Introduction
Hepatocellular carcinoma (HCC) accounts for ~80–90%
of the primary liver cancer cases worldwide. Additionally, it is
the third most common cause of cancer-related mortality after
gastric and esophageal cancer (1).
Surgery is considered to be the primary treatment option. Without
liver transplantation, the 5-year survival rate of patients with
HCC is <5%. Therefore, research focused on HCC is critical, and
this disease has attracted considerable attention from
international medical groups (2).
Tumor development in HCC is a complex process involving multiple
factors and stages, while the detailed mechanism of HCC tumor
development has not yet been fully elucidated. However, cell
proliferation and apoptosis are the primary drivers of HCC
tumorigenesis, as in most types of cancer. The tumor suppressor
phosphatase and tensin homolog (PTEN) and the newly discovered
hepatocellular biomarker miR-92 represent the apoptosis-related
factors that may be important in HCC development.
The PTEN gene is located on chromosome 10q23.3. Its
protein product is a tumor suppressor with a dual phosphatase
activity. It dephosphorylates the D3 position on
phosphatidylinositol 3,4,5-trisphosphate (PIP3) to negatively
regulate the phosphatidylinositol 3-kinase (PI3K)/Akt signaling
pathway. This antagonizes the growth and survival signals elicited
by the PI3K pathway and induces cell death and cell cycle arrest.
Thus, PTEN activity inhibits tumor development (3). The mutation and inactivation of tumor
suppressor genes, such as PTEN, leads to uncontrolled cell
proliferation and survival, which subsequently increases the
likelihood of tumor formation. Moreover, PTEN mutation is
potentially associated with the histological grading of HCC tumors
and the invasive metastatic phenotype of the disease. PTEN
mutations have been identified in a number of other types of
cancer, including gastric, hepatic, breast, endometrial and
prostate cancer (4,5). In these types of tumors, loss of PTEN
function is critical for tumorigenesis since it has been shown to
regulate the cell cycle, apoptosis, invasion and metastasis of
tumor cells (6).
MicroRNAs (miRNAs) are a class of non-coding
single-stranded RNAs (20–24 nucleotides), which are encoded by the
genomes of higher eukaryotes. Mature miRNAs are formed through a
series of processing steps in the nucleus and cytoplasm (7). miRNAs exert their regulatory effects
by binding to the 3′ untranslated regions (UTRs) of target gene
mRNAs. This results in degradation of the target mRNA by the
RNA-induced silencing complex (RISC) or by inhibition of the target
mRNA translation (8). Previous
studies have demonstrated that the expression levels of various
miRNAs differ significantly in different tissues and at different
developmental stages. Notably, each miRNA may have multiple target
genes, and multiple miRNAs may regulate a single gene. The
microRNA-17-92 (miR-17-92) gene family is considered to play a
critical role in the normal development of the lung, heart and
immune system. This family may also be significant in tumor
formation, since several miRNA family members have been shown to be
upregulated in multiple tumor types. miR-92 is a member of the
miR-17-92 family and has been previously described to be a
proto-oncogene. In fact, it has been shown to increase
proliferation and inhibit apoptosis, thus leading to tumorigenesis
(9). Mice with specific expression
of the miR-17-92 gene family in lymphocytes exhibited an increased
proliferation of this cell type, developed autoimmune disease, and
died prematurely. In these mice, miR-17-92 promoted the
proliferation of lymphocytes and attenuated apoptosis by inhibiting
PTEN and increasing Bim expression (10). Since miR-92 plays a specific role
in apoptosis, PTEN may also be critical in HCC. Therefore, both may
have a negative correlation in HCC. In this study, we used
immunohistochemistry and quantitative reverse
transcription-polymerase chain reaction (qRT-PCR) to investigate
the expression of miR-92 and PTEN as well as the correlation
between the two. Their influence on HCC tumor development was
examined and our data were related to useful clinical parameters,
such as diagnosis and prevention.
Materials and methods
Subjects
A total of 15 patients who underwent surgery at the
Department of General Surgery, Qilu Hospital of Shandong University
(Jinan, China) between March 2008 and April 2009 with integrated
medical records were included in this study. The study was approved
by the ethics committee of Shandong University. Resected tissue
diagnosis for HCC was performed using postoperative pathological
methods. The patients did not receive any hormonal therapy prior to
surgery, and did not suffer from any complicating diseases in the
nervous or endocrine systems. The differentiation status of each
tumor was defined according to the Edmondson grading system, in
which the HCC is defined as grade I, II, III or IV. Grades I and II
are considered to be highly differentiated, while grades III and IV
are considered to be less differentiated. Fresh specimens of the
HCC and paracancerous tissues (2 cm in distance from the edge of
the tumor) were obtained by resection, fixed in 4% formalin and
prepared into paraffin sections. The remaining tissues were
preserved in a −80°C freezer after freezing in liquid nitrogen.
Reagents
The following reagents were used: rabbit anti-human
PTEN monoclonal antibody (Cell Signaling Technology, Inc., Beverly,
MA. USA); streptomycin avidin-peroxidase kit and concentrated DAB
kit (Beijing Zhongshan Goldenbridge Biotechnology, Co., Ltd.,
Beijing, China); diethyl pyrocarbonate (DEPC; Takara Biotechnology,
Dalian, China); TRIzol, SYBR fluorescent real-time PCR kit and
reverse transcription kit (Invitrogen, Carlsbad, CA, USA);
TaqMan® MicroRNA kit (Applied Biosystems, Foster City,
CA, USA); and primers for PTEN and β-actin (BGI Life Technologies,
Beijing, China).
Methods
PTEN and miR-92 expression in HCC and
paracancerous tissues using streptavidin-peroxidase (SP)
methods
Experimental procedures were performed according to
the manufacturer’s instructions for two-step detection. Positive
results were defined as yellow-brown staining in the cytoplasm of
hepatocytes. Under a light microscope (magnification, ×400), 10
different non-overlapping visual fields were randomly chosen in
each specimen. Stained cells were counted by artificial counts, and
the mean values were used for comparisons among the groups. The
positive cells were divided into 4 different grades based on their
number and intensity: cells with no obvious difference in staining
intensity and background were defined as (−); numbers of positive
cells <10% with mostly weak staining intensity were defined as
(+); numbers of positive cells >50% with mild to strong staining
intensity were defined as (+++); numbers of positive cells and
staining intensity between (+) and (+++) were considered (++). The
criteria used to assess the immunohistochemical results have been
previously described (11).
PTEN and miR-92 expression in HCC and
paracancerous tissues as detected using qRT-PCR
Extraction of total RNA from carcinoma
and paracancerous tissues
Tissues (100 μg) were harvested and pulverized into
powder. TRIzol (1 ml) was added to mix evenly, and the powder was
placed into a 1.5-ml Eppendorf tube. Chloroform (0.2 ml) was added
and the tube was vigorously mixed for 15 sec. The tube was then
placed at room temperature for 2 min and centrifuged at 12,000 × g
for 30 min. The upper aqueous phase was obtained and placed into a
new EP tube. The same proportion of isopropyl alcohol was added.
The EP tube was inverted 10 times, and then centrifuged at 12,000 ×
g, 4°C for 30 min. Supernatants were discarded, ethanol was added
and mixed evenly, and the solution was centrifuged at 12,000 × g
4°C, for 10 min. The supernatants were again discarded, and the
remaining portion was dried at room temperature for 10 min. The
extracted RNA was dissolved in 30 μl DEPC water. The purity and
density of RNA were determined using UV spectrophotometry and 1%
agarose gel electrophoresis was used to detect the integrity of the
RNA. Finally, the RNA was preserved in a −80°C freezer.
Reverse transcription of synthetic
cDNA and qRT-PCR
The TaqMan® MicroRNA kit was used. U6
snRNA was used as an internal control to perform qRT-PCR for the
detection of the differential expression of miR-92. The primer used
for miR-92 was obtained from Applied Biosystems. The reaction
system and conditions used were as described in the manufacturer’s
instructions. Three replicates were used for each detection index,
and the formula 2−ΔΔCT was used for the analysis of the
final data. cDNA synthesis was performed by collecting equal
amounts of total RNA, followed by the application of reverse
transcription when qRT-PCR was performed. A reaction volume of 50
μl was used for each gene amplification. Each reaction mixture
consisted of 2 μl PCR products, 5 μl 10X buffer, 39.5 μl
dH2O, 0.5 μl Taq enzyme, 1 μl dNTPs, and 10 μl primers
for PTEN and control β-actin. The primers used were the following:
PTEN: upstream, 5′-TCCACAAA CAGAACAAGATG-3′ and downstream,
5′-CTGGTCCTGG TATGAAGAAT-3′; β-actin: upstream, 5′-CTAAGTCATAG
TTCCGCCTAGAAGCA-3′ and downstream, 5′-TGGCACC CAGCACAATGAA-3′. The
qRT-PCR conditions for PTEN and β-actin were: 95°C for 10 min, 95°C
for 10 sec, and then 60°C for 60 sec for 24 repeated cycles.
Statistical analysis
SPSS 16.0 software (SPSS, Inc., Chicago, IL, USA)
was used to conduct the analysis, and the Student’s t-test was used
for comparison between groups. ANOVA was used for comparisons among
groups. Pearson’s correlation analysis was used to analyze the
correlation of the two indices. P<0.05 was considered to
indicate a statistically significant difference.
Results
Expression of PTEN protein and PTEN mRNA
in the HCC and paracancerous tissues
PTEN mRNA was detected as a clear band at 517 bp in
the 15 cases of HCC and normal hepatic tissues. A band at 712 bp
was detected for β-actin, which served as an internal control.
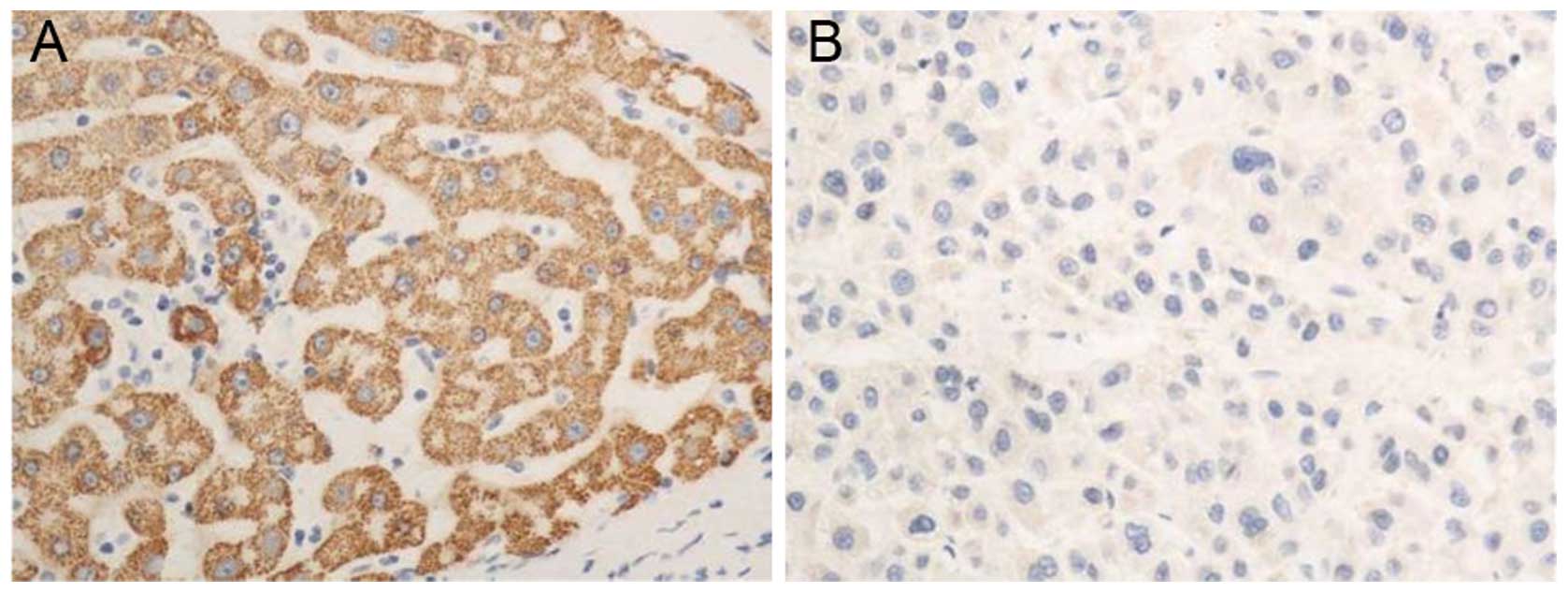
The immunohistochemical results demonstrated
positive staining for PTEN protein in the nuclei of tumor cells,
and yellow-brown particles were generated. The expression level of
PTEN protein in each HCC tissue sample was significantly lower
compared with that in the corresponding paracancerous tissue
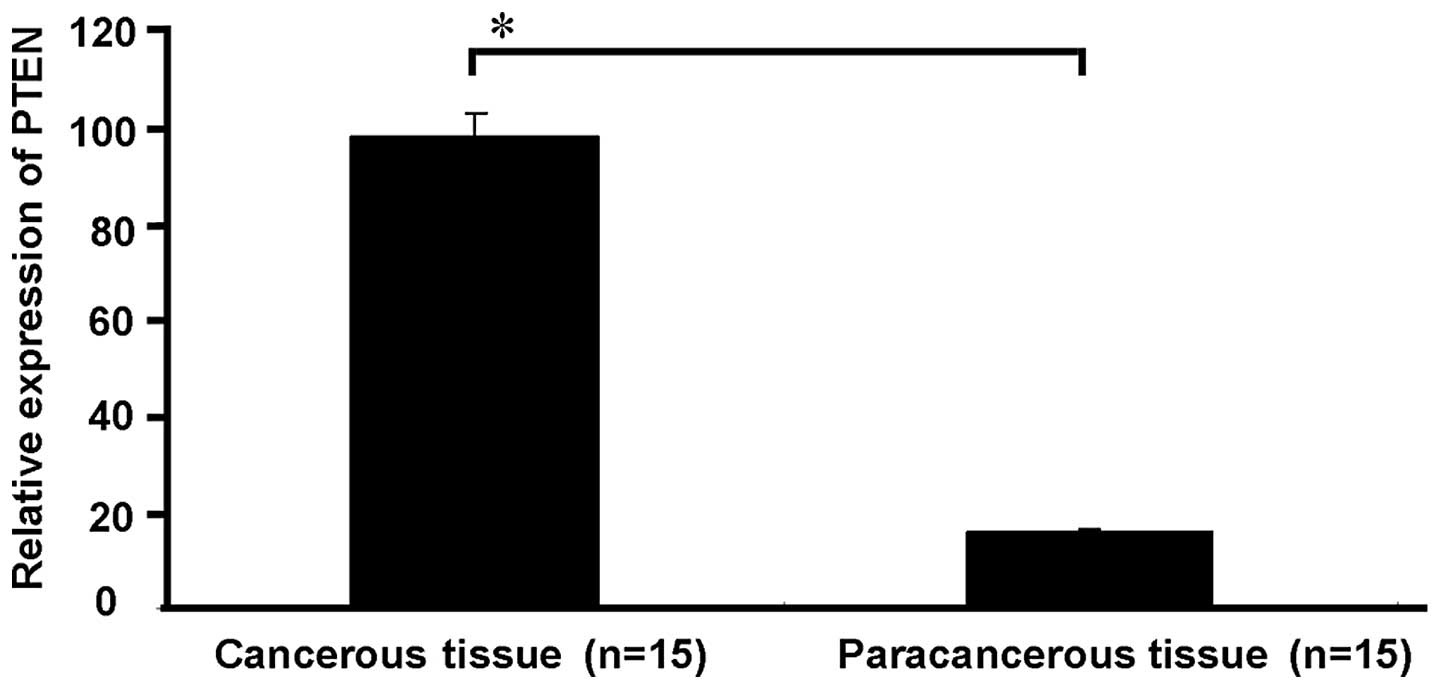
(P<0.05, Fig. 1). The qRT-PCR
results indicated that the PTEN mRNA expression level in 13 (86.7%)
of the cases of HCC was significantly lower compared with the PTEN
mRNA expression level in the corresponding paracancerous tissue.
The expression level of PTEN showed a significant difference
between the HCC and paracancerous tissues (P<0.05, Fig. 2).
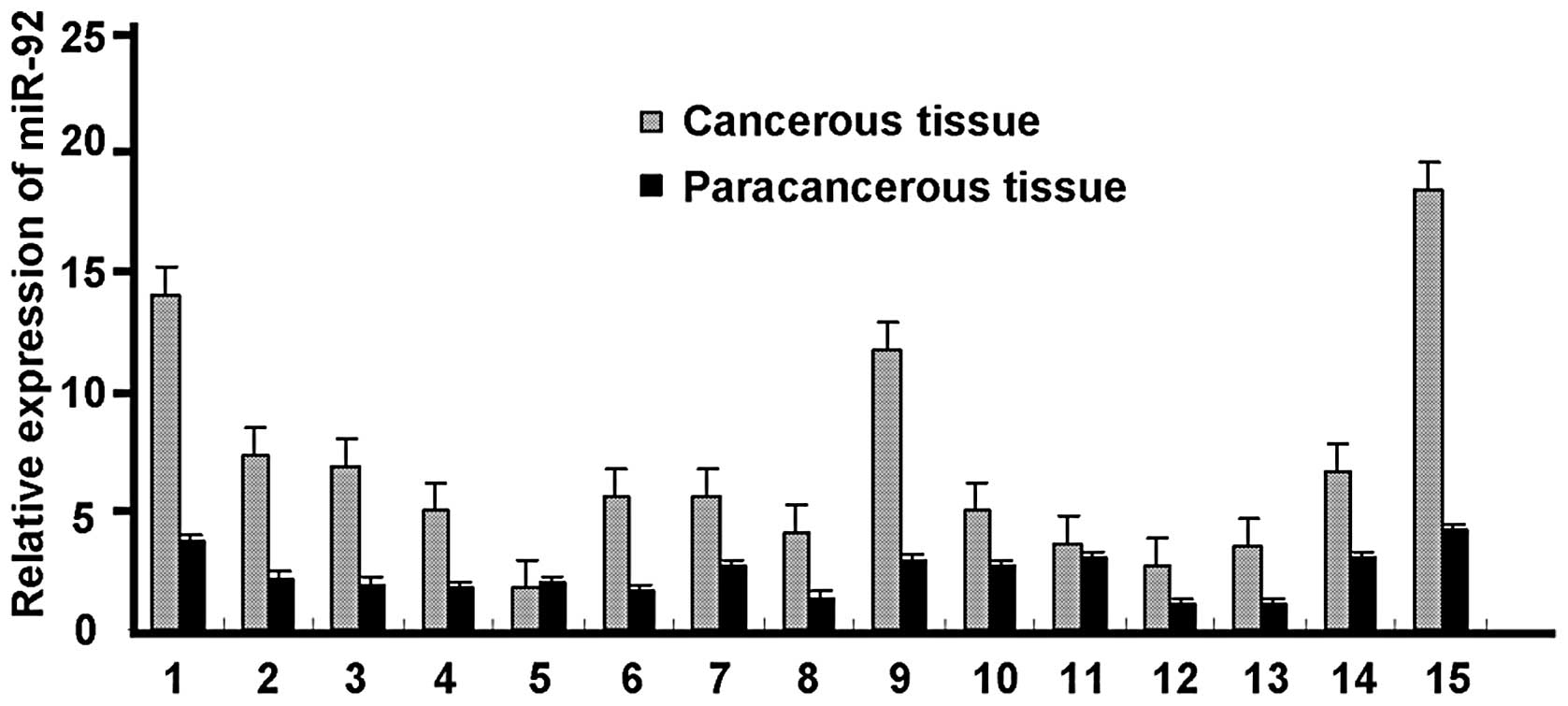
Expression of miR-92 mRNA in HCC and
paracancerous tissues
The miR-92 mRNA expression level was significantly
higher in 14 (93.3%) of the cases of HCC than in the corresponding
paracancerous tissue. However, in one case there was no significant
difference in the miR-92 expression level between the HCC and
paracancerous tissues (Fig.
3).
Correlation of PTEN and miR-92 expression
levels in HCC
The relative amounts of miR-92 expression were
gradually increased with the degree of malignancy in HCC, while the
relative amounts of PTEN gene expression gradually decreased.
Pearson’s correlation analysis indicated that the expression of
miR-92 showed a significant negative correlation with PTEN
expression in HCC (r=−0.858, P<0.05, Table I).
 | Table ICorrelation analysis between miR-92
and PTEN in hepatocellular carcinoma tissues (r=−0.858, P<0.05;
n=15). |
Table I
Correlation analysis between miR-92
and PTEN in hepatocellular carcinoma tissues (r=−0.858, P<0.05;
n=15).
| PTEN, n | miR-92, n |
|---|
| High expression | 2 | 14 |
| Low expression | 13 | 1 |
Discussion
Tumorigenesis is a complex process influenced by
various genes and involving various stages. Studies have
demonstrated that the abnormal expression of multiple genes is
implicated in the development of HCC. Proliferation and apoptosis
are known to be critical for tumor formation and growth (12). The development of HCC is associated
with high malignancy, poor therapies and gene expression changes.
The PTEN gene is a tumor suppressor gene with dual-specific
phosphatase activity, which is a critical signaling molecule in the
regulation of cell proliferation and apoptosis, thus inhibiting
tumorigenesis. The PTEN gene regulates normal physiology mainly
through the PIP3, FAK and MAPK signaling pathways (13). A study demonstrated that multiple
human tumors have PTEN mutations, which alter signal transduction,
increase tumorigenesis, and lead to a worse patient prognosis
(11). The results of the present
study indicate that PTEN mRNA and protein expression levels were
significantly lower in HCC compared with paracancerous tissues
(P<0.05). These results indicated that the inactivation of the
PTEN gene may play a critical role in the development of HCC. The
loss of function of PTEN is mostly due to genetic changes,
including point mutations, deletions, splicing alterations,
frameshift mutations, missense mutations, or small fragment
insertions and deletions (14).
PTEN may also be post-transcriptionally regulated by miRNAs
(15). We observed that PTEN
expression was lower in poorly differentiated HCC with high
malignancy. By contrast, it was higher in more differentiated
tumors, suggesting a critical clinical significance.
miR-92 is a member of the microRNA-17-92 family and
has been described to be a proto-oncogene. miR-92 is also known to
promote cell proliferation and inhibit apoptosis. The present study
indicates that miR-92 expression levels in HCC are significantly
higher compared with those in the corresponding paracancerous
tissue, which is in agreement with the study by Huang et
al(16), where miR-92 was
demonstrated to play a role in the development of HCC.
Despite the studies that have been performed, it
remains unclear whether the regulation by miR-92 of PTEN is
involved in the development of HCC. Our results indicate that the
tumor suppressor gene PTEN is negatively correlated with the
expression of miR-92 in HCC clinical tissues, which indicates that
PTEN and miR-92 have opposing roles in HCC development which is in
agreement with previous studies on other diseases (17). This result also indicates that the
upregulation of miR-92 may inhibit the expression of PTEN, thereby
inducing tumorigenesis through downstream genes. Consequently, the
following potential mechanism of miR-92 function is proposed. The
expression of miR-92 is regulated by the PI3K/Akt signaling
pathway. PTEN is a phosphatase that inhibits the PI3K/Akt pathway
in normal physiological conditions, thereby inhibiting the
expression of miR-92. When PTEN expression is lost, PI3K/Akt is no
longer inhibited, and miR-92 expression is upregulated. Cell
division is promoted and the the number of cells in the S and G2/M
phases is increased. miR-92 enters the cell nucleus and binds to
cell cycle-dependent protein kinases, inhibits apoptosis and
promotes the proliferation of tumor cells. In the progression of
HCC, miR-92 and PTEN show mutual correlation and restriction. Both
miR-92 and PTEN are involved in the regulation of the cell cycle
and apoptosis, whereas their biological effects are opposite
(18,19). In addition, it is possible that
miR-92 affects Myc-induced E2F1 expression, and that it plays a
role in the apoptotic response. In this case, PTEN may also play an
opposing role to miR-92 (3,20).
Thus, examining the regulation by miR-92 of PTEN is beneficial in
elucidating the complex regulatory mechanisms governing HCC
tumorigenesis. Additionally, the combined detection of miR-92 and
PTEN may have clinical significance in terms of diagnosis,
prognostic judgment and therapeutic options for primary HCC.
Acknowledgements
This study was supported by grants from the 973
Program of China (no. 2011CB504302) and the Natural Science
Foundation of Shandong (nos. 2007GG200002043 and Y2007C122). The
authors appreciate valuable comments from other members of their
laboratories.
References
|
1
|
El-Serag HB and Rudolph KL: Hepatocellular
carcinoma: epidemiology and molecular carcinogenesis.
Gastroenterology. 132:2557–2576. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
El-Serag HB and Mason AC: Rising incidence
of hepatocellular carcinoma in the United States. N Engl J Med.
340:745–750. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Tamguney T and Stokoe D: New insights into
PTEN. J Cell Sci. 120:4071–4079. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Martin J and Dufour JF: Tumor suppressor
and hepatocellular carcinoma. World J Gastroenterol. 14:1720–1733.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Yao YJ, Ping XL, Zhang H, et al:
PTEN/MMAC1 mutations in hepatocellular carcinomas. Oncogene.
18:3181–3185. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Steck PA, Pershouse MA, Jasser SA, et al:
Identification of a candidate tumour suppressor gene, MMAC1, at
chromosome 10q23.3 that is mutated in multiple advanced cancers.
Nat Genet. 15:356–362. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Lee Y, Kim M, Han J, et al: MicroRNA genes
are transcribed by RNA polymerase II. EMBO J. 23:4051–4060. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Xie X, Lu J, Kulbokas EJ, et al:
Systematic discovery of regulatory motifs in human promoters and 3′
UTRs by comparison of several mammals. Nature. 434:338–345.
2005.
|
|
9
|
Mendell JT: miRiad roles for the miR-17-92
cluster in development and disease. Cell. 133:217–222. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Xiao C, Srinivasan L, Calado DP, et al:
Lymphoproliferative disease and autoimmunity in mice with increased
miR-17-92 expression in lymphocytes. Nat Immunol. 9:405–414. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Slipicevic A, Holm R, Nguyen MT, Bohler
PJ, Davidson B and Florenes VA: Expression of activated Akt and
PTEN in malignant melanomas: relationship with clinical outcome. Am
J Clin Pathol. 124:528–536. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kerr JF, Winterford CM and Harmon BV:
Apoptosis. Its significance in cancer and cancer therapy. Cancer.
73:2013–2026. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Xu Z, Stokoe D, Kane LP and Weiss A: The
inducible expression of the tumor suppressor gene PTEN promotes
apoptosis and decreases cell size by inhibiting the PI3K/Akt
pathway in Jurkat T cells. Cell Growth Differ. 13:285–296.
2002.PubMed/NCBI
|
|
14
|
Smith JS and Jenkins RB: Genetic
alterations in adult diffuse glioma: occurrence, significance, and
prognostic implications. Front Biosci. 5:D213–D231. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Meng F, Henson R, Wehbe-Janek H, Ghoshal
K, Jacob ST and Patel T: MicroRNA-21 regulates expression of the
PTEN tumor suppressor gene in human hepatocellular cancer.
Gastroenterology. 133:647–658. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Huang YS, Dai Y, Yu XF, et al: Microarray
analysis of microRNA expression in hepatocellular carcinoma and
non-tumorous tissues without viral hepatitis. J Gastroenterol
Hepatol. 23:87–94. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Huang GL, Zhang XH, Guo GL, et al:
Expression of microRNA-21 in invasive ductal carcinoma of the
breast and its association with phosphatase and tensin homolog
deleted from chromosome expression and clinicopathologic features.
Zhonghua Yi Xue Za Zhi. 88:2833–2837. 2008.
|
|
18
|
Fang J, Ding M, Yang L, Liu LZ and Jiang
BH: PI3K/PTEN/AKT signaling regulates prostate tumor angiogenesis.
Cell Signal. 19:2487–2497. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Mann CD, Neal CP, Garcea G, Manson MM,
Dennison AR and Berry DP: Prognostic molecular markers in
hepatocellular carcinoma: a systematic review. Eur J Cancer.
43:979–992. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
O’Donnell KA, Wentzel EA, Zeller KI, Dang
CV and Mendell JT: c-Myc-regulated microRNAs modulate E2F1
expression. Nature. 435:839–843. 2005.PubMed/NCBI
|