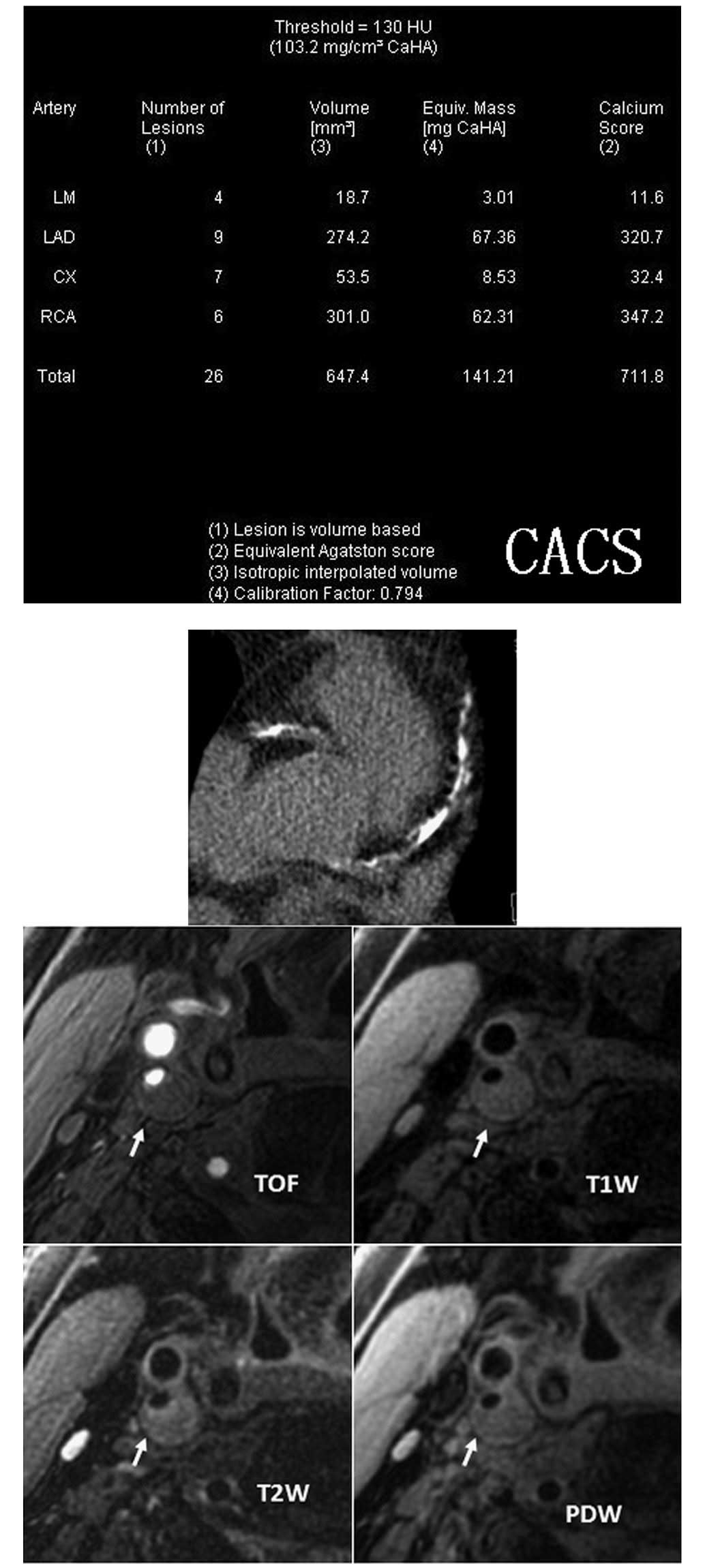
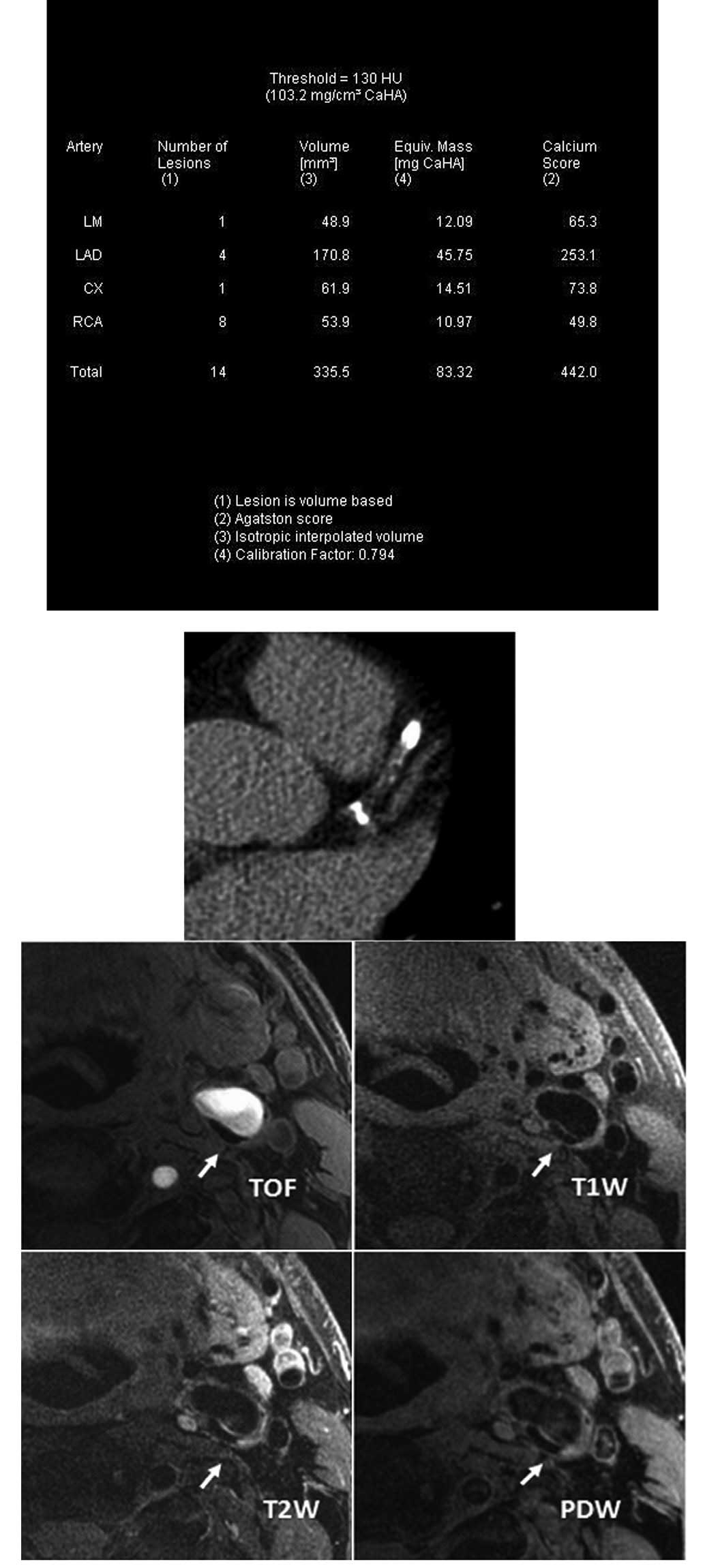
Introduction
Atherosclerotic disease has been demonstrated to be
associated with cardiovascular events, including myocardial
infarction, transient ischemia attack (TIA) and ischemic stroke
(1,2). Atherosclerosis is a systemic arterial
disease which frequently involves multiple vascular beds (3,4).
Etiologically, cases of atherosclerosis occurring in different
arterial vascular beds may share the same set of traditional risk
factors (5). Therefore,
individuals who develop atherosclerotic disease in one vascular bed
may have concomitant atherosclerosis in other vasculatures.
Therefore, atherosclerotic incidence in one arterial circulation
may be a predictor of atherosclerosis in other arterial
territories.
A number of previous studies demonstrated a
significant correlation between coronary and carotid
atherosclerosis (6–8). Coronary artery calcium score (CACS)
was demonstrated to represent an effective predictor of carotid
atherosclerotic disease as measured by ultrasound intima-media
thickness (IMT) (9,10). In addition to measurement of plaque
burden, more recently, characterization of carotid compositional
features, including lipid-rich necrotic core (LRNC), intraplaque
hemorrhage (IPH) and surface disruption, as determined by
high-resolution, multi-contrast magnetic resonance imaging (MRI),
have been hypothesized to be clinically significant due to their
associations with neurovascular events (11–13).
However, the predictive value of CACS for carotid compositional
features remains unknown.
The present study aimed to determine the association
between CACS and carotid atherosclerotic disease and the predictive
value of CACS for plaque burden and compositional features in
carotid arteries as determined by high-resolution, multi-contrast
MRI.
Materials and methods
Study population
Individuals who were suspected to suffer from
coronary artery disease (CHD) with chest pain were recruited. The
study was conducted in accordance with the declaration of Helsinki
and the study protocol was approved by the Ethics Committee and the
Institutional Review Board of the Chinese PLA General Hospital. All
patients provided written informed consent. The exclusion criteria
included contraindications to MR scan. The subjects underwent CT
scan for CACS exam and carotid high-resolution, multi-contrast,
bilateral carotid MR imaging within 2 weeks.
CACS data acquisition and image
processing
A low-dose CACS protocol was performed to capture
coronary pre-contrast images for CACS measurement using a Dual
Source CT scanner (Dual Source CT; Siemens, Forchheim, Germany).
The parameters of the low-dose CACS protocol were as follows: 120
kV; 80 mA/rot; pitch, 0.2–2.0; rotation time, 0.33 sec/rot; and
slice thickness, 3.0 mm. CACS was computed using commercial
software (Syngo MultiModality Workplace; Siemens) and Agatston
algorithm (14) by an experienced
radiologist.
Carotid MR imaging
Carotid MR imaging was performed using a dedicated,
phased-array, surface coil and a 3.0T scanner (GE Signa Excite; GE
Healthcare, Waukesha, WI, USA). Cross-sectional images of the
carotid arteries were captured using four contrast-weighted images:
T1-weighted (T1W), proton density-weighted (PDW), T2-weighted (T2W)
and three-dimensional (3D) time of flight (TOF). Parameters for the
imaging sequences were as follows: T1W: quadruple
inversion-recovery (QIR), black-blood, 2D fast spin-echo, TR/TE
800/8.8 msec; PDW and T2W: double echo, TR=3,000 msec, TE 13.1 msec
for PDW and 56.9 msec for T2W; and 3D TOF: TR/TE 29/2.1 msec, flip
angle 20º. Images were captured under a field of view of 14 cm and
matrix size 256×256 for an in-plane acquisition resolution of
0.55×0.55 mm2. Axial images of the bilateral carotid
arteries were captured with a 2-mm slice thickness over a
longitudinal coverage of 32 mm.
Interpretation of carotid MR images
The carotid MR images were interpreted by two
trained reviewers via consensus opinion blinded to clinical
information and CACS results. For MR image review, CASCADE image
analysis software (15) was used
to outline the lumen and outer wall boundaries. The lumen area
(LA), wall area (WA), total vessel area (TVA), mean wall thickness
(MWT) and normalized wall index (NWI = WA/TVA) were measured for
each axial location. Carotid artery was divided into three
segments: internal carotid artery (ICA), defined as the segment
above the bifurcation; bulb, defined as the segment from the
bifurcation to 4 cm below; and common carotid artery (CCA), defined
as the segment below the bulb. The mean values of LA, WA, TVA, MWT
and NWI of bilateral arteries for each subject and each carotid
segment were calculated. The presence or absence of carotid plaque
compositions, including calcification, LRNC, IPH and surface
disruption were also identified and recorded for each carotid
segment and each subject, respectively, using criteria described
previously (16).
Statistical analysis
The correlation of CACS with carotid morphological
measurements, including LA, WA, TVA, MWT and NWI and the presence
or absence of plaque compositions, such as calcification, LRNC and
IPH were evaluated. The correlation between CACS and the volumes of
carotid calcification, LRNC and IPH in subjects was also
determined. CACS was divided into three categories: CACS=0, =1–399
and >400 (17). Morphological
measurements and the prevalence of plaque compositions in each CACS
category were determined. Univariate regression was used to
determine the correlation between CACS and carotid plaque burden
and compositional features. Multivariate regression was also
conducted to evaluate the correlation between CACS and carotid
plaque burden and compositional features following adjustment for
confounding factors. The odds ratios (OR) and corresponding 95%
confidential interval (CI) of CACS were calculated to predict the
presence of carotid calcification, LRNC and IPH with an increment
of 100. P<0.05 was considered to indicate a statistically
significant difference. Analyses were performed with SPSS software
for windows (version 12.0; SPSS, Inc., Chicago, IL, USA).
Results
Patient characteristics
Between May 2008 and February 2009, 128 patients
with suspected CAD were recruited. Of 128 patients, 5 were excluded
from the final analysis due to poor MR image quality. Of the
remaining 123 patients, 96 were male (mean age, 58.0±9.5 years), 60
had carotid calcification, 79 had LRNC, 12 had IPH and 4 had
surface disruption. The demographic characteristics for the
patients are provided in Table I.
Surface disruption was not included in the statistical analysis due
to a limited number of cases.
 | Table IDemographic characteristics of the
study population (n=123). |
Table I
Demographic characteristics of the
study population (n=123).
| Characteristics | Mean ± SD or n
(%) | Range (where
applicable) |
|---|
| Gender, male | 96 (78) | - |
| Age, years | 58.0±9.5 | 44–84 |
| Height, cm | 170.1±6.9 | 152–188 |
| Weight, kg | 75.4±10.8 | 50–100 |
| BMI | 26.0±2.9 | 19.0–33.8 |
| TC, mg/dl | 192.3±41.9 | 116.0–382.8 |
| HDL, mg/dl | 45.8±14.3 | 18.2–144.2 |
| LDL, mg/dl | 104.9±29.2 | 35.2–191.0 |
| SBP, mmHg | 148.6±26.4 | 100–240 |
| DBP, mmHg | 91.5±16.7 | 62–130 |
| Hypertension | 72 (58.5) | - |
| Diabetes | 30 (24.4) | - |
| Smoking | 67 (54.5) | - |
Correlation between CACS and carotid
morphology
CACS was found to significantly correlate with WA,
TVA, MWT and NWI for carotid artery (r=0.208–0.529; P<0.05;
Fig. 1). Similarly, significant
correlations between CACS with WA, TVA, MWT and NWI for ICA, bulb
and CCA were also found. In addition, CACS was found to positively
correlate with LA for carotid artery and CCA, but not for ICA and
bulb. Of note, CACS was observed to correlate more closely with NWI
of ICA and bulb than that of CCA.
Following adjustment for confounding factors,
including age, gender, BMI, hypertension, hyperlipidemia, diabetes
and smoking, CACS remained significantly correlated with MWT for
carotid artery and all its segments (P<0.01). Similarly, a
significant correlation of CACS with NWI for carotid artery and
segments ICA and bulb were also found. CACS positively correlated
with NWI for CCA (β=0.238; P=0.028) prior to adjustment for
confounding factors, but not following adjustment (β=0.178;
P=0.115). Of note, CACS was found to correlate more closely with
NWI of ICA and bulb than that of CCA.
Correlation between CACS and carotid
plaque compositions
A significant moderate correlation was observed
between CACS and the presence of calcification for carotid artery
and its segments (ICA, bulb and CCA; r=0.426–0.510; P<0.05;
Table II). In addition, a weak
correlation between CACS and the presence of LRNC in carotid artery
and its three segments was observed (r=0.237–0.394; P<0.05). For
IPH, a significant weak correlation with CACS was found in carotid
artery (r=0.208; P=0.021) and bulb (r=0.205; P=0.023). However, no
arteries in patients with CACS=0 developed IPH. In patients with
carotid calcification (n=60), a marked correlation was found
between carotid calcification volume and CACS (r=0.747; P<0.001;
Fig. 2). For arteries with LRNC
(n=79), CACS did not correlate with LRNC volume (r=0.183; P=0.107).
Similarly, there was no significant correlation between CACS and
IPH volume (r=−0.055; P=0.865) in arteries with IPH (n=12).
 | Table IICorrelation between carotid plaque
compositions and CACS. |
Table II
Correlation between carotid plaque
compositions and CACS.
| CACS categories | | |
|---|
|
| | |
|---|
| Parameter | 0 (n=35) | 1–399 (n=66) | >400 (n=22) | r | P-value |
|---|
| Carotid artery |
| Calcification | 11.4 | 60.6 | 72.7 | 0.479 | <0.001 |
| LRNC | 25.7 | 78.8 | 81.8 | 0.394 | <0.001 |
| IPH | 0 | 13.6 | 13.6 | 0.208 | 0.021 |
| ICA |
| Calcification | 5.7 | 33.8 | 54.5 | 0.426 | <0.001 |
| LRNC | 5.7 | 33.8 | 40.9 | 0.237 | 0.009 |
| IPH | 0 | 6.2 | 9.1 | 0.138 | 0.130 |
| Bulb |
| Calcification | 8.6 | 48.5 | 63.6 | 0.466 | <0.001 |
| LRNC | 17.1 | 66.7 | 68.2 | 0.368 | <0.001 |
| IPH | 0 | 7.6 | 9.1 | 0.205 | 0.023 |
| CCA |
| Calcification | 0 | 25.8 | 68.2 | 0.510 | <0.001 |
| LRNC | 22.9 | 59.1 | 59.1 | 0.244 | 0.007 |
| IPH | 0 | 3.0 | 0 | 0.044 | 0.629 |
In addition, in CACS predicting the presence of
carotid calcification, LRNC and IPH, OR was 1.32 (95% CI,
1.11–1.57; P=0.002), 1.21 (95% CI, 1.02–1.44; P=0.029) and 1.06
(95% CI, 0.98–1.15; P=0.156), respectively. However, following
adjustment for confounding factors, OR was 1.148 (P>0.05) in
predicting presence of carotid LRNC (Table III).
 | Table IIICorrelation between CACS and carotid
plaque composition prior to and following adjustment for
confounding factors. |
Table III
Correlation between CACS and carotid
plaque composition prior to and following adjustment for
confounding factors.
| CACS prior to
adjustment | CACS following
adjustment |
|---|
|
|
|
|---|
| Parameters | OR | P-value | OR | P-value |
|---|
| Calcification | 1.320
(1.110–1.570) | 0.002 | 1.369
(1.153–1.624) | <0.001 |
| LRNC | 1.209
(1.019–1.435) | 0.030 | 1.148
(0.962–1.369) | 0.126 |
| IPH | 1.060
(0.978–1.148) | 0.156 | 1.032
(0.9421–1.132) | 0.498 |
| Surface
disruption | 0.925
(0.636–1.346) | 0.684 | 0.206
(0.022–1.939) | 0.167 |
Discussion
In the present study, the association of CACS with
carotid atherosclerosis and the predictive value of CACS for
carotid atherosclerotic disease was determined. Results indicate
that CACS is significantly associated with carotid plaque burden
and compositional features. In addition, CACS was found to be an
effective predictor of the presence of carotid calcification and
LRNC. These observations are consistent with the hypothesis that
atherosclerosis is a systemic disease that frequently involves
multiple vascular territories. In addition, results indicate that
CACS may be a useful predictor of carotid plaque burden and
compositional features, particularly calcification and LRNC.
The identification of a correlation between CACS and
carotid plaque burden is consistent with previous studies (6,10,18).
In a study using IMT to measure carotid plaque burden by B-mode
ultrasound, El-Saed et al(10) demonstrated that CACS significantly
correlates with carotid IMT (r=0.47, P<0.001). Similar results
have also been reported in additional studies (7,19).
In the present study, MWT and NWI were used as a surrogate of IMT
for plaque burden measurement due to consistencies between IMT and
MWT (20). In addition, the
correlation between CACS and various segments of carotid artery was
investigated and ICA and bulb, particularly bulb, were found to
reveal a slightly stronger correlation with CACS than CCA. This
correlation difference may be explained by the high prevalence of
atherosclerotic plaques and the complex hemodynamic properties in
the bulb region (21). Since CACS
has been demonstrated to be a powerful measure of coronary disease
severity, current observations of a positive correlation between
CACS and carotid plaque burden indicate that the severity of
atherosclerotic disease in coronary and carotid arteries may be
parallel. In their study, Bauer et al reported a weak
correlation between CACS and IMT (r=0.26; P<0.0001) among 1,620
males without CAD and stroke, aged 45–75 years, indicating that
CACS is not sufficient to predict carotid IMT. However, the study
population examined by Bauer et al was different to that of
the present study, as all participants were male, and had no CAD or
history of stroke (22).
In the present study, a significant correlation
between CACS and prevalence and volume of carotid plaque
calcification was identified. Consistent with a study by Odink
et al(18), CACS was found
to positively correlate with carotid calcification volume. In
addition, results of the present study are also supported by a
study by Allison et al(23), in which 650 asymptomatic patients
receiving whole-body electron beam computed tomography scanning
were analyzed to assess the carotid, coronary, proximal and distal
aorta and iliac vessels for atherosclerotic calcification. The
authors of that study found that age and hypertension represented
the dominant risk factors for systemic calcified atherosclerosis
and a positive correlation existed between the coronary and carotid
beds (0.28–0.29). Etiologically, atherosclerosis occurring in
various arterial vascular beds may share the same set of
traditional risk factors. Schlieper et al(24) reported that age, male gender,
dialysis vintage, smoking, calcium-phosphate product and
high-sensitivity CRP are independent risk factors for
cardiovascular calcifications, including coronary and carotid
calcification. Observations of the present study confirm the
presence of significant correlations and risk factor associations
for calcified atherosclerosis in various vascular beds. However,
carotid calcification plays a controversial role in plaque
vulnerability. Although the possible contribution of calcification
to plaque stabilization requires additional clarification, a number
of previous studies have reported that calcification is markedly
associated with plaque rupture and subsequent thrombosis. Demer
et al(25) found that
calcified human vessels were less distensible in vivo and
ex vivo compared with non-calcified vessels. Studies
performed by Richardson et al(26), based on computer modeling, as well
as Lee et al(27), with use
of computer-assisted analysis of tensile stress on excised plaques,
demonstrated that sites prone to rupture were often associated with
the greatest tensile stress in the vessel wall, which occurs at the
interphases between two tissues with differing elastic
properties.
To the best of our knowledge, no studies have
investigated the link between CACS and carotid LRNC and IPH. In the
current study, a positive correlation was identified between CACS
and the volume of carotid LRNC and presence of IPH. In addition,
our results indicate that CACS correlates with carotid plaque
stability.
It is evident from a series of studies that carotid
plaque complexity and composition may be associated with plaque
stability. Previous pathological studies established that a large
LRNC or IPH is an important feature of vulnerable atherosclerotic
plaque. The size of carotid LRNC and presence of IPH were
demonstrated to be high-risk features due to their correlation with
neurovascular events. Cappendijk et al(28) reported that symptomatic patients
exhibited wider ranges in LRNC scores than asymptomatic
individuals. Altaf et al(29) prospectively studied symptomatic
patients with mild to moderate carotid stenosis and found that
patients with carotid IPH were prone to recurrent neurological
events, including TIA and stroke. In addition, a study by the same
research group identified a significant and marked association
between recurrent clinical ischemia and intraplaque hemorrhage in
patients with symptomatic high-grade carotid disease (30).
In the present study, the prevalence and volume of
carotid LRNC and presence of IPH revealed an increasing trend with
CACS, indicating that CACS may be associated with carotid plaque
vulnerability.
This study has concentrated on a specific time
point, and mainly analyzed the correlation between CACS and carotid
atherosclerosis, however, the predictive value of CACS to carotid
atherosclerosis requires a larger prospective study to confirm.
Although CACS was revealed to predict carotid atherosclerosis,
additional studies must be performed. In addition, only four
patients developed carotid surface disruption. In future studies
the correlation between CACS and carotid surface disruption must be
determined using a larger study population.
References
|
1
|
Dalager-Pedersen S, Ravn HB and Falk E:
Atherosclerosis and acute coronary events. Am J Cardiol. 82:37–40.
1998. View Article : Google Scholar
|
|
2
|
Iannuzzi A, Wilcosky T, Mercuri M, et al:
Ultrasonographic correlates of carotid atherosclerosis in transient
ischemic attack and stroke. Stroke. 26:614–619. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
CAPRIE Steering Committee. A randomised,
blinded, trial of clopidogrel versus aspirin in patients at risk of
ischaemic events (CAPRIE). CAPRIE Steering Committee. Lancet.
348:1329–1339. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kannel WB and Wolf PA: Peripheral and
cerebral atherothrombosis and cardiovascular events in different
vascular territories: insights from the Framingham Study. Curr
Atheroscler Rep. 8:317–323. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Meco JF, Pintó X, Escribà JM, et al:
Cardiovascular risk factors associated with clinically isolated and
diffuse atherosclerosis in Spanish patients with coronary artery
disease. Eur J Clin Invest. 28:643–650. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Rampersaud E, Bielak LF, Parsa A, et al:
The association of coronary artery calcification and carotid artery
intima-media thickness with distinct, traditional coronary artery
disease risk factors in asymptomatic adults. Am J Epidemiol.
168:1016–1023. 2008. View Article : Google Scholar
|
|
7
|
Taylor AJ, Bindeman J, Le TP, et al:
Progression of calcified coronary atherosclerosis: relationship to
coronary risk factors and carotid intima-media thickness.
Atherosclerosis. 197:339–345. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Underhill HR, Yuan C, Terry JG, et al:
Differences in carotid arterial morphology and composition between
individuals with and without obstructive coronary artery disease: a
cardiovascular magnetic resonance study. J Cardiovasc Magn Reson.
10:312008. View Article : Google Scholar
|
|
9
|
Manolio TA, Arnold AM, Post W, et al:
Ethnic differences in the relationship of carotid atherosclerosis
to coronary calcification: the Multi-Ethnic Study of
Atherosclerosis. Atherosclerosis. 197:132–138. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
El-Saed A, Sekikawa A, Edmundowicz D, et
al: Coronary calcification is more predictive of carotid intimal
medial thickness in black compared to white middle aged men.
Atherosclerosis. 196:913–918. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Cai J, Hatsukami TS, Ferguson MS, et al:
In vivo quantitative measurement of intact fibrous cap and
lipid-rich necrotic core size in atherosclerotic carotid plaque:
comparison of high-resolution, contrast-enhanced magnetic resonance
imaging and histology. Circulation. 112:3437–3444. 2005. View Article : Google Scholar
|
|
12
|
Chu BC, Kampschulte A, Ferguson MS, et al:
Hemorrhage in the atherosclerotic carotid plaque: a high-resolution
MRI study. Stroke. 35:1079–1084. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Chu B, Phan BA, Balu N, et al:
Reproducibility of carotid atherosclerotic lesion type
characterization using high resolution multicontrast weighted
cardiovascular magnetic resonance. J Cardiovasc Magn Reson.
8:793–799. 2006. View Article : Google Scholar
|
|
14
|
Agatston AS, Janowitz WR, Hildner FJ, et
al: Quantification of coronary artery calcium using ultrafast
computed tomography. J Am Coll Cardiol. 15:827–832. 1990.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kerwin W, Xu D, Liu F, et al: Magnetic
resonance imaging of carotid atherosclerosis: plaque analysis. Top
Magn Reson Imaging. 18:371–378. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Saam T, Ferguson MS, Yarnykh VL, et al:
Quantitative evaluation of carotid plaque composition by in vivo
MRI. Arterioscler Thromb Vasc Biol. 25:234–239. 2005.PubMed/NCBI
|
|
17
|
Schenker MP, Dorbala S, Hong EC, et al:
Interrelation of coronary calcification, myocardial ischemia and
outcomes in patients with intermediate likelihood of coronary
artery disease: a combined positron emission tomography/computed
tomography study. Circulation. 117:1693–1700. 2008. View Article : Google Scholar
|
|
18
|
Odink AE, van der Lugt A, Hofman A, et al:
Association between calcification in the coronary arteries, aortic
arch and carotid arteries: the Rotterdam study. Atherosclerosis.
193:408–413. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Newman AB, Naydeck BL, Ives DG, et al:
Coronary artery calcium, carotid artery wall thickness, and
cardiovascular disease outcomes in adults 70 to 99 years old. Am J
Cardiol. 101:186–192. 2008.PubMed/NCBI
|
|
20
|
Underhill HR, Kerwin WS, Hatsukami TS and
Yuan C: Automated measurement of mean wall thickness in the common
carotid artery by MRI: a comparison to intima-media thickness by
B-mode ultrasound. J Magn Reson Imaging. 24:379–387. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Salzar RS, Thubrikar MJ and Eppink RT:
Pressure-induced mechanical stress in the carotid artery
bifurcation: a possible correlation to atherosclerosis. J Biomech.
28:1333–1340. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Bauer M, Mohlenkamp S, Lehmann N, et al:
The effect of age and risk factors on coronary and carotid artery
atherosclerotic burden in males - results of the Heinz Nixdorf
recall study. Atherosclerosis. 205:595–602. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Allison MA, Criqui MH and Wright CM:
Patterns and risk factors for systemic calcified atherosclerosis.
Arterioscler Thromb Vasc Biol. 24:331–336. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Schlieper G, Brandenburg V, Djuric Z, et
al: Risk factors for cardiovascular calcifications in non-diabetic
Caucasian haemodialysis patients. Kidney Blood Press Res.
32:161–168. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Demer LL: Effect of calcification on in
vivo mechanical response of rabbit arteries to balloon dilation.
Circulation. 83:2083–2093. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Richardson PD, Davies MJ and Born GV:
Influence of plaque configuration and stress distribution on
fissuring of coronary atherosclerotic plaques. Lancet. 2:941–944.
1989. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Lee RT, Schoen FJ, Loree HM, et al:
Circumferential stress and matrix metalloproteinase 1 in human
coronary atherosclerosis. Implications for plaque rupture.
Arterioscler Thromb Vasc Biol. 16:1070–1073. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Cappendijk VC, Kessels AG, Heenemao S, et
al: Comparison of lipid-rich necrotic core size in symptomatic and
asymptomatic carotid atherosclerotic plaque: initial results. J
Magn Reson Imaging. 27:1356–1361. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Altaf N, Daniels L, Morgan PS, et al:
Detection of intraplaque hemorrhage by magnetic resonance imaging
in symptomatic patients with mild to moderate carotid stenosis
predicts recurrent neurological events. J Vasc Surg. 47:337–342.
2008. View Article : Google Scholar
|
|
30
|
Altaf N, MacSweeney ST, Gladman J and Auer
DP: Carotid intraplaque hemorrhage predicts recurrent symptoms in
patients with high-grade carotid stenosis. Stroke. 38:1633–1635.
2007. View Article : Google Scholar : PubMed/NCBI
|