Introduction
Opioids, including morphine and fentanyl, have been
widely used to treat various types of acute and chronic pain for a
number of years. However, the analgesic efficacy of opioids is well
known to vary widely among individuals (1). In recent years, with the development
of pharmacogenomics, it has been hypothesized that opioids exhibit
various interindividual reactions due to gene sequence differences
in drug-metabolizing enzymes and drug transporters and targets
(2,3). Various factors, including gender,
age, weight, organ function and disease severity, are associated
with differences in individual response to these drugs, however,
30–95% of drug response and disposal differences is due to genetic
factors (4).
Fentanyl is mainly metabolised to norfentanyl by the
cytochrome P450 3A4 (CYP3A4) enzyme and is the primary route of
hepatic biotransformation (5,6).
Interindividual differences in the activity and expression of the
CYP3A4 enzyme contribute to its metabolising substrate
pharmacokinetics (6,7). Previous studies showed that 30–90% of
the interindividual variability in the activity and expression of
CYP3A4 is predominantly attributed to genetic polymorphisms
encoding their genes (2,4,8).
Forty single nucleotide polymorphisms (SNPs) of CYP3A4 alleles have
been identified. CYP3A4*18B, a SNP in intron 10 of the CYP3A4 gene,
was initially identified in Japanese patients and is present at
high frequency in Asian populations (9,10).
This SNP creates a G→A substitution at position 82,266, which
correlates with increased CYP3A4 enzymatic activity (9,10).
Based on these studies, it was hypothesized that the CYP3A4*18B
allele may be associated with variations in fentanyl metabolism. To
date, a limited number of association studies between the
CYP3A4*18B polymorphism and postoperative fentanyl requirements
have been performed (11,12).
The human micro-opioid receptor gene (OPRM1) encodes
the μ opioid receptor, the main target of analgesia for fentanyl. A
large number of SNPs (>700) have been identified in the OPRM1
gene (refer to the dbSNP database, the NCBI database of genetic
variations). The OPRM1 A118G mutation is the most common functional
variation at position 118 in exon 1. The mutation leads to an
exchange of the amino acid asparagine to aspartate at position 40
(N40D) on the extracellular N-terminal domain, affecting a putative
glycosylation site of the receptor (13). Studies on fentanyl and the A118G
SNP remain scarce although fentanyl is one of the most commonly
used opioid analgesics in clinical anesthesia and acute pain
therapy. Authors have reported that subjects with the G allele of
the OPRM1 A118G SNP were less sensitive to fentanyl or that the
fentanyl dose was greater compared with AA subjects in patients
with postoperative pain or labor analgesia (14–16).
However, results of the additional three studies on this
association were not consistent with these observations (17–19).
The majority of studies have analyzed the
association between individual SNPs selected from a single gene
region and morphine effects. Pain is a complex human experience and
it is likely that the interaction of multiple genes and
environmental factors affect the clinical efficacy of opioids.
Therefore, a number of studies have aimed to determine the
correlation between gene-gene interactions and morphine responses
(20,21). To the best of our knowledge no
studies have analyzed whether the combined mutation of OPRM1 and
CYP3A4 polymorphisms affects fentanyl consumption and
fentanyl-associated side effects. In the present study, patients
undergoing radical gastrectomy were selected to evaluate whether
the genetic polymorphisms of CYP3A4*18B and OPRM1 A118G affect
postoperative fentanyl requirements for analgesia. In addition, the
combined effects of OPRM1 and CYP3A4 polymorphisms on fentanyl
analgesia and side effects in Chinese Han patients were
explored.
Materials and methods
Patients
Between May 2008 and January 2010, 128 patients with
an American Society of Anesthesiologists physical status of I-III,
aged 20–75 years and undergoing radical gastrectomy were enrolled
in the present study. Liver and renal functions were normal in all
patients and no history of chronic pain, long-term application of
analgesic and cortisol drugs, alcohol or drug abuse or allergies to
fentanyl were found. Patients with a history of severe
cardiovascular disease, diabetes mellitus, psychiatric disorders or
were currently pregnant or at lactation period were excluded.
Patients who had consumed drugs (1 week) or foods (3 days) known to
inhibit or induce the expression of CYP3A4 enzymes prior to surgery
were also excluded. The study design was approved by the
Institutional Ethics Committee of the Third Xiangya Hospital of
Central South University (Changsha, China). Signed informed consent
was obtained from all patients.
Preoperative psychological
evaluation
Evaluation of patient preoperative trait/state
anxiety and depression was performed 1 night prior to surgery by
two psychiatric doctors using the State-Trait Anxiety Inventory
(22) and Self-Rating Depression
Scale (23).
Anesthetic methods
No premedication was used. All patients received
general anesthesia of 0.05 mg/kg midazolam, 0.2 mg/kg etomidate, 5
μg/kg fentanyl and 0.12 mg/kg vecuronium. End tidal CO2
partial pressure was maintained between 35 and 40 mmHg by
mechanical ventilation. Propofol and remifentanil were infused
through micropumps and sevoflurane was inhaled to maintain
anesthesia. Drug dosage was adjusted according to alterations in
auditory-evoked potential and hemodynamics. An intermittent dose of
0.03–0.06 mg/kg vecuronium was administered to maintain adequate
surgical muscle relaxation. During surgery, fentanyl infusion was
discontinued following abdominal cavity opening and the total dose
of fentanyl remained at 12 μg/kg to avoid a residual effect on
postoperative fentanyl dose. The electrocardiogram, non-invasive
blood pressure, pulse oximetry and arterial blood gas analysis were
monitored.
Postoperative analgesia
Following radical gastrectomy, patients were sent to
the postanesthesia care unit (PACU). The trachea was extubated and
the pain severity of patients was assessed. Patients were
intravenously injected with a 20-μg bolus of fentanyl until a
visual analog scale (VAS: O, no pain; 10, unbearable pain) until a
visual analog scale value< 3 was obtained. Patient-controlled
intravenous analgesia (PCIA) commenced when patients perceived
slight pain (VAS 1–3). That is, patients were administered fentanyl
by PCIA when a slightly painful state (VAS 1–3) was reported.
Patients were excluded if their recovery time of pain (VAS>0)
exceeded 3 h or opioid receptor antagonist was provided. The
electronic patient controlled analgesia (PCA) pump was filled with
30 μg/kg fentanyl with 0.9% normal saline diluted to 240 ml. The
PCA was programmed to administer 1.5 ml/h background infusion with
a 20-μg bolus of fentanyl solution, with a 5-min lockout time. PCA
was continued for 48-h following surgery. Postoperative pain was
maintained at VAS<3 at rest. When patients experienced VAS>3,
despite being on PCA, the dose of fentanyl was increased by the
patient by pushing the bolus button, no other rescue drugs other
than intravenous fentanyl were used within the first 48 h following
surgery. Nausea and vomiting following radical gastrectomy is
common, therefore all patients were intravenously administered 8 mg
ondansetron. Post-operative non-invasive blood pressure, heart
rate, pulse oxygen saturation and VAS were documented 2, 6, 12, 24
and 48 h following surgery. The total amount of PCA fentanyl was
recorded at 24 and 48 h. Adverse effects associated with fentanyl
administration, including nausea, vomiting and dizziness, were
assessed as events for 48 h.
Genotyping assays
Peripheral venous blood (2 ml) was obtained from
each patient and placed in an EDTA tube. DNA was extracted from
leukocytes using a standard phenol-chloroform procedure and was
stored at 4–8°C. OPRM1 A118G genotyping was performed by DNA
sequence analysis of polymerase chain reaction (PCR)-amplified DNA.
Primers were designed by primer design software Oligo 6.0 to cover
the polymorphic site. OPRM1 A118G was amplified by PCR using the
following primers: forward, GAAAAGTCTCGGTGCTCCTG and reverse,
GGAGTAGAGGGCCATGATCG. DNA sequence of the fragment was determined
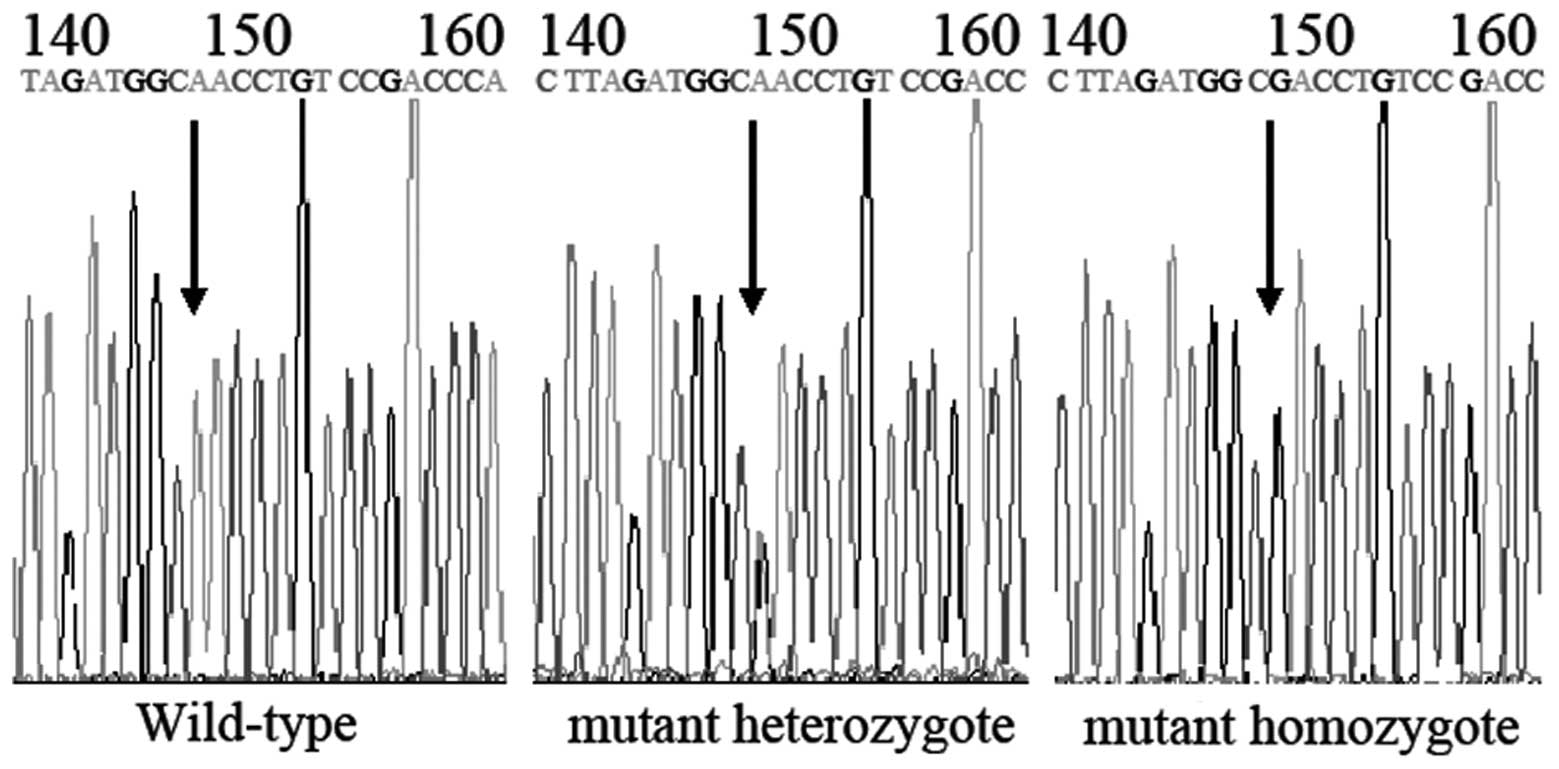
using the automated sequencer ABI-PRISM 3730 Genetic Analyzer
(Sangon Biotech Co. Ltd., Shanghai, China; Fig. 1). CYP3A4*18B was amplified by PCR
using the following primers: forward, CACCCTGATGTCCAGCAGAAACT and
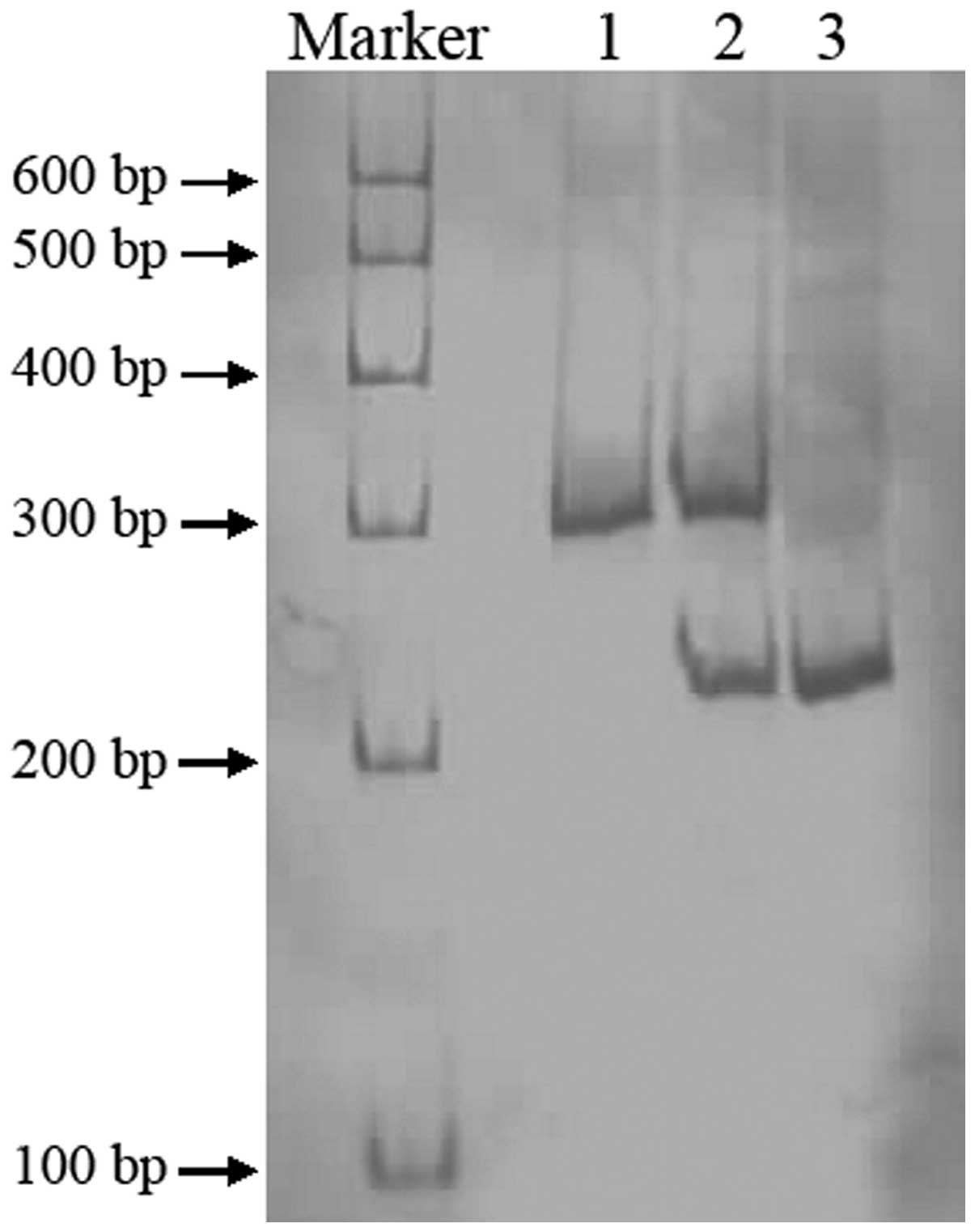
reverse, AAT AGAAAGCAGATGAACCAGAGCC. PCR products of CYP3A4*18B
were digested by RsaI, resolved by electrophoresis on a
non-denaturing polyacrylamide gel and stained by silver nitrate
(Fig. 2).
Statistical analysis
Clinical studies and genotyping were performed blind
with regards to patient clinical outcome. Statistical analysis was
performed using SPSS 16.0 (SPSS Inc., Chicago, IL, USA).
Demographic and clinical data were presented as the mean ± SD,
median (interquartiles) and counts, as appropriate. P<0.05 was
considered to indicate a statistically significant difference. The
Chi-square test was used to detect Hardy-Weinberg equilibrium.
Depending on the data form, Fisher's exact, Mann-Whitney U or
t-tests were used to evaluate whether clinical parameters
(including age, gender, body weight, height, surgery duration,
incision length, preoperative state anxiety, trait anxiety,
depression and 24 and 48 h VAS pain score) were significantly
different between genotypes. In the present study, 24- and 48-h
postoperative fentanyl dose was not normally distributed.
Therefore, non-parametric analyses, including the Mann-Whitney U
and Kruskal Wallis tests, were performed to detect the effect of
genotype on PCA fentanyl dose. The incidence of adverse effects was
analyzed using the Chi-square or Fisher's exact tests.
Results
Patient information
In the present study, 128 patients were enrolled. Of
these, 31 were not included due to no available blood sample or
failure to measure the blood sample (n=14), condition changes
(n=6), severe dizziness or vomiting (n=3) or the patient withdrawal
from the study (n=8). In total, 97 patients were analyzed in this
study. All 97 patients were of Chinese Han ethnicity. Biological
and clinical data of the patients are summarized in Tables I and II and no significant differences were
detected among the genotype groups (P>0.05).
 | Table IDemographic and clinical data for
OPRM1 A118G and CYP3A4*18B. |
Table I
Demographic and clinical data for
OPRM1 A118G and CYP3A4*18B.
| Characteristics | OPRM1 A118G | P-value | CYP3A4*18B | P-value |
|---|
|
|
|---|
| AA | AG | GG | *1*1 | *1/*18B | *18B/*18B |
|---|
| Patients, n | 42 | 41 | 14 | | 48 | 43 | 6 | |
| Age, years | 51.0±12.4 | 53.8±13.5 | 51.7±13.2 | 0.622 | 53.2±12.1 | 52.1±13.5 | 45.5±15.6 | 0.395 |
| Male/female | 22/20 | 27/14 | 11/3 | 0.171 | 28/20 | 29/14 | 3/3 | 0.555 |
| Height, cm | 161.3±7.8 | 162.3±7.3 | 165.4±7.0 | 0.210 | 162.2±7.4 | 162.8±8.1 | 159.8±4.3 | 0.664 |
| Weight, kg | 53.9±10.8 | 55.5±9.9 | 59.7±10.7 | 0.195 | 56.7±11.1 | 54.8±10.0 | 49.7±8.2 | 0.271 |
| Duration of surgery,
min | 211.5±60.4 | 190.2±74.4 | 181.8±67.3 | 0.225 | 194.0±73.2 | 205.1±62.7 | 182.5±66.7 | 0.627 |
| Length of incision,
cm | 19.0±2.5 | 19.6±3.4 | 20.6±4.6 | 0.287 | 20.0±3.7 | 19.0±2.9 | 19.3±2.0 | 0.348 |
| Preoperative state
anxiety | 41.5±7.9 | 42.1±7.8 | 40.6±6.5 | 0.847 | 42.7±7.3 | 41.2±7.4 | 34.5±8.7 | 0.112 |
| Preoperative trait
anxiety | 43.9±8.0 | 45.1±9.0 | 45.8±5.8 | 0.756 | 45.5±7.7 | 44.7±7.5 | 38.5±11.9 | 0.250 |
| Preoperative
depression | 37.0±7.8 | 35.1±6.2 | 37.0±6.0 | 0.559 | 35.8±6.7 | 37.8±6.7 | 30.0±5.3 | 0.087 |
| VAS pain score 24
h | 2.0 (4.2) | 2.0 (4.3) | 1.9 (4.2) | 0.969 | 1.9 (4.3) | 2.0 (4.0) | 2.1 (4.5) | 0.606 |
| VAS pain score 48
h | 1.9 (3.4) | 2.0 (3.4) | 1.9 (3.7) | 0.808 | 1.9 (3.7) | 2.0 (3.2) | 2.0 (3.6) | 0.733 |
 | Table IIDemographic and clinical data for
combined genetic variation of OPRM1 A118G and CYP3A4*18B. |
Table II
Demographic and clinical data for
combined genetic variation of OPRM1 A118G and CYP3A4*18B.
|
Characteristics | AA + *1/*1 | AG + *1/*18B | AG + *1/*1 | AA + *1/*18B | *18B/*18B | P-value |
|---|
| Patients, n | 21 | 17 | 22 | 18 | 6 | - |
| Age, years | 52.1±10.6 | 54.2±13.2 | 54.0±14.4 | 52.0±13.4 | 45.5±15.6 | 0.680 |
| Male/female | 11/10 | 11/6 | 14/8 | 11/7 | 3/3 | 0.905 |
| Height, cm | 160.2±7.8 | 160.1±8.3 | 163.7±6.5 | 163.3±8.2 | 159.8±4.3 | 0.363 |
| Weight, kg | 54.0±12.3 | 51.0±8.3 | 59.3±10.0 | 55.4±9.0 | 49.7±8.2 | 0.081 |
| Duration of
surgery, min | 214.4±68.2 | 204.0±72.0 | 185.9±77.1 | 210.3±53.4 | 182.5±66.7 | 0.617 |
| Length of incision,
cm | 19.5±2.2 | 19.0±2.5 | 20.2±4.0 | 18.4±3.0 | 19.3±2.0 | 0.474 |
| Preoperative state
anxiety | 44.5±5.2 | 44.0±5.6 | 42.4±8.2 | 39.7±8.6 | 34.5±8.7 | 0.156 |
| Preoperative trait
anxiety | 45.3±8.2 | 45.0±10.4 | 45.7±8.3 | 44.0±6.4 | 38.5±11.9 | 0.650 |
| Preoperative
depression | 36.3±8.9 | 36.1±6.5 | 35.4±5.8 | 38.0±7.2 | 30.0±5.3 | 0.406 |
| VAS pain score 24
h | 1.9 (3.9) | 2.1 (3.1) | 1.9 (4.9) | 1.9 (4.7) | 2.1 (4.5) | 0.586 |
| VAS pain score 48
h | 1.9 (3.4) | 2.1 (2.5) | 1.9 (3.9) | 1.9 (3.5) | 2.0 (3.6) | 0.460 |
Genotype and allele frequency of OPRM1
A118G, CYP3A4*18B and the genetic combination of these two
SNPs
Of the 97 patients, 42 patients were wild-type
homozygotes (AA), 41 heterozygotes (AG) and 14 mutant homozygotes
(GG), with the frequency of the G allele being 35.6% in the OPRM1
A118G polymorphism. There were 48 wild-type homozygotes (*1/*1), 43
heterozygotes (*1/*18B) and 6 mutant homozygotes (*18B/*18B), the
frequency of the *18B allele was 28.4% in the CYP3A4*18B
polymorphism. Allele frequency was found to be in Hardy-Weinberg
equilibrium (P>0.05) (Table
III). To analyze gene-gene interactions, SNPs were combined in
pairs in 9 possible combinations in 97 patients, including 21 with
AA + *1/*1 (21.6%), 18 with AA + *1/*18B (18.6%), 3 with AA +
*18B/*18B (3.1%), 22 with AG + *1/*1 (22.7%), 17 with AG + *1/*18B
(17.5%), 2 with AG + *18B/*18B (2.1%), 5 with GG + *1/*1 (5.2%), 8
with GG + *1/*18B (8.2%) and 1 with GG + *18B/*18B (1.0%). Since
only 6 subjects were identified with *18B/*18B, they were combined
into a single group, thus 7 groups of combinations were compared in
total.
 | Table IIIOPRM1 A118G and CYP3A4*18B genetic
variation in patients. |
Table III
OPRM1 A118G and CYP3A4*18B genetic
variation in patients.
| Genotypes/
allele | No. of
patients | Frequency (%) | Fentanyl dose for
24 h (μg/kg) | P-value | Fentanyl dose for
48 h (μg/kg) | P-value |
|---|
| OPRM1 A118G | 97 | 100 | | | | |
| AA | 42 | 43.3 | 11.8±6.1 | 0.857 | 21.3±8.6 | 0.997 |
| AG | 41 | 42.3 | 10.6±4.5 | | 20.8±7.5 | |
| GG | 14 | 14.4 | 10.5±3.8 | | 20.8±6.5 | |
| CYP3A4*18B | 97 | 100 | | | | |
| *1/*1 | 48 | 49.5 | 11.5±5.5 | 0.432 | 22.5±7.4 | 0.032a |
| *1/*18B | 43 | 44.3 | 11.0±5.1 | | 20.3±8.1 | |
| *18B/*18B | 6 | 6.2 | 9.1±3.7 | | 16.3±4.0 | |
| Joint genotype
combination | 84 | 86.6 | | | | |
| AA + *1/*1 | 21 | 21.6 | 12.8±7.1 | 0.535 | 23.2±9.0 | 0.021a |
| AG + *1/*18B | 17 | 17.5 | 10.5±5.0 | | 19.4±8.2 | |
| AG + *1/*1 | 22 | 22.7 | 11.2±4.1 | | 23.5±5.9 | |
| AA + *1/*18B | 18 | 18.6 | 11.3±5.3 | | 20.4±8.4 | |
| *18B/*18B | 6 | 6.2 | 9.1±3.7 | | 16.3±4.0 | |
Postoperative fentanyl dose and
polymorphisms of OPRM1 A118G and CYP3A4*18B
Fentanyl dose was 5.2–17.8 and 12.2–30.7 μg/kg at
postoperative 24 and 48 h, respectively. No correlation was
observed in the fentanyl analgesic dose at postoperative 24 and 48
h between the genotype groups of OPRM1 A118G (P>0.05). In
addition, the fentanyl analgesic dose at postoperative 24 h was not
found to be significantly different between the genotype groups of
CYP3A4*18B (P>0.05). However, 48 h following surgery, patients
with CYP3A4*18B/*18B required less fentanyl than patients with
CYP3A4*1/*1 (P=0.032; Table
III), but no significant difference in fentanyl dose was noted
between CYP3A4*18B/*18B and CYP3A4*1/*18B or between CYP3A4*1/*18B
and CYP3A4*1/*1 (P>0.05). In the joint genetic effect, there
were four statistically different groups: 1, patients with OPRM1 AA
and CYP3A4*1*18B polymorphisms received fewer fentanyl doses during
the 48-h period compared with those with OPRM1 AG and CYP3A4*1*1
(P=0.049); 2, patients with OPRM1 AG and CYP3A4*1*18B polymorphisms
received significantly fewer fentanyl doses during the 48-h period
compared with those with OPRM1 AG and CYP3A4*1*1 (P=0.010); 3 and
4, patients with CYP3A4*18B*18B polymorphisms received
significantly fewer fentanyl doses during the 48-h period compared
with those with OPRM1 AA and CYP3A4*1*1 (P=0.024) or those with
OPRM1 AG and CYP3A4*1*1 polymorphisms (P=0.006). No significant
differences were detected in the demographics, including gender
ratio, preoperative anxiety, depression and postoperative VAS pain
scores, across genotypes and joint genotype combination (P>0.05;
Tables I-III).
Side effects and polymorphisms of OPRM1
A118G and CYP3A4*18B
At 48 h following surgery, the main side effects of
fentanyl were nausea, vomiting and dizziness in 38.1, 18.6 and
32.0% of patients, respectively. No significant differences were
found in nausea, vomiting and dizziness among the genotype groups
of OPRM1 A118G and CYP3A4*18B (P>0.05) or the groups of joint
genotype combination (P>0.05; Tables IV and V). Only 2/97 patients (2.1%) developed
mild pruritus. None of the patients developed respiratory
depression or any other serious side effects.
 | Table IVComparison of incidence of nausea,
vomiting and dizziness between the genotype groups. |
Table IV
Comparison of incidence of nausea,
vomiting and dizziness between the genotype groups.
| Symptoms | OPRM1 A118G | P-value | CYP3A4*18B | P-value |
|---|
|
|
|---|
| AA | AG | GG | *1/*1 | *1/*18B | *18B/*18B |
|---|
| Patients, n | 42 | 41 | 14 | 48 | 43 | 6 | | |
| Nausea, frequency
(%) |
| No | 28 (66.7) | 24 (58.5) | 8 (57.1) | 0.692 | 27 (56.3) | 30 (69.8) | 3 (50.0) | 0.343 |
| Yes | 14 (33.3) | 17 (41.5) | 6 (42.9) | | 21 (43.7) | 13 (30.2) | 3 (50.0) | |
| Vomiting, frequency
(%) |
| No | 34 (81.0) | 33 (80.5) | 12 (85.7) | 0.905 | 39 (81.3) | 36 (83.7) | 4 (66.7) | 0.602 |
| Yes | 8 (19.0) | 8 (19.5) | 2 (14.3) | | 9 (18.7) | 7 (16.3) | 2 (33.3) | |
| Dizziness,
frequency (%) |
| No | 30 (71.4) | 26 (63.4) | 10 (71.4) | 0.705 | 28 (58.3) | 33 (76.7) | 5 (83.3) | 0.121 |
| Yes | 12 (28.6) | 15 (36.6) | 4 (28.6) | | 20 (41.7) | 10 (23.3) | 1 (16.7) | |
 | Table VIncidence of nausea, vomiting and
dizziness among genetic combination groups. |
Table V
Incidence of nausea, vomiting and
dizziness among genetic combination groups.
| Symptoms | AA + *1/*1 | AG + *1/*18B | AG + *1/*1 | AA + *1/*18B | *18B/*18B | P-value |
|---|
| Patients, n | 21 | 17 | 22 | 18 | 6 | |
| Nausea, frequency
(%) |
| No | 13 (61.9) | 11 (64.7) | 12 (54.5) | 13 (72.2) | 3 (50) | 0.784 |
| Yes | 8 (38.1) | 6 (35.3) | 10 (45.5) | 5 (27.8) | 3 (50) | |
| Vomiting, frequency
(%) |
| No | 18 (85.7) | 15 (88.2) | 17 (77.3) | 14 (77.8) | 4 (66.7) | 0.736 |
| Yes | 3 (14.3) | 2 (11.8) | 5 (22.7) | 4 (22.2) | 2 (33.3) | |
| Dizziness,
frequency (%) |
| No | 13 (61.9) | 12 (70.6) | 12 (54.5) | 14 (77.8) | 5 (83.3) | 0.472 |
| Yes | 8 (38.1) | 5 (29.4) | 10 (45.5) | 4 (22.2) | 1 (16.7) | |
Discussion
In the present study, fentanyl dose in 97 patients
was 5.2–17.8 and 12.2–30.7 μg/kg 24 and 48 h following surgery,
respectively, indicating that postoperative fentanyl doses vary
greatly among individuals. A number of factors, including the
character and intensity of external pain stimuli, age, gender,
weight and genetic variation, may affect analgesic requirements.
Among these, the contribution of variations in the CYP3A4 and OPRM1
genes has attracted considerable attention (10–21,24).
The CYP3A4*18B polymorphism was identified to be
associated with significant variability in fentanyl consumption 48
h following radical gastrectomy in Chinese Han patients. This
polymorphism was previously found at high frequencies in Asian
populations (9,10). The CYP3A4*18B mutant allele
frequency is 28.4% in patients of this study, consistent with
previous reports of 24.9% in 416 Japanese individuals (9), 22.7% in 176 Chinese patients
undergoing lower abdominal surgery (11) and 26.9% in 143 Chinese
gynecological patients (12).
Results indicate that patients with *18B/*18B consumed
significantly less fentanyl than patients with *1/*1, consistent
with previous studies (11,12).
Authors of those studies (11,12)
found that the CYP3A4*18B (CYP3A4*1G) genetic polymorphism
decreased CYP3A4 activity or fentanyl pharmacokinetics and
therefore, patients with the CYP3A4*18B/*18B genotype required
significantly less fentanyl for postoperative pain control than
patients with the wild-type or CYP3A4 *1/*18B genotype. However,
there were two differences in the present study. Firstly, in the
discussed previous studies, variations in fentanyl dose were
identified at postoperative 24 h, while in the present study,
fentanyl dose was different at postoperative 48 h only. A recent
study by Dong et al(24)
reported that patients with the *18B allele consumed less fentanyl
than the *1/*1 group at postoperative 2 and 4 h, but not at 24 and
48 h, among the three genotype groups. Second, although our results
indicate that patients with the CYP3A4*1/*18B required less
fentanyl than those with CYP3A4*1/*1, the difference was not found
to be statistically significant (P>0.05). This discrepancy may,
in part, be explained by variations in surgery types (upper vs.
lower abdominal surgery), small sample size [97 (present study) and
79 (22) vs. 176 (11) and 143 (12) patients], analgesic formula
[fentanyl only vs. fentanyl + droperidol (11,12)]
and additional unknown factors. By contrast, Hu et
al(10) reported that the
CYP3A4*18B genotype significantly affects cyclosporine
pharmacokinetics and is likely to result from higher enzymatic
activity associated with this mutation. Results of both previous
studies and the present study indicate that the CYP3A4*18B variant
allele may be critical for polymorphic CYP3A4 enzymatic activities
and thus variations in the consumption of fentanyl. The exact
functional significance of the CYP3A4*18B polymorphism requires
additional studies using different drugs in large sample sizes.
In the present study, no correlation was detected
between OPRM1 A118G and fentanyl dose at postoperative 24 and 48 h,
consistent with studies on labor analgesia and cancer pain
(17,18). However, results of this study are
inconsistent with findings of other studies (14–16)
which found that fentanyl was less effective in subjects with the G
allele of the OPRM1 A118G and these individuals required more
fentanyl for adequate postoperative pain control compared with
those with the A allele. These inconsistencies may be due to
variations in the analgesia method and the analgesic used for
postoperative pain. In this study, fentanyl only was administered
by PCIA and no rescue analgesics were used. In Hayashida et
al(16), 138 patients received
continous epidural analgesia with fentanyl or morphine with rescue
analgesics, including buprenorphine, pentazocine and pethidine, as
well as non-steroidal anti-inflamatory drugs (NSAIDs). Analgesic
requirement was determined as the sum of systemic fentanyl
equivalent doses of all opioids and NSAIDs used for analgesia
during the first 24 h following surgery. This conversion may not
reflect precise fentanyl doses due to variations in properties and
routes of different analgesics. In Fukuda et al(15), although the analgesic effects of
fentanyl were evaluated using a cold pressor prior to surgery and
found to be reduced in subjects carrying the G allele compared with
subjects not carrying this allele, the A118G SNP revealed no
significant association with postoperative 24 h fentanyl,
perioperative fentanyl or total perioperative analgesic use in
patients undergoing orofacial cosmetic surgery. This result was
consistent with present observations only with regards to the
association between the OPRM1 A118G polymorphism and postoperative
dose of fentanyl. It is likely that gene-gene interactions, not
single genes, in addition to the effect of environmental factors,
affect the clinical efficacy of opioids. Therefore, the joint
effect of OPRM1 A118G and CYP3A4*18B in predicting clinical
efficacy of fentanyl for postoperative pain control was determined
in the present study.
Significant variability among the combined genetic
groups was observed. First, patients with OPRM1 AA and CYP3A4*1*18B
polymorphisms received fewer fentanyl doses during the 48-h period
compared with those with OPRM1 AG and CYP3A4*1*1 (P=0.049). With
respect to a single gene, no significant difference in fentanyl
dose was found between patients with AA or AG in OPRM1 A118G or
between patients with *1*1 and those with *1*18B in CYP3A4*18B.
However, the association between these polymorphisms and fentanyl
consumption became significant when combined respectively. This
observation indicates that variability in fentanyl consumption is
not associated with genetic variation in a single gene, but in two
or more genes and this was demonstrated further in the second
group, in which although the fentanyl dose was not different
between patients with *1*18B and those with *1*1, combination with
OPRM1 AG led to a significant difference (P=0.010). These results
are similar to a previous study in which Kolesnikov et
al(21) reported that when
48-h PCA morphine consumption was analyzed in 102 patients who
underwent abdominal surgery, no significant statistical difference
among the catechol-O-methyltransferase (COMT) G1947A or OPRM1 A118G
groups was detected, however, patients with OPRM1 A118G and COMT
G1947A polymorphisms received significantly fewer morphine doses
compared with patients with OPRM1 wild-type homozygous. These
results indicate that genetic effects on opioid analgesic dose are
under the control of multiple, not single genes and may account for
discrepancies between genetic associations with pain modulatory
effects in clinical studies. In addition, patients with
CYP3A4*18B*18B polymorphisms (groups 3 and 4) received
significantly fewer fentanyl doses during the 48-h period compared,
not only with those with OPRM1 A118 and CYP3A4*1*1 (P=0.024), but
also with those with OPRM1 A118G and CYP3A4*1*1 polymorphisms
(P=0.006). Moreover, of the 7 combinations, patients with
CYP3A4*18B*18B consumed the lowest fentanyl dose, demonstrating
that 48 h following surgery, variations in fentanyl consumption
were largely determined by the CYP3A4*18B*18B genotype in the
patient population. The CYP3A4*18B polymorphism was therefore an
important determinant of fentanyl variation for postoperative pain
control.
The correlation between fentanyl-associated side
effects and CYP3A4 and OPRM1 genes in the patient cohort was also
investigated. Incidence of nausea, vomiting and dizziness was 38.1,
18.6 and 32.0%, respectively. Analysis revealed no significant
association between side effects (nausea, vomiting and dizziness)
and genetic variation in CYP3A4*18B and OPRM1 genes, including
their joint genetic variation. In the present study, the adverse
effects were assessed by events, not rating scale, therefore, it
was not a standard and precise assessment. However, results were
consistent with previous studies (14,25),
in which A118G and CYP3A4*18B were found to have no effect on
nausea or vomiting, did not reduce frequency of these side effects
and had no effect on sedation scores. This indicated that the OPRM1
A118G and CYP3A4*18B genetic polymorphisms are not the main factors
affecting nausea, vomiting and dizziness during PCIA with
fentanyl.
Findings of this study provide preliminary insight
into the effect of the CYP3A4*18B polymorphisms and its joint
effects with OPRM1 A118G in the clinical efficacy of fentanyl. To
the best of our knowledge, this is the first study to have explored
the joint effects of OPRM1 and CYP3A4*18B in fentanyl analgesia.
However, the present study is associated with a number of
limitations. Firstly, the activity of CYP3A4 and plasma fentanyl
concentration in these patients was not detected and the mechanisms
associated with differences noted between various CYP3A4 phenotypes
have yet to be elucidated. Secondly, the mixed-gender study
population may have increased variability in postoperative fentanyl
requirement, although no statistically significant differences in
gender were found between the different genotypes. In addition,
only two SNPs of two genes associated with pain treatment, OPRM1
A118G and CYP3A4*18B, were analyzed, leaving a number of genes with
functional significance to be assessed in future studies. Moreover,
the sample size was too small, thus a decisive conclusion could not
be drawn from the results. Future studies with larger cohorts are
required to further characterize the joint effects of the OPRM1 and
CYP3A4 mutations, in addition to environmental factors.
In the present study, patients with the
CYP3A4*18B/*18B polymorphism required a reduced dose of fentanyl
for pain relief compared with patients with other polymorphisms.
Although postoperative fentanyl analgesic dose, nausea, vomiting
and dizziness were not found to be significantly different between
OPRM1 A118G polymorphisms, the combination of OPRM1 A118G and
CYP3A4*18B affected the fentanyl requirements of Chinese Han
patients following radical gastrectomy.
References
|
1
|
Ikeda K, Ide S, Han W, Hayashida M, Uhl GR
and Sora I: How individual sensitivity to opiates can be predicted
by gene analyses. Trends Pharmacol Sci. 26:311–317. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Evans WE and McLeod HL: Pharmacogenomics -
drug disposition, drug targets and side effects. N Engl J Med.
348:538–549. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Weinshilboum R: Inheritance and drug
response. N Engl J Med. 348:529–537. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hu YF, Qiu W, Liu ZQ, et al: Effects of
genetic polymorphisms of CYP3A4, CYP3A5 and MDR1 on cyclosporine
pharmacokinetics after renal transplantation. Clin Exp Pharmacol
Physiol. 33:1093–1098. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Feierman DE and Lasker JM: Metabolism of
fentanyl, a synthetic opioid analgesic, by human liver microsomes.
Role of CYP3A4. Drug Metab Dispos. 24:932–939. 1996.PubMed/NCBI
|
|
6
|
Tateishi T, Krivoruk Y, Ueng YF, Wood AJ,
Guengerich FP and Wood M: Identification of human liver cytochrome
P-450 3A4 as the enzyme responsible for fentanyl and sufentanil
N-dealkylation. Anesth Analg. 82:167–172. 1996.PubMed/NCBI
|
|
7
|
Labroo RB, Paine MF, Thummel KE and
Kharasch ED: Fentanyl metabolism by human hepatic and intestinal
cytochrome P450 3A4: implications for interindividual variability
in disposition, efficacy and drug interactions. Drug Metab Dispos.
25:1072–1080. 1997.PubMed/NCBI
|
|
8
|
Agrawal V, Choi JH, Giacomini KM and
Miller WL: Substrate-specific modulation of CYP3A4 activity by
genetic variants of cytochrome P450 oxidoreductase. Pharmacogenet
Genom. 20:611–618. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Fukushima-Uesaka H, Saito Y, Watanabe H,
et al: Haplotypes of CYP3A4 and their close linkage with CYP3A5
haplotypes in a Japanese population. Hum Mutat. 23:1002004.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Hu YF, Tu JH, Tan ZR, et al: Association
of CYP3A4*18B polymorphisms with the pharmacokinetics of
cyclosporine in healthy subjects. Xenobiotica. 37:315–327.
2007.
|
|
11
|
Yuan R, Zhang X, Deng Q, Wu Y and Xiang G:
Impact of CYP3A4*1G polymorphism on metabolism of fentanyl in
Chinese patients undergoing lower abdominal surgery. Clin Chim
Acta. 412:755–760. 2011.
|
|
12
|
Zhang W, Chang YZ, Kan QC, et al:
CYP3A4*1G genetic polymorphism influences CYP3A activity and
response to fentanyl in Chinese gynecologic patients. Eur J Clin
Pharmacol. 66:61–66. 2010.
|
|
13
|
Walter C and Lötsch J: Meta-analysis of
the relevance of the OPRM1 118A>G genetic variant for pain
treatment. Pain. 146:270–275. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhang W, Chang YZ, Kan QC, et al:
Association of human micro-opioid receptor gene polymorphism A118G
with fentanyl analgesia consumption in Chinese gynaecological
patients. Anaesthesia. 65:130–135. 2010. View Article : Google Scholar
|
|
15
|
Fukuda K, Hayashida M, Ide S, et al:
Association between OPRM1 gene polymorphisms and fentanyl
sensitivity in patients undergoing painful cosmetic surgery. Pain.
147:194–201. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Hayashida M, Nagashima M, Satoh Y, et al:
Analgesic requirements after major abdominal surgery are associated
with OPRM1 gene polymorphism genotype and haplotype.
Pharmacogenomics. 9:1605–1616. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wong CA, McCarthy RJ, Blouin J and Landau
R: Observational study of the effect of mu-opioid receptor genetic
polymorphism on intrathecal opioid labor analgesia and
post-cesarean delivery analgesia. Int J Obstet Anesth. 19:246–253.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Klepstad P, Fladvad T, Skorpen F, et al;
European Palliative Care Research Collaborative (EPCRC); European
Association for Palliative Care Research Network. Influence from
genetic variability on opioid use for cancer pain: a
Europeangenetic association study of 2294 cancer pain patients.
Pain. 152:1139–1145. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Landau R, Kern C, Columb MO, Smiley RM and
Blouin JL: Genetic variability of the mu-opioid receptor influences
intrathecal fentanyl analgesia requirements in laboring women.
Pain. 30:5–14. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Reyes-Gibby CC, Shete S, Rakvag T, et al:
Exploring joint effects of gene and the clinical efficacy of
morphine for cancer pain. Pain. 130:25–30. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kolesnikov Y, Gabovits B, Levin A, Voiko E
and Veske A: Combined catechol-O-methyltransferase and mu-opioid
receptor gene polymorphisms affect morphine postoperative analgesia
and central side effects. Anesth Analg. 112:448–453. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Spielberger CD: Manual for the State-Trait
Anxiety Inventory. Consulting Psychologists Press; Palo Alto,
California, USA: 1983
|
|
23
|
Zung WW: A self-rating depression scale.
Arch Gen Psychiatry. 12:63–70. 1965. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Dong ZL, Li H, Chen QX, et al: Effect of
CYP3A4*1G on the fentanyl consumption for intravenous
patient-controlled analgesia after total abdominal hysterectomy in
Chinese Han population. J Clin Pharm Ther. 37:153–156. 2012.
|
|
25
|
Tan PC, Hassan SK, Mohamad NA and Gan SH:
Cytochrome P450 3A4 genetic polymorphisms and post-operative
fentanyl requirements. J Clin Pharm Ther. 37:100–104. 2012.
View Article : Google Scholar : PubMed/NCBI
|