Introduction
With an incidence of approximately 1 million new
cases each year, breast cancer (BC) constitutes a major cause of
mortality in females diagnosed at the stage of infiltrating disease
(1). BC is classically classified
as either lobular or ductal in form, with scirrhous, medullary and
mucinous variants. Their biological and clinical heterogeneity and
variable response to therapy lead to refined classifications based
on receptor status (2) as luminal
A and B type [estrogen receptor (ER)-positive]; human epidermal
growth factor receptor (HER)2 overexpressing epidermal growth
factor (EGF) receptors; and basal type [not expressing ER,
progesterone (PR) and HER, also known as triple-negative breast
cancer (TNBC)] and normal-like cancer. Attempts to devise
additional classifications are based on markers for diagnosis and
prognosis (cytokeratins and chaperonins), in relation to specific
mutations detected by proteomic and cDNA microarray techniques
(3). Such studies are currently in
progress.
In the present study, we performed a proteomic
analysis of normal and BC tissue from individual patients
undergoing mastectomy at the Ferrara University Hospital (Ferrara,
Italy), during the last 2 years. Changes in the expression of
specific proteins in the majority of patients support the
investigation of their role as tissue and serological markers for
the identification of aggressive tumors and as targets for therapy
refractory cases.
Materials and methods
Tissue specimens
Samples of normal and cancerous tissue were
collected from 28 patients (represented as P1 to P28) with ductal
BC for proteomic analysis. The study was approved by the
Institutional Ethics Committee of the Ferrara University Hospital.
Diagnosis was confirmed by histopathological analysis, which
demonstrated that the tumor specimens contained >50% tumor
cells. Samples from 10 patients providing large quantities of
tissue were snap-frozen in liquid nitrogen and stored at −80°C
until proteomic analysis was performed.
Proteomic analysis by 2 dimensional (2D)
electrophoresis and mass spectrometry
Proteomic analysis of BC and normal tissue from
individual patients was performed by homogenization in 2.5 volumes
of 7 M urea, 2 M thiourea, 4% CHAPS, 40 mM Tris, 1 mM benzamidine,
1 mM iodacetamide and 1 mM EDTA, pH 8.8. Following centrifugation,
portions of supernatant corresponding to 260 μg protein were
separated by isoelectric focusing (IEF) on precast pH 3–10 linear
IPG strips at 40,000 Vh, according to the manufacturer's
instructions. Following thiol reduction by 1% dithiothreitol (DTT)
and alkylation by 4% iodoacetamide, the strips were separated on 2D
SDS-PAGE on 12.5% polyacrylamide gels for 1 h at 200 V. Gels were
stained with colloidal Coomassie, scanned with the Molecular Imager
PharosFX System and analyzed using the ProteomeWeaver 4 program
(Bio-Rad, Hercules, CA, USA). Spots excised from the gels were
processed by trypsin digestion for mass spectrometry-based peptide
identification. The gel fragments were briefly rinsed in buffered
acetonitrile and then dried. Following thiol group reduction and
alkylation, the peptides were digested overnight with 12.5 ng/μl
trypsin, resuspended in aqueous formic acid and analyzed with an
Ultimate 3000 Nano/micro HPLC apparatus coupled with a LTQ-Orbitrap
XL mass spectrometer (Thermo Scientific, Sunnyvale, CA, USA)
(4,5). Using Excalibur 2.0.7 software,
spectra were submitted for peptide identification to the MASCOT
program against the NCBI database.
Results
The clinical characteristics of each individual
patient associated with altered protein expression are summarized
in Table I. The proteins with
altered expression are also listed, as recognized by 2D gel
electrophoresis. We achieved optimal resolution at a total load of
260 μg of protein on the IEF strip, running 2D electrophoresis at a
constant voltage of 200 V for 1 h in order to avoid proteins <20
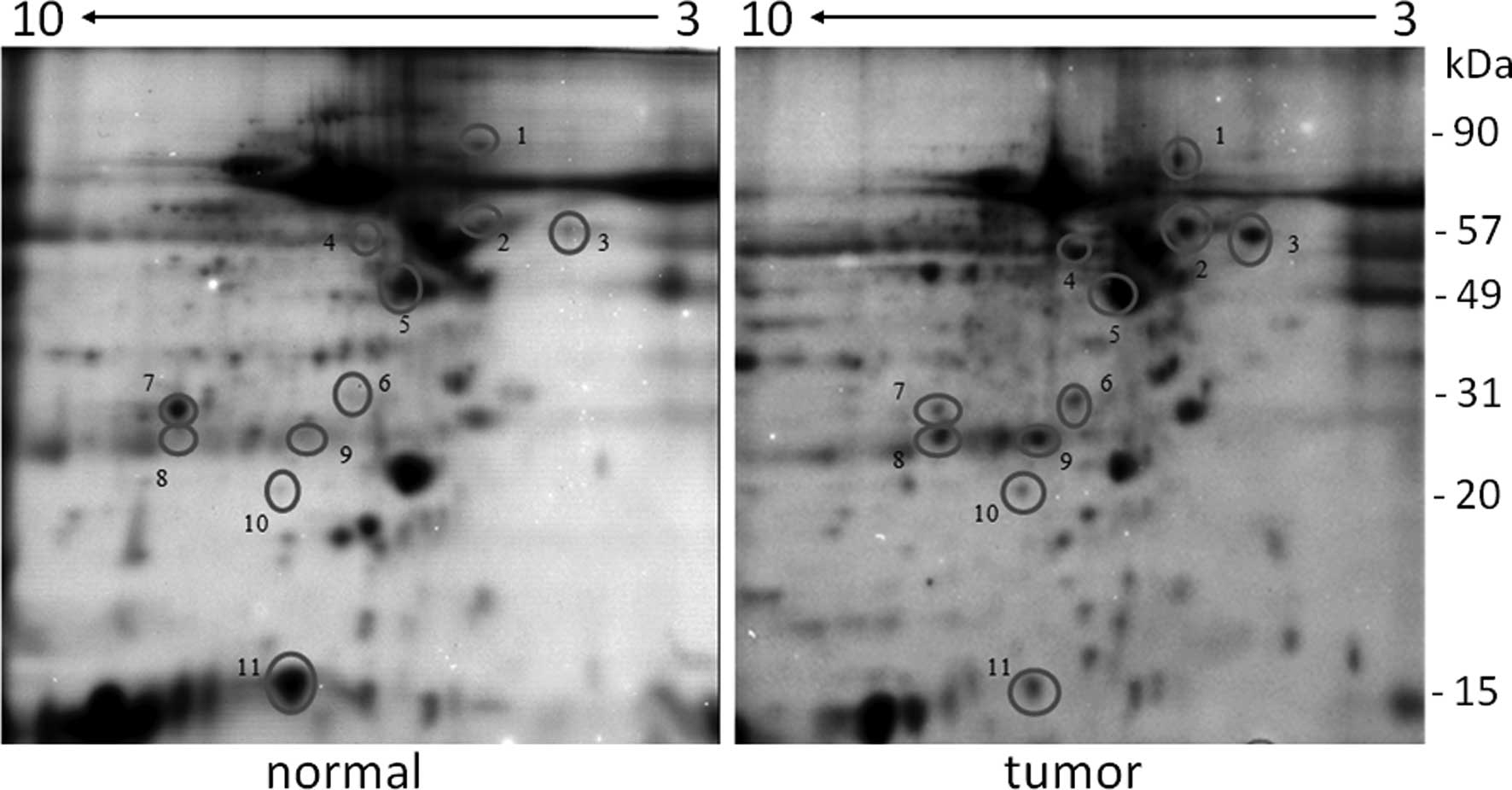
kDa running out of the gel. Typically, 2D electrophoresis of tumor
and normal samples of individual patients (Fig. 1) resolved several hundreds of
proteins. By visual inspection, 11 proteins had altered expression
in BC tissue (circles, Fig. 1); 2
proteins had a decreased expression and 9 had an increased
expression. These proteins were identified by mass spectrometry.
The 9 proteins with an increased expression (Table I) were endoplasmin precursor
(gp96), protein disulfide isomerase (PDI), TCP-1 subunit θ
(TCP1-θ), F actin-capping protein subunit β (FACP-β), heat shock
protein β 1 (namely, HSP27), triosephosphate isomerase (TPI),
RS/DJ-1, β-tubulin (Tub-β) and β-actin (Act-β), while carbonic
anhydrase-1 (CA-1) and adipocyte fatty acid binding-protein
(A-FABP) were downregulated. These proteins included molecular
chaperones, cytoskeletal proteins and metabolic enzymes. We also
identified several cytokeratin peptides; however, these were
ignored due to possible contamination during sample processing.
Cytokeratins are relevant in BC immunocytochemical approaches,
however, not when searching for circulating biomarker discovery
(6).
 | Table IPopulation characteristics and
identification of significantly altered protein expression levels
in BC. |
Table I
Population characteristics and
identification of significantly altered protein expression levels
in BC.
|
Clinical
setting |
|---|
|
|
|---|
| pT1c-pN0(i)(sn) | pT2-pN0(i−)(sn) | pT1c-pN0(i+)(sn) | pT1c-pN0(i−)(sn) |
pT1mic-pN0(i−)(sn) | pT2-pN1a | pT1c-pN0(i+)(sn) | pT1c-pN0(i−)(sn) | pT1c-pN0(i−)(sn) | pT2-pN3a |
|---|
| Case | P10 | P13 | P18 | P19 | P20 | P21 | P22 | P23 | P27 | P28 |
| Cytology |
| NCI | n.d. | 4.5 | 3.3 | 3.32 | n.d. | 5.56 | 3.28 | 3.3 | 3.24 | 6.44 |
| Grade | G2 | G3 | G2 | G2 | micro | G3 | G2 | G2 | G2 | G3 |
| ER | 90 | 0 | 96 | 97 | 0 | 21 | 99 | 72 | 99 | 52 |
| PR | 50 | 0 | 36 | 94 | 0 | 8 | 98 | 64 | 56 | 68 |
| HER2 | 1+ | 1+ | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 3+ |
| Identified
proteins |
| gp96 | 2.43 | 2.00 | 4.86 | 8.13 | 5.00 | 4.67 | 1.14 | 5.00 | 1.27 | 1.18 |
| PDI | 8.25 | 3.38 | 6.00 | 4.33 | n.d. | 9.33 | 2 | 4.76 | n.d. | 4.55 |
| TCP1-θ | 2.22 | 2.00 | 2.11 | 4.33 | 2.89 | 7.19 | 4.80 | 12.68 | 3.86 | 5.38 |
| FACP-β | 3.50 | 1.44 | 2.75 | 2.00 | 2.00 | 2.92 | 1.30 | 3.33 | 2.89 | 2.00 |
| HSP27 | 2.43 | 1.29 | 5.33 | 6.77 | 2.57 | 2.00 | 2.29 | 4.67 | 3.60 | 2.73 |
| TPI | 3.50 | n.d. | 5.75 | 2.41 | 1.38 | 3.50 | n.d. | 3.33 | 6.00 | 3.93 |
| RS/DJ 1 | 2.25 | 0.91 | 4.00 | 2.53 | 3.58 | 2.33 | 2.80 | 4.80 | 6.00 | 2.42 |
| TUB-β | 2.53 | 1.40 | 6.50 | 2.00 | 2.02 | 3.50 | 0.80 | 4.98 | 4.32 | 2.18 |
| ACT-β | 6.25 | 3.60 | 8.00 | 2.28 | 2.75 | 3.89 | 2.00 | 13.33 | 3.52 | 3.64 |
| CA-1 | 0.50 | 0.28 | 1.11 | 0.50 | 0.50 | 0.18 | 0.50 | 0.19 | 0.50 | 0.45 |
| A-FABP | 0.23 | 0.18 | 0.50 | 0.11 | 0.25 | 0.13 | 0.36 | 0.89 | 0.15 | 0.18 |
The estimation of the abundance of proteins with
altered expression was achieved through normalization of the
intensity of spots from different gels against a virtual spot
calculated as an average on the same gel of 5–6 spots with
identical expression in all analyzed samples. Ratios of proteins
present were obtained in spots of BC and corresponding normal
tissue in 2D electrophoresis gels (Table I). Ratios >2 or <0.5 denote
proteins significantly upregulated or downregulated in BC versus
normal tissue, respectively. Thus, gp96 and TPI proteins were
consistently modulated in at least 7 out of 10 patients, even if
the score for TPI should be higher due to its expression not being
constantly detectable in normal tissue, thus precluding the
calculation of a precise ratio. PDI, FACP-β and Tub-β were highly
expressed in 8 out of 10 tumors. HSP27, RS/DJ-1, CA-1 and A-FABP
were consistently modulated in 9 out of 10 patients. Act-β and
TCP1-θ were elevated in all tumors analyzed.
Discussion
In the present study, the abundance of numerous
proteins in normal and cancerous breast tissue was analyzed, with
11 proteins exhibiting a consistently altered expression in tumors.
The proteins which exhibited an altered expression were as
follows:
Chaperonins, which are notably involved in increased
tumorigenicity, metastatic potential and resistance to
chemotherapy. Chaperonins include HSP27 induced under unfavorable
conditions to protect cells from death, preventing aggregation of
denatured proteins, regulating caspase activity, intracellular
redox state, polymerization of actin and cytoskeletal dynamics
(7). HSP27 is a proposed
immunocytochemical discriminator to refine C3 and C4 categories in
suspect BC aspirates (8) and gp96
of the HSP90 family (9), which
represents a tool for active immunization against tumors by
associating with cell surface peptides for presentation to
cytolytic T lymphocytes and cell destruction. Notably Vitespen, a
peptide vaccine based on gp96, prolongs survival in patients with
early-stage melanoma or renal cancer (10). The presence of gp96 in infiltrating
ductal BC is attractive for vaccine treatment in TNBC patients
resistant to classical therapy while maintaining the expression of
gp96 (patient P20).
The cytoskeletal proteins, Act-β, FACP-β, Tub-β and
TCP1-θ, of which the latter protein assists in the ATP-dependent
folding of actin and tubulin (11). Notably, some cytoskeletal proteins
upregulated in BC are involved in ER activation: Act-β binds to the
ER-α complex, contributing to ER nuclear functions (12); chaperonin TCP1-θ (spot 3) is
involved in the folding of Act-β (13) and estrogen-regulated HSP27 controls
the palmitoylation of ER, which is required for its interaction
with membranes (14). This
indicates that cytoskeletal rearrangement is a key step in the
motility mechanisms leading to metastatic spreading; however, it
may also be involved in hormone receptivity (15).
Signaling proteins, including RS/DJ-1, TPI and
A-FABP. RS/DJ-1 is an oncogene protein regulating RNA protein
interaction, present in sera from BC patients but not from healthy
patients, along with circulating antibodies against this protein
(16). Similarly, autoantibodies
against TPI are present in BC patients (17) and also in patients with oral cavity
and lung squamous cell carcinoma (18,19).
PDI catalyzes the formation and rearrangement of protein disulfide
bonds, acting as a reductase at the cell surface cleaving disulfide
bonds with structural modifications of cell-associated proteins and
as chaperonin (inhibiting aggregation of misfolded proteins) or
antichaperonin (facilitating aggregation) inside cells, depending
on the concentration. In addition, PDI binds estrogen and thyroid
hormones (20). Notably, the
knockdown of PDI in MCF7 BC cells induces caspase-dependent
apoptosis (21). A-FABP is another
protein whose expression is negatively affected. It plays a role in
intracellular lipid transport and metabolism as well as signaling.
The prognostic value for A-FABP has been reported in bladder cancer
since its decreased expression correlates with poor prognosis
(22). It is induced by PPAR
ligands [the promoter region of the A-FABP gene contains functional
peroxisome proliferator-responsive elements (23)] and its overexpression is likely
beneficial in the treatment of bladder cancer. Contrasting results
have been reported in BC since serum levels of A-FABP have been
associated with tumor risk and aggressive behavior (24); however, comparable values of
expression have been reported in ductal infiltrating carcinoma and
normal tissue (25). Our data
instead report the decreased levels of A-FABP in ductal BC compared
with normal tissues in 9 out of 10 patients, including the TNBC
patient in our population. The association between BC and A-FABP,
and if the data are confirmed in larger populations, the possible
selective induction by PPAR ligands may impact on the development
of future strategies for the treatment of TNBC.
In conclusion, despite the limited number of
patients investigated in the present study, the robustness of our
results suggests that similar results may be observed in future
studies with a larger sample size, including other tumor types and
their multidrug-resistant (MDR) counterparts. This is an aspect of
particular interest, since one of the drawbacks of conventional
anticancer therapy is the development of drug resistance. The
proteins which we observed to exhibit an altered expression in
infiltrating ductal BC may be exploited as novel targets for
therapeutic interventions or represent novel diagnostic/prognostic
markers for the early detection of aggressive tumors, particularly
those with MDR phenotypes during the earlier stages of the
disease.
Acknowledgements
This study was supported by grants from Fondazione
Cassa Risparmio di Ferrara to Professor Paolo Carcoforo.
References
|
1
|
Pisani P, Bray F and Parkin DM: Estimates
of the world-wide prevalence of cancer for 25 sites in the adult
population. Int J Cancer. 97:72–81. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Sørlie T, Perou CM, Tibshirani R, et al:
Gene expression patterns of breast carcinomas distinguish tumor
subclasses with clinical implications. Proc Natl Acad Sci USA.
98:10869–10874. 2001.PubMed/NCBI
|
|
3
|
Gast MC, Schellens JH and Beijnen JH:
Clinical proteomics in breast cancer: a review. Breast Cancer Res
Treat. 116:17–29. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Bozzo C, Spolaore B, Toniolo L, et al:
Nerve influence on myosin light chain phosphorylation in slow and
fast skeletal muscles. FEBS J. 272:5771–5785. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Shevchenko A, Tomas H, Havlis J, Olsen JV
and Mann M: In-gel digestion for mass spectrometric
characterization of proteins and proteomes. Nat Protoc.
1:2856–2860. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Galvão ER, Martins LM, Ibiapina JO,
Andrade HM and Monte SJ: Breast cancer proteomics: a review for
clinicians. J Cancer Res Clin Oncol. 137:915–925. 2011.
|
|
7
|
Acunzo J, Katsogiannou M and Rocchi P:
Small heat shock proteins HSP27 (HspB1), αB-crystallin (HspB5) and
HSP22 (HspB8) as regulators of cell death. Int J Biochem Cell Biol.
44:1622–1631. 2012.
|
|
8
|
Keeling J and McKee GT: Heat shock protein
(HSP)27: a further refinement in the diagnosis of suspicious fine
needle aspirates of breast. Cytopathology. 10:40–49. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Yang Y and Li Z: Roles of heat shock
protein gp96 in the ER quality control: redundant or unique
function? Mol Cells. 20:173–182. 2005.PubMed/NCBI
|
|
10
|
Randazzo M, Terness P, Opelz G and Kleist
C: Active-specific immunotherapy of human cancers with the heat
shock protein Gp96-revisited. Int J Cancer. 130:2219–2231. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Brackley KI and Grantham J: Activities of
the chaperonin containing TCP-1 (CCT): implications for cell cycle
progression and cytoskeletal organisation. Cell Stress Chaperones.
14:23–31. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ambrosino C, Tarallo R, Bamundo A, et al:
Identification of a hormone-regulated dynamic nuclear actin network
associated with estrogen receptor alpha in human breast cancer cell
nuclei. Mol Cell Proteomics. 9:1352–1367. 2010. View Article : Google Scholar
|
|
13
|
Vainberg IE, Lewis SA, Rommelaere H, et
al: Prefoldin, a chaperone that delivers unfolded proteins to
cytosolic chaperonin. Cell. 93:863–873. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Razandi M, Pedram A and Levin ER: Heat
shock protein 27 is required for sex steroid receptor trafficking
to and functioning at the plasma membrane. Mol Cell Biol.
30:3249–3261. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Jiang P, Enomoto A and Takahashi M: Cell
biology of the movement of breast cancer cells: intracellular
signalling and the actin cytoskeleton. Cancer Lett. 284:122–130.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Le Naour F, Misek DE, Krause MC, Deneux L,
Giordano TJ, Scholl S and Hanash SM: Proteomics-based
identification of RS/DJ-1 as a novel circulating tumor antigen in
breast cancer. Clin Cancer Res. 7:3328–3335. 2001.PubMed/NCBI
|
|
17
|
Tamesa MS, Kuramitsu Y, Fujimoto M, et al:
Detection of autoantibodies against cyclophilin A and
triosephosphate isomerase in sera from breast cancer patients by
proteomic analysis. Electrophoresis. 30:2168–2181. 2009. View Article : Google Scholar
|
|
18
|
Shukla S, Pranay A, D'Cruz AK, Chaturvedi
P, Kane SV and Zingde SM: Immunoproteomics reveals that cancer of
the tongue and the gingivobuccal complex exhibit differential
autoantibody response. Cancer Biomark. 5:127–135. 2009.
|
|
19
|
Zhang XZ, Xiao ZF, Li C, et al:
Triosephosphate isomerase and peroxiredoxin 6, two novel serum
markers for human lung squamous cell carcinoma. Cancer Sci.
100:2396–2401. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Fu XM, Wang P and Zhu BT: Characterization
of the estradiol-binding site structure of human protein disulfide
isomerase (PDI). PLoS One. 6:e271852011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Hashida T, Kotake Y and Ohta S: Protein
disulfide isomerase knockdown-induced cell death is
cell-line-dependent and involves apoptosis in MCF7 cells. J Toxicol
Sci. 1:1–7. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
22
|
Boiteux G, Lascombe I, Roche E,
Plissonnier ML, Clairotte A, Bittard H and Fauconnet S: A-FABP, a
candidate progression marker of human transitional cell carcinoma
of the bladder, is differentially regulated by PPAR in urothelial
cancer cells. Int J Cancer. 124:1820–1828. 2009. View Article : Google Scholar
|
|
23
|
Schachtrup C, Emmler T, Bleck B, Sandqvist
A and Spener F: Functional analysis of
peroxisome-proliferator-responsive element motifs in genes of fatty
acid-binding proteins. Biochem J. 382:239–245. 2004. View Article : Google Scholar
|
|
24
|
Hancke K, Grubeck D, Hauser N, Kreienberg
R and Weiss JM: Adipocyte fatty acid-binding protein as a novel
prognostic factor in obese breast cancer patients. Breast Cancer
Res Treat. 119:367–377. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Li H, Lu Q, Dong LH, Xue H, Zhou HY and
Yang HJ: Expression of fatty acid binding protein in human breast
cancer tissues. Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi. 23:312–316.
2007.PubMed/NCBI
|
|
26
|
Elston CW and Ellis IO: Pathological
prognostic factors in breast cancer. I The value of histological
grade in breast cancer: experience from a large study with
long-term follow-up. Histopathology. 19:403–410. 1991. View Article : Google Scholar
|