Introduction
Recent advances in diagnostic techniques have
allowed gastric cancer to be detected early in an increasing number
of cases and the treatment outcomes of gastric cancer as a whole
have been significantly improved. However, diffuse growth-type
malignant tumors, such as scirrhous gastric cancer, remain
difficult to diagnose during the early stages of development and
are usually at an advanced stage at the time of diagnosis, leading
to poor treatment outcomes.
Cell motility is an important factor in primary
tumor isolation and invasion; the cell adhesion factors that have
been identified to promote cell motility include epidermal growth
factor (EGF), transforming growth factor (TGF)-β and hepatocyte
growth factor (HGF) (1–7). The present study investigated
fibroblast growth factor (FGF), which is involved in fibroblast
proliferation (8–11). With regard to the signal
transmission pathways involved in scirrhous gastric cancer,
scirrhous gastric cancer cells grow in the submucosa (SM), which is
dense with lymph nodes, rather than on the mucosal surface where
they would be exposed to gastric acid (12). FGF7 is produced by fibroblasts
inside the stomach and promotes proliferation of scirrhous gastric
cancer cells via FGFR2 receptors; the production of HGF and TGF-β
promotes infiltration in a similar manner. Since TGF-β also
promotes the proliferation of fibroblasts, these factors have the
potential to induce the rapid infiltration and proliferation of
scirrhous gastric cancer cells (13–15).
The K-sam gene, which is known to exacerbate
scirrhous gastric cancer, has also been revealed to be homologous
to other genes, including FGFR2, KGFR and Bek. An evaluation of the
association of K-sam with keratinocyte growth factor (KGF)
expression may enable the identification of malignant tumors with a
poor prognosis (16–20).
Materials and methods
Patients and sample collection
A total of 86 patients underwent gastrectomy for
carcinoma (early cancer, 49 cases; advanced cancer, 37 cases)
between 1999 and 2003. The clinical characteristics of the patients
are listed in Table I. A total of
31 patients who underwent surgery for a benign disease (e.g.,
inguinal hernia, gallbladder stone) and did not have cancer, as
confirmed on prior screening, were enrolled as the control group in
this study. Blood samples were obtained preoperatively from the 86
gastric carcinoma and 31 control patients. These samples were
centrifuged and then preserved at −80°C in a freezer. The serum KGF
concentration was estimated using a KGF ELISA kit and a
clinical/oncological analysis was completed >5 years after the
surgery.
 | Table IPatient characteristics. |
Table I
Patient characteristics.
| Characteristics | n (%) |
|---|
| Age, mean (SD) | 63.33 (9.5) |
| Gender |
| Male | 66 (76.74) |
| Female | 20 (23.26) |
| Macroscopic
types |
| Type 0 | 53 (61.63) |
| Type 1 | 4 (4.65) |
| Type 2 | 15 (17.44) |
| Type 3 | 8 (9.30) |
| Type 4 | 5 (5.81) |
| Type 5 | 1 (1.16) |
| Histological
types |
| Papillary | 2 (2.33) |
| Well-differentiated
(tub1) | 27 (31.40) |
| Moderately
differentiated (tub2) | 16 (18.60) |
| Poorly
differentiated (por1) | 20 (23.26) |
| Poorly
differentiated (por2) | 12 (13.95) |
| Signet-ring
cell | 6 (6.98) |
| Mucinous | 3 (3.49) |
| Depth of tumor
invasion |
| M | 22 (25.58) |
| SM | 27 (31.40) |
| MP | 8 (9.30) |
| SS | 13 (15.12) |
| SE | 16 (18.61) |
| Lymphatic
invasion |
| ly0 | 37 (43.02) |
| ly1 | 31 (36.05) |
| ly2 | 14 (16.28) |
| ly3 | 4 (4.65) |
| Venous invasion |
| v0 | 69 (80.23) |
| v1 | 16 (18.61) |
| v2 | 1 (1.16) |
| Lymph node
metastasis |
| n0 | 63 (73.26) |
| n1 | 14 (16.28) |
| n2 | 6 (6.98) |
| n3 | 1 (1.16) |
| n4 | 2 (2.33) |
| Japanese clinical
stage |
| Ia | 46 (53.49) |
| Ib | 14 (16.28) |
| II | 12 (13.95) |
| IIIa | 5 (5.81) |
| IIIb | 3 (3.49) |
| IVa | 2 (2.33) |
| IVb | 4 (4.65) |
A total of 86 patients who had undergone a primary
tumor resection and were histologically diagnosed with sporadic
gastric cancer were included in this study. None of the patients
had received preoperative radiation therapy and/or chemotherapy.
The samples were fixed in 10% formaldehyde solution and embedded in
paraffin. The samples were sectioned (4-μm) and mounted on glass
slides. Pathological diagnosis and classifications were determined
according to the Japanese Classification of Gastric Carcinoma by
the Japanese Gastric Cancer Association (21). Additionally, based on this
classification, tumor invasion in the mucosa (M) and SM was
determined as early-stage cancer, while tumor invasion in the
muscularis propria (MP), subserosa (SS), serosa (SE), and adjacent
structures (SI) was evaluated as advanced-stage cancer, regardless
of lymph node or distant metastases.
Antibodies and reagents
A rabbit polyclonal antibody against K-sam was
purchased from Immuno-Biological Laboratories Co., Ltd. (9G-915,
dilution 1:40; Gunma, Japan). A mouse monoclonal antibody against
KGF was obtained from R&D Systems, Inc. (MAB2511, dilution
1:50; Minneapolis, MN, USA). Skim milk, Dako EnVision + System-HRP
(DAB; catalog nos. K4006 and K4011), Target Retrieval Solution pH
9.0 and diaminobenzidine were purchased from Dako (Carpinteria, CA,
USA).
Amplifying Wash Buffer™ 20× (catalog no. AA4;
ProHisto, Columbia, SC, USA), xylene, ethanol,
H2O2, coverslips (24×50 mm, no. 1 thickness;
Chase Scientific Glass, Inc., Rockwood, TN, USA) and hematoxylin
(Gill-1, catalog no. 23-245653; Thermo Fisher Scientific, Inc.,
Kanagawa, Japan) were also used in this study.
Serological investigation
The venous blood samples, which were obtained prior
to surgery, were centrifuged and the purified serum was stored at
−80°C. The frozen serum was allowed to thaw naturally prior to
examination. Serum KGF levels were measured using the ELISA method
with a Quantikine Human KGF ELISA kit. A cuvette port internal
microplate spectrophotometer was used, with a biquadratic
approximation formula.
Subsequently, 100 μl assay diluent and 100 μl of
sample were added to each well. The wells were covered with the
adhesive strip provided and then incubated for 3 h at room
temperature. Each well was aspirated and washed 4 times; for
washing, each well was filled with wash buffer (400 μl).
KGF conjugate (200 μl) was added to each well. The
wells were covered with a new adhesive strip and incubated for 2 h
at room temperature. Each well was then aspirated and washed 4
times, as previously described. Substrate solution (200 μl) was
then added to each well, followed by incubation.
Immunohistochemical techniques
The immunohistochemical detection of K-sam and KGF
was performed according to the manufacturer’s instructions.
Briefly, the slides were deparaffinized in xylene and hydrated in
decreasing concentrations of ethyl alcohol. The tissues were heated
for 20 min at 105°C by autoclave in Target Retrieval Solution pH
9.0. Then, the sections were deparaffinized and incubated with 3%
hydrogen peroxide in methanol for 15 min to block the endogenous
peroxidase activity. The sections were washed in phosphate-buffered
saline (PBS) and incubated in skim milk for 10 min to reduce the
non-specific antibody binding.
The specimens were incubated with K-sam (2.5 mg/ml)
or KGF antibody (2.5 mg/ml) overnight at 4°C, followed by 3 washes
with PBS. The sections were incubated with labeled polymer-HRP
(bottle 2) for 30 min at room temperature, followed by 3 washes
with PBS. The slides were treated with ready-to-use AEC
substrate-chromogen solution for 3 min and washed with PBS 3 times.
Finally, the slides were incubated in PBS diaminobenzidine and 1%
hydrogen peroxide v/v for 60 or 90 sec, counterstained with Mayer’s
hematoxylin and mounted.
Identification of K-sam and KGF using
immunohistochemical staining
Known cases of scirrhous gastric cancer were used as
controls for KGF and K-sam expression. KGF was immunologically
localized mainly in the cytoplasm, while K-sam was in the cell
membrane and cytoplasm (22).
Hematoxylin and eosin (H&E) staining was also used on the
control slides to select infiltrative regions. Infiltrative regions
were defined as areas on the serosal side where cancer cells were
initially observed. Microscopy revealed that cancer cells were
continuously present from the mucosa to the serosa.
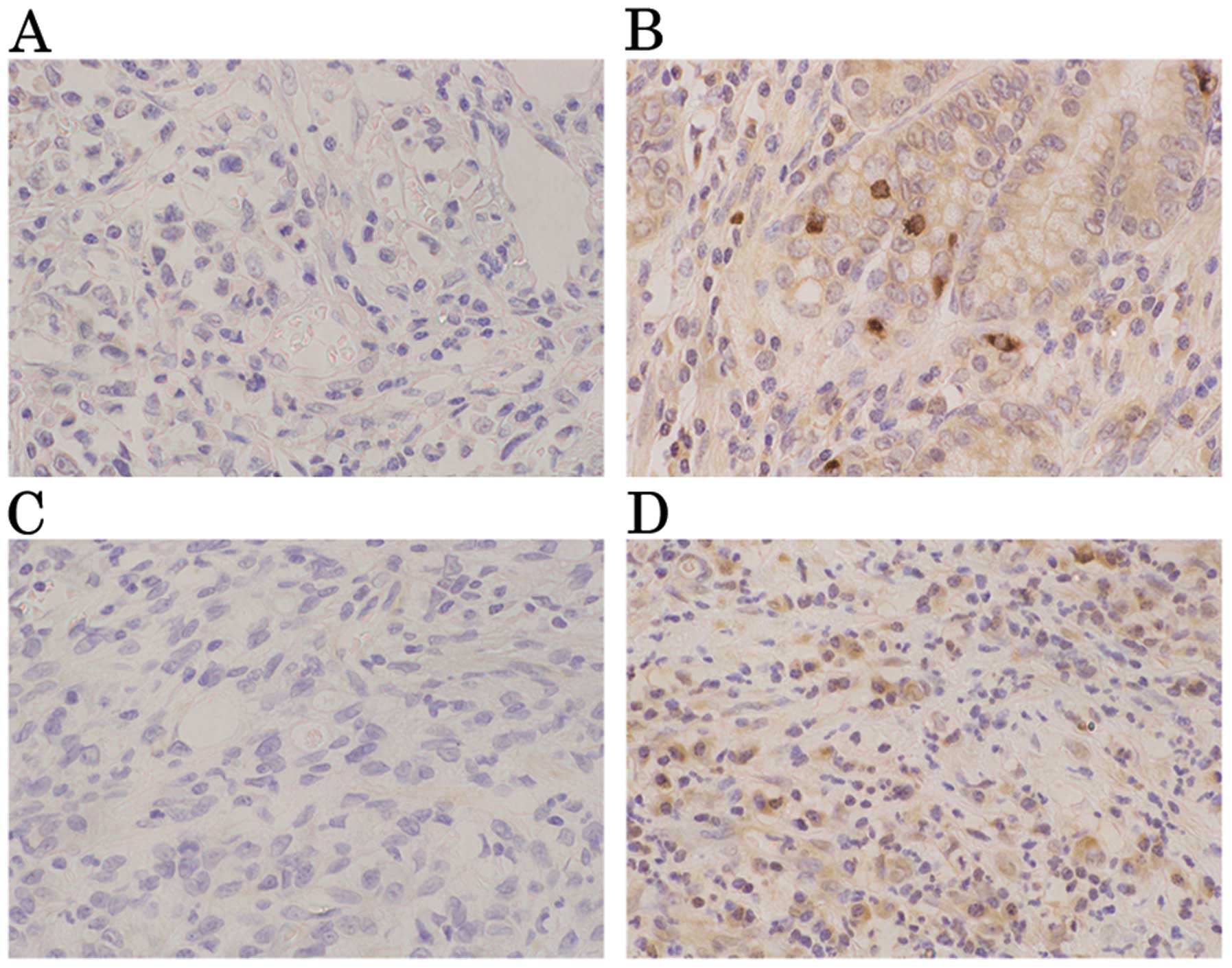
K-sam antibodies were weakly stained in the gastric
cancer mucosa. Tumors were evaluated as positive for K-sam when
≥50% of tumor cells in the infiltrative region were stained more
intensely than healthy epithelial cells in the same region
(Fig. 1A and B). In the
infiltrative region, tumors were evaluated as positive for KGF when
≥2 fibroblasts in the interstitium were stained more intensely than
the fundic gland at a magnification of ×200 (Fig. 1C and D).
Statistical analysis
The Chi-square and Mann-Whitney U tests were used to
determine the significance of the differences between the
covariates. The survival durations were calculated using the
Kaplan-Meier method and analyzed using the log-rank test to compare
the cumulative survival durations in the patient groups.
Additionally, a Cox proportional hazards model was used to
determine multivariate hazard ratios for the study parameters. In
all the tests, P<0.05 was considered to indicate a statistically
significant difference. The JMP 8.0.1 software program (SAS
Institute, Inc., Cary, NC, USA) was used for the analysis.
Results
Association of serological KGF levels
with clinicopathological factors
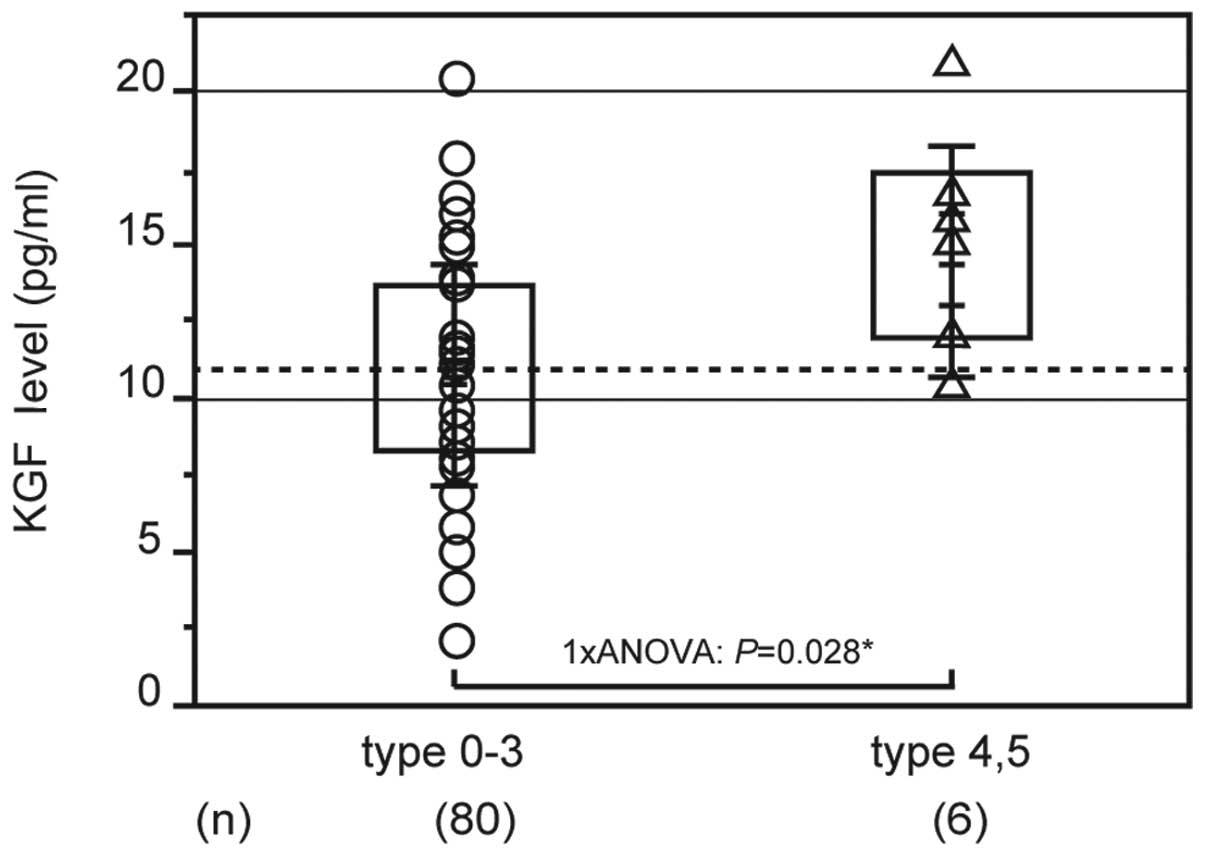
Fig. 2 shows the
association between clinical/oncological factors and serum KGF
levels. The average KGF level in early-stage gastric cancer was
11.191±3.808 pg/ml and in advanced gastric cancer was 10.715±3.4991
pg/ml; the difference between these results was small. Analysis of
the macroscopic type revealed that KGF levels were significantly
higher in types 4 and 5 (14.498±3.812 pg/ml, n=6) compared with
types 1, 2 and 3 (10.747±3.571 pg/ml, n=80; P=0.028; Fig. 2). With regard to the serum KGF
levels, there were no significant differences in histological type,
invasion depth, lymph node infiltration, vascular infiltration,
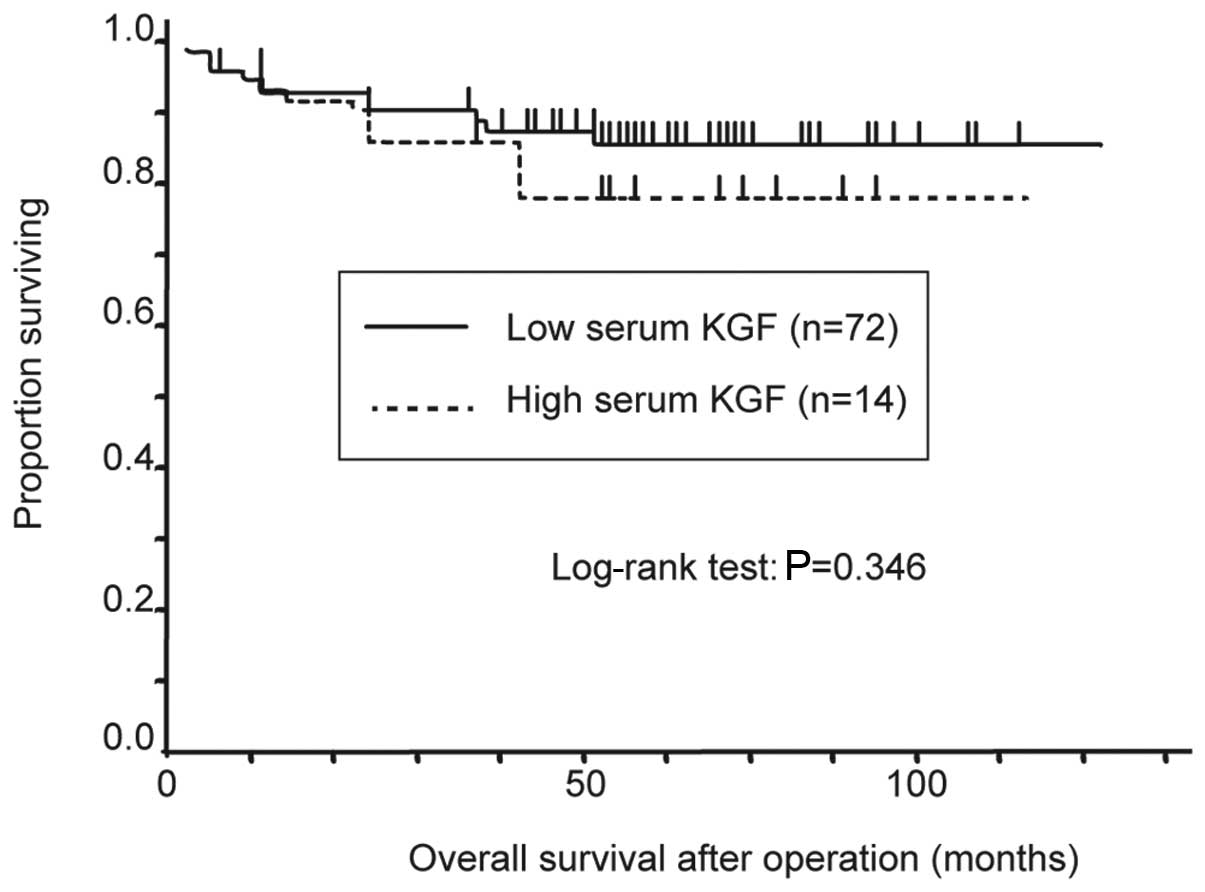
lymph node metastasis or stage classification (Table II). For the overall survival rate,
stage classification was the only significant factor that
determined prognosis (Table
III). Tumors were classified as high- or low-KGF with a cut-off
value of 14.608 pg/ml (the mean + 1xSD). The Kaplan-Meier method
was used to calculate the survival rate curves, with the high-KGF
group trending towards a poorer prognosis (Fig. 3).
 | Table IIUnivariate analysis of serum KGF. |
Table II
Univariate analysis of serum KGF.
| Factors | n | Mean | SD | P-value |
|---|
| Histological
types |
| Papillary/well- to
moderately differentiated | 45 | 11.242 | 3.797 | NS |
| Poorly
differentiated/signet-ring cell/mucinous | 41 | 10.753 | 3.601 | |
| Depth of tumor
invasion |
| M/SM | 49 | 11.191 | 3.808 | NS |
| MP/SS/SE | 37 | 10.768 | 3.568 | |
| Lymphatic
invasion |
| ly0/ly1 | 68 | 10.949 | 3.72 | NS |
| ly2/ly3 | 18 | 11.235 | 3.68 | |
| Venous invasion |
| v0 | 69 | 10.758 | 3.445 | NS |
| v1/v2 | 17 | 12.029 | 4.537 | |
| Lymph node
metastasis |
| No metastasis | 64 | 11.253 | 3.594 | NS |
| Metastasis | 22 | 10.3 | 3.963 | |
| Japanese clinical
stage |
| Ia/Ib/II | 72 | 11.015 | 3.573 | NS |
|
IIIa/IIIb/IVa/IVb | 14 | 10.977 | 4.403 | |
| Macroscopic
types |
| Type 0/1/2/3 | 80 | 10.747 | 3.571 | P=0.028 |
| Type 4/5 | 6 | 14.498 | 3.812 | |
 | Table IIIUnivariate and multivariate analysis
of overall survival. |
Table III
Univariate and multivariate analysis
of overall survival.
| Multivariate | Univariate | |
|---|
|
|
| |
|---|
| Factors | Risk ratio | 95% CI | Risk ratio | 95% CI | P-value |
|---|
| Depth of tumor
invasion (M, SM) vs. (MP, SS, SE) | 1.159 | 0.033–35.213 | 16.778 | 3.337–304.762 | NS |
| Infiltration (α, β)
vs. (γ) | 1.468 | 0.049–43.966 | 18.568 | 3.692–377.311 | NS |
| Vascular invasion
(v0) vs. (v1, v2) | 5.133 | 0.728–34.157 | 8.614 | 2.858–28.649 | NS |
| Lymphatic invasion
(ly0, ly1) vs. (ly2, ly3) | 0.329 | 0.054–1.757 | 7.278 | 2.474–23.928 | NS |
| Histological type
(Papillary/well- to moderately differentiated) vs. (poorly
differentiated/signet-ring cell/mucinous) | 2.158 | 0.478–15.611 | 6.389 | 1.714–41.301 | NS |
| Lymph node
metastasis (No metastasis) vs. (metastasis) | 0.532 | 0.040–6.389 | 0.053 | 0.008–0.197 | NS |
| Japanese clinical
stage (Ia, Ib, II) vs. (IIIa, IIIb, IVa, IVb) | 28.004 | 2.770–937.265 | 48.436 | 12.788–315.979 | 0.00031 |
| Macroscopic type
(Type 0, 1, 2, 3) vs. (type 4, 5) | 0.714 | 0.092–6.209 | 20.306 | 6.193–66.794 | NS |
| Serum KGF level
(≥mean + 1xSD) vs. (<mean + 1xSD) | 1.124 | 0.225–5.807 | 1.731 | 0.462–5.300 | NS |
Pathological KGF/K-sam expression
For the association between pathological KGF
expression and histological type, there was a tendency for
KGF-positive tumors to be poorly differentiated; however, this
difference was not significant.
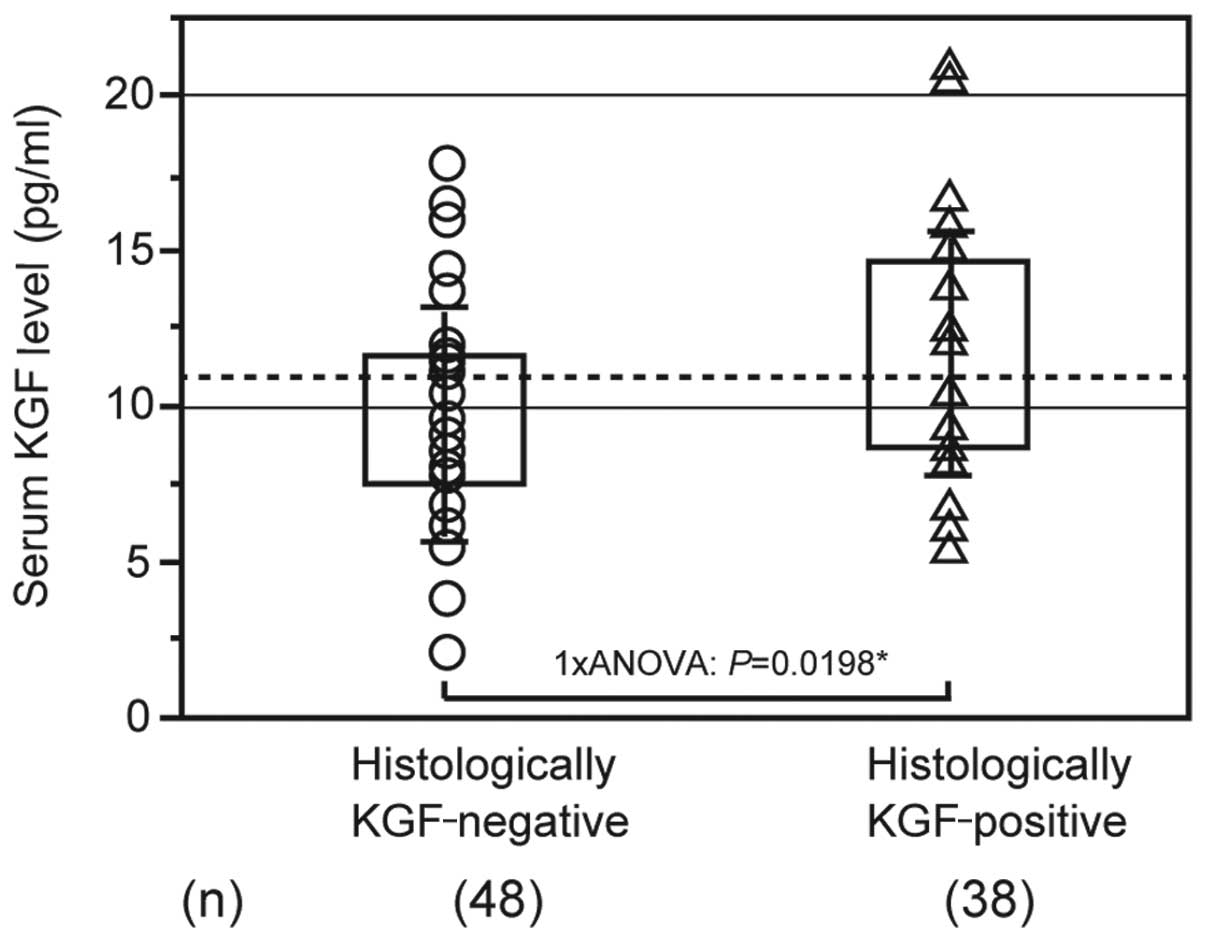
Exploring the association between pathological KGF
expression and serum KGF levels revealed that the mean level for
patients with KGF-negative tumors was 10.121±3.329 pg/ml, while it
was 12.131±3.861 pg/ml for patients with KGF-positive tumors.
Patients with KGF-positive tumors had significantly higher KGF
levels compared with patients with KGF-negative tumors (Fig. 4).
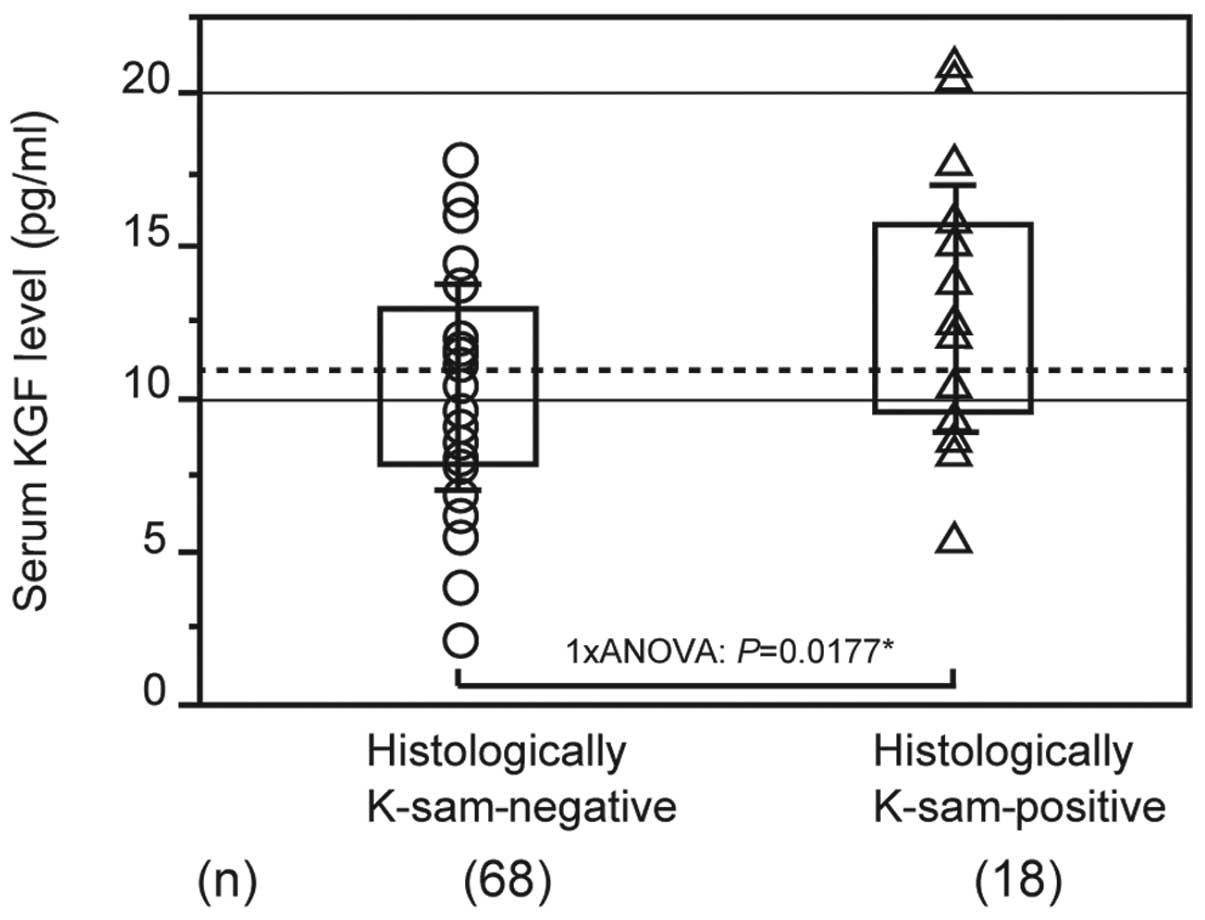
As demonstrated in previous studies, the K-sam gene
is highly expressed in scirrhous gastric cancer (22). The mean KGF level was 10.456±3.362
pg/ml in patients with K-sam-negative tumors and 13.099±4.212 pg/ml
in patients with K-sam-positive tumors. Patients with
K-sam-positive tumors had significantly higher KGF levels than
those with K-sam-negative tumors (Fig.
5).
Pathological KGF expression was not significantly
correlated with the degree of differentiation, while there was a
positive correlation between high K-sam expression and serum KGF
levels in scirrhous gastric tumors.
Patients with a high expression of the K-sam gene
also tended to have high serum KGF levels. It is possible that
serum KGF levels may be correlated with high K-sam expression and
diffuse infiltrative gastric cancer.
Discussion
Various mechanisms have been hypothesized to exist
in the interactions between cancer and interstitial cells.
Interstitial cell growth and angiogenesis factors produced by
cancer cells are considered to trigger interstitial cell
recruitment and angiogenesis. It has also been shown that cells
recruited by cancer cells produce various tumor growth factors
which promote growth and give rise to the invasive capacity of
cancer cells. In a number of these molecular mechanisms, the
surrounding cells are hypothesized to have paracrine-like
functions, resulting from the molecules produced and released by
cancer or interstitial cells (15).
The growth factors previously investigated,
including KGF and K-samII, have been shown to mainly contribute to
fibrosis. The molecules involved in this process have an impact on
cancer cells, fibroblasts and inflammatory cells, creating the
properties required for rapidly progressing, diffuse infiltrative
gastric cancer cells (13).
In the present study, the mechanisms that control
the development of diffuse infiltrative gastric cancer were
examined through the serological and pathological evaluation of
fibroblast growth factors and their receptors and coding genes.
Serum KGF levels in patients with gastric cancer were demonstrated
to be higher in those who had tumors with a poor prognosis and
exhibited significant cell proliferation and infiltration. This
suggests that the K-sam gene, which codes for the pathological KGF
receptor KGFR, was highly expressed. Therefore, it is possible that
KGFR expression may also be high and that KGF is released into the
serum, leading to the association between the high expression of
the K-sam gene and serum KGF levels.
Serum KGF was significantly higher in patients with
macroscopic types 4 and 5, regardless of the stage of cancer
progression. This finding indicates that it may be possible to
search serologically for malignant tumors with a poor prognosis
that exhibit significant proliferation and infiltration, such as
scirrhous gastric cancer.
Previous studies have shown that the types of
gastric cancer that have a high pathological expression of K-sam or
KGF are malignant tumors with a poor prognosis that induce
significant proliferation and infiltration, such as scirrhous
gastric cancer. Therefore, this has been identified as one factor
which may determine prognosis (23,24).
In the present study, there was a positive
correlation between pathological KGF expression and the serum KGF
levels, in addition to a positive correlation between pathological
K-sam expression and KGF levels, which indicates an association
between serum KGF and diffuse malignant tumors. Since there was no
correlation with cancer invasion depth or the stage of progression,
it may be possible to identify gastric cancer patients who have the
potential to develop malignant tumors with a poor prognosis that
exhibit significant proliferation and infiltration, such as
scirrhous gastric cancer, at the precursor lesion stage. However,
the investigation of survival rates conducted in this study did not
identify serum KGF as a factor determining prognosis. Further
studies are required to elucidate its clinical significance as a
biomarker.
Currently, diffuse growth-type malignant tumors,
such as scirrhous gastric cancer, remain difficult to diagnose
during the early stages of development and are usually at an
advanced stage at the time of diagnosis, which leads to poor
treatment outcomes.
The results of the present study suggest that serum
KGF is a risk factor for diffuse infiltrative gastric cancer and
may provide a simple method of identifying patients with a poor
prognosis among previously diagnosed preoperative gastric cancer
patients.
Acknowledgements
The authors would like to thank Mrs. Tabe (SRL
Laboratory, Tokyo, Japan) for kindly providing instrumentation. The
authors also thank Mr. Sakurada and Mr. Karita (Department of
Pathology, Tokyo Women’s Medical University, Tokyo, Japan) for
their technical advice.
References
|
1
|
Yamada A, Saito N, Kameoka S and Kobayashi
M: Clinical significance of epidermal growth factor (EGF)
expression in gastric cancer. Hepatogastroenterology. 54:1049–1052.
2007.PubMed/NCBI
|
|
2
|
Hirosawa T, Saito N, Kameoka S and
Kobayashi M: Clinical significance of epidermal growth factor (EGF)
expression for assessing the spreading of human colon cancer.
Nippon Daicho Komonbyo Gakkai Zasshi. 55:402–412. 2002. View Article : Google Scholar
|
|
3
|
Soyama K, Saito N and Kameoka K: Study on
adhesion molecule beta1 integrin in colorectal
cancer-quantification of blood levels and immunohistological
staining. Nippon Daicho Komonbyo Gakkai Zasshi. 52:119–127. 1999.
View Article : Google Scholar
|
|
4
|
Suda A, Saito N, Seshimo A, Kameoka S and
Kobayashi M: Examination of transforming growth factor beta1
expression in the serum and tumor tissue of gastric cancer. Int
Surg. 94:182–188. 2009.PubMed/NCBI
|
|
5
|
Daiko W, Saito N and Kameoka S: Clinical
significance of TGF-beta1 expression in evaluation of the
malignancy of colorectal cancer. Nippon Daicho Komonbyo Gakkai
Zasshi. 58:377–382. 2005. View Article : Google Scholar
|
|
6
|
Hashimoto T, Saito N, Kameoka S, Shibata N
and Kobayashi M: Clinical significance of hepatocyte growth factor
and its specific receptor c-Met expression in colorectal cancer
progression. Acta Histochem Cytochem. 37:139–146. 2004. View Article : Google Scholar
|
|
7
|
Saito N, Nishimura H and Kameoka S:
Clinical significance of fibronectin expression in colorectal
cancer. Mol Med Rep. 1:77–81. 2008.PubMed/NCBI
|
|
8
|
Yashiro M, Chung YS and Sowa M: Role of
orthotopic fibroblasts in the development of scirrhous gastric
carcinoma. Jpn J Cancer Res. 85:883–886. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Finch PW and Rubin JS: Keratinocyte growth
factor expression and activity in cancer: implications for use in
patients with solid tumors. J Natl Cancer Inst. 98:812–824. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Presta M, Dell’Era P, Mitola S, Moroni E,
Ronca R and Rusnati M: Fibroblast growth factor/fibroblast growth
factor receptor system in angiogenesis. Cytokine Growth Factor Rev.
16:159–178. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Szabo S and Sandor Z: Basic fibroblast
growth factor and PDGF in GI diseases. Baillières Clin
Gastroenterol. 10:97–112. 1996.PubMed/NCBI
|
|
12
|
Katoh M and Katoh M: FGF signaling network
in the gastrointestinal tract (Review). Int J Oncol. 29:163–168.
2006.PubMed/NCBI
|
|
13
|
Nakazawa K, Yashiro M and Hirakawa K:
Keratinocyte growth factor produced by gastric fibroblasts
specifically stimulates proliferation of cancer cells from
scirrhous gastric carcinoma. Cancer Res. 63:8848–8852. 2003.
|
|
14
|
Kunii K, Davis L, Gorenstein J, Hatch H,
Yashiro M, Di Bacco A, Elbi C and Lutterbach B: FGFR2-amplified
gastric cancer cell lines require FGFR2 and Erbb3 signaling for
growth and survival. Cancer Res. 68:2340–2348. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Yashiro M, Chung Y, Kubo T, Hato F and
Sowa M: Differential responses of scirrhous and well-differentiated
gastric cancer cells to orthotopic fibroblasts. J Cancer.
74:1096–1103. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Nakatani H, Tahara E, Yoshida T, Sakamoto
H, Suzuki T, Watanabe H, Sekiguchi M, Kaneko Y, Sakurai M, Terada
M, et al: Detection of amplified DNA sequences in gastric cancers
by a DNA renaturation method in gel. Jpn J Cancer Res. 77:849–853.
1986.PubMed/NCBI
|
|
17
|
Nakatani H, Sakamoto H, Yoshida T, Yokota
J, Tahara E, Sugimura T and Terada M: Isolation of an amplified DNA
sequence in stomach cancer. Jpn J Cancer Res. 81:707–710. 1990.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ishii H, Hattori Y, Itoh H, Kishi T,
Yoshida T, Sakamoto H, Oh H, Yoshida S, Sugimura T and Terada M:
Preferential expression of the third immunoglobulin-like domain of
K-sam product provides keratinocyte growth factor-dependent growth
in carcinoma cell lines. Cancer Res. 54:518–522. 1994.PubMed/NCBI
|
|
19
|
Ishii H, Yoshida T, Oh H, Yoshida S and
Terada M: A truncated K-sam product lacking the distal
carboxyl-terminal portion provides a reduced level of
autophosphorylation and greater resistance against induction of
differentiation. Mol Cell Biol. 15:3664–3671. 1995.
|
|
20
|
Itoh H, Hattori Y, Sakamoto H, Ishii H,
Kishi T, Sasaki H, Yoshida T, Koono M, Sugimura T and Terada M:
Preferential alternative splicing in cancer generates a K-sam
messenger RNA with higher transforming activity. Cancer Res.
54:3237–3241. 1994.PubMed/NCBI
|
|
21
|
Japanese Gastric Cancer Association.
Japanese Classification of Gastric Carcinoma - 2nd English Edition.
Gastric Cancer. 1:10–24. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Hattori Y, Itoh H, Uchino S, Hosokawa K,
Ochiai A, Ino Y, Ishii H, Sakamoto H, Yamaguchi N, Yanagihara K,
Hirohashi S, Sugimura T and Terada M: Immunohistochemical detection
of K-sam protein in stomach cancer. Clin Cancer Res. 2:1373–1381.
1996.PubMed/NCBI
|
|
23
|
Toyokawa T, Yashiro M and Hirakawa K:
Co-expression of keratinocyte growth factor and K-sam is an
independent prognostic factor in gastric carcinoma. Oncol Rep.
21:875–880. 2009.PubMed/NCBI
|
|
24
|
Nakamura K, Yashiro M, Matsuoka T, Tendo
M, Shimizu T, Miwa A and Hirakawa K: A novel molecular targeting
compound as K-samII/FGF-R2 phosphorylation inhibitor, Ki23057, for
scirrhous gastric cancer. Gastroenterology. 131:1530–1541. 2006.
View Article : Google Scholar : PubMed/NCBI
|