on day 2. In unseparated plasma in LH
tubes, 4 cytokines increased markedly. EGF levels increased
extremely rapidly to >6 times that of baseline following storage
at 4°C for 6 h and its concentration was >500 ng/l at day 6.
VEGF increased on day 1 and by day 6 had increased by >25-fold.
IL-8 levels increased from day 2 and were 25 times higher than
baseline at day 6. TNFα increased from day 2. These observations
may be due to blood cells secreting these cytokines continuously,
particularly EGF and VEGF. In unseparated serum samples in glass
tubes, EGF concentration more than doubled following storage at 4°C
for 6 h and at 6 days its concentration was 281.92 ng/l. IL-8 and
TNFα increased from day 3. These results demonstrate that plasma or
serum must be separated immediately following centrifugation and
cytokine concentrations in the sample must be measured as soon as
possible.
CV values <10% were obtained for the within- and
between-run precisions of the simultaneous immunoassays for the 12
cytokines assessed on EVIDENCE 180 for 3 concentrations. This
result indicated that the cytokine biochip array had good stability
and precision in measuring cytokines.
The use of a multi-analytical approach for the
simultaneous measurement of 12 cytokines/sample using biochip array
technology on the fully automated analyzer EVIDENCE 180 allows
determination of cytokine levels in real time, which is
advantageous for studies on the complex cytokine network and the
role played by cytokines in normal and pathological processes.
Assaying multiple cytokines in a single sample is likely to become
an important technique in laboratory medicine. The knowledge gained
from multiple cytokine analysis may enable improved diagnosis and
disease management (15,16).
In conclusion, the current study has identified
improved methods for the detection of multiple cytokines in
clinical and research analyses. Firstly, plasma cytokines
accurately reflect levels of cytokines in the human body and a
reduced number of factors compromise plasma levels compared with
serum. Therefore, plasma is the most appropiate and stable sample
type for the determination of multiple cytokine levels. Secondly,
repeated freeze/thaw cycles of the samples must be avoided.
Thirdly, storage at 4°C is likely to affect the concentration of a
number of cytokines, therefore, plasma and serum must be separated
immediately following centrifugation and the concentration of
cytokines in the sample must be measured as soon as possible.
Acknowledgements
The current study was supported by grants from the
Doctor Innovation Funding (11BCZ07) and the National Natural
Science Funding (81071413), supported by the Chinese PLA General
Hospital and Military Postgrad Medical College and the National
Natural Science Foundation of China, respectively.
References
|
1
|
Berrahmoune H, Lamont JV, Herbeth B,
FitzGerald PS and Visvikis-Siest S: Biological determinants of and
reference values for plasma interleukin-8, monocyte chemoattractant
protein-1, epidermal growth factor and vascular endothelial growth
factor: Results from the STANISLAS cohort. Clin Chem. 52:504–510.
2006. View Article : Google Scholar
|
|
2
|
Beamer GL, Flaherty DK, Assogba BD,
Stromberg P, Gonzalez-Juarrero M, de Waal Malefyt R, Vesosky B and
Turner J: Interleukin-10 promotes Mycobacterium tuberculosis
disease progression in CBA/J mice. J Immunol. 181:5545–5550.
2008.PubMed/NCBI
|
|
3
|
Londono D, Carvajal J, Arguelles-Grande C,
Marques A and Cadavid D: Interleukin 10 protects the brain
microcirculation from spirochetal injury. J Neuropathol Exp Neurol.
67:976–983. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Rautert R, Schinkothe T, Franklin J,
Weihrauch M, Boll B, Pogge E, Bredenfeld H, Engert A, Diehl V and
Re D: Elevated pretreatment interleukin-10 serum level is an
International Prognostic Score (IPS)-independent risk factor for
early treatment failure in advanced stage Hodgkin lymphoma. Leuk
Lymphoma. 49:2091–2098. 2008. View Article : Google Scholar
|
|
5
|
Karadag F, Karul AB, Cildag O, Yilmaz M
and Ozcan H: Biomarkers of systemic inflammation in stable and
exacerbation phases of COPD. Lung. 186:403–409. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kosmala W, Derzhko R, Przewlocka-Kosmala
M, Orda A and Mazurek W: Plasma levels of TNF-alpha, IL-6 and IL-10
and their relationship with left ventricular diastolic function in
patients with stable angina pectoris and preserved left ventricular
systolic performance. Coron Artery Dis. 19:375–382. 2008.
View Article : Google Scholar
|
|
7
|
Jha HC, Srivastava P, Sarkar R, Prasad J
and Mittal A: Chlamydia pneumoniae IgA and elevated level of
IL-6 may synergize to accelerate coronary artery disease. J
Cardiol. 52:140–145. 2008. View Article : Google Scholar
|
|
8
|
Moreno I, Mir A, Vicente R, Pajares A,
Ramos F, Vicente JL and Barbera M: Analysis of interleukin-6 and
interleukin-8 in lung transplantation: correlation with nitric
oxide administration. Transplant Proc. 40:3082–3084. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Nee L, O'Connell S, Nolan S, Ryan MP and
McMorrow T: Nitric oxide involvement in TNF-alpha and IL-1
beta-mediated changes in human mesangial cell MMP-9 and TIMP-1.
Nephron Exp Nephrol. 110:e59–e66. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Cheung GT, Siow YL and OK: Homocysteine
stimulates monocyte chemoattractant protein-1 expression in
mesangial cells via NF-kappaB activation. Can J Physiol Pharmacol.
86:88–96. 2008. View
Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yasui T, Uemura H, Yamada M, Matsuzaki T,
Tsuchiya N, Noguchi M, Yuzurihara M, Kase Y and Irahara M:
Associations of interleukin-6 with interleukin-1beta, interleukin-8
and macrophage inflammatory protein-1beta in midlife women.
Cytokine. 41:302–306. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lee KY, Lee KS, Park SJ, Kim SR, Min KH,
Choe YH and Lee YC: Clinical significance of plasma and serum
vascular endothelial growth factor in asthma. J Asthma. 45:735–739.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Schoonenboom NS, Mulder C, Vanderstichele
H, Van Elk EJ, Kok A, Van Kamp GJ, Scheltens P and Blankenstein MA:
Effects of processing and storage conditions on amyloid beta (1–42)
and tau concentrations in cerebrospinal fluid: implications for use
in clinical practice. Clin Chem. 51:189–195. 2005.
|
|
14
|
Fitzgerald SP, Lamont JV, McConnell RI and
Benchikhel O: Development of a high-throughput automated analyzer
using biochip array technology. Clin Chem. 51:1165–1176. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Matei I and Matei L: Cytokine patterns and
pathogenicity in autoimmune diseases. Rom J Intern Med. 40:27–41.
2002.PubMed/NCBI
|
|
16
|
Moser B and Willimann K: Chemokines: role
in inflammation and immune surveillance. Ann Rheum Dis. 63(Suppl
2): ii84–ii89. 2004. View Article : Google Scholar : PubMed/NCBI
|