Introduction
Diabetes mellitus (DM) is a common metabolic
disorder with acute and chronic complications, that affects
survival and quality of life of the patient (1–3).
Diabetic cardiomyopathy is one of the major causes of morbidity and
mortality in diabetic patients, and may cause cardiac glucose
metabolism disorders, calcium overload and cardiac fibrosis, which
induce cardiac structural and functional abnormalities. However,
the mechanisms underlying diabetic cardiomyopathy remain to be
elucidated.
Long-term high blood glucose levels may lead to
complications in cardiac glucose uptake and utilization, affect the
metabolism of cardiac myocytes, and ultimately lead to myocardial
systolic dysfunction (4).
Hyperglycemia may also increase the overproduction of reactive
oxygen species (ROS), including superoxide anion, hydroxyl radicals
and hydrogen peroxide (H2O2). Oxidative
stress caused by these free radicals has been suggested to play a
crucial role in the pathogenesis of diabetic cardiomyopathy
(5,6).
Mitochondrial aldehyde dehydrogenase 2 (ALDH2) is
one of the key enzymes in alcohol metabolism that catalyzes the
conversion of aldehyde to acetic acid (7). ALDH2 plays a crucial metabolic role
in the detoxification and oxidation of aldehydes, such as
4-hydroxynon-2-enal (4-HNE). 4-HNE is a highly cytotoxic aldehyde
generated during oxidative stress as a result of lipid peroxidation
(8–10). ALDH2 has been found to attenuate
ethanol-induced myocardial dysfunction by regulating Akt and AMPK
signaling pathways (11–13). The activation of ALDH2 has been
shown to lead to cardiac protection against ischemia and
reperfusion injury (14–16). A previous study also indicated that
inhibition of ALDH2 by oxidative stress leads to cardiac
dysfunction in diabetes mellitus (17). Nevertheless, the association
between ALDH2 and myocardial injury in diabetic cardiomyopathy
mediated by oxidative stress has yet to be fully elucidated.
Inflammatory injury is involved in the pathology and
complications of diabetes. Hyperglycemia and oxidative stress cause
inflammatory injury, eventually inducing cell death. Interleukin
(IL)-1 is a pro-inflammatory cytokine that regulates inflammatory
reaction (18,19) and IL-4 is a cytokine with
anti-inflammatory properties.
Consequently, we hypothesized that cardiac ALDH2
activity is altered with DM progression, and that this is
associated with inflammatory injury. To test this hypothesis, we
determined the changes in ALDH2 activity in relation to oxidative
stress and inflammatory injury in different stages of DM in
rats.
Materials and methods
Animals
Male Sprague-Dawley (SD) rats of 200–250 g were
purchased from the Animal Center of Bengbu Medical College (Bengbu,
China). The rats were fed normal chow and had free access to water.
The rats were maintained at a constant temperature of 21±1°C on a
fixed 12-h light/dark cycle. All the animal procedures were in
accordance with the United States National Institutes of Health
Guide and were approved by the Animal Use and Care Committee of
Bengbu Medical College.
Chemicals and reagents
Streptozotocin (STZ) was purchased from Sigma (St.
Louis, MO, USA). IL-1, IL-4 and 4-HNE kits were purchased from
R&D Systems (Minneapolis, MN, USA). The ALDH2 kit was purchased
from Genmed Scientific Inc. (Shanghai, China). The primers used
were: Bax 5′-GGA TCG AGC AGA GAG GAT GG-3′ and 5′-GCT CAT TGC CGA
TAG TGA TGA CT-3′, with an amplified fragment length of 464 bp;
Bcl-2: 5′-CTG GTG GAC AAC ATC GCT CTG-3′, 5′-GGT CTG CTG ACC TCA
CTT GTG-3′, with an amplified fragment length of 227 bp; β-actin:
5′-GAT GGT GGG TAT GGG TCA GAA GGA C-3′ and 5′-GCT CAT TGC CGA TAG
TGA TGA CT-3′, with an amplified fragment length of 630 bp. Any
additional chemicals were of the highest purity available.
Induction of diabetes and experimental
protocol
Diabetes was induced in overnight fasted rats by the
administration of a single intraperitoneal (i.p.) injection of 55
mg/kg STZ freshly dissolved in 0.1 mol/l sodium citrate buffer (pH
4.5). The rats in the control group were intraperitoneally injected
with the same dose of sodium citrate buffer alone. The rats with a
fasting blood glucose (FBG) level of >16.7 mmol/l 72 h after the
injection were considered diabetic.
The rats were randomly allocated into a control
group, as well as into DM4w, DM8w and DM12w groups containing DM
rats 4, 8 and 12 weeks after DM induction, respectively (n=6
rats/group).
Isolated perfused heart preparation
After the rats were anesthetized (chloral hydrate,
4g/kg, i.p.), the hearts were rapidly excised, placed in ice-cold
Krebs-Henseleit (K-H) buffer, mounted on a Langendorff apparatus,
and perfused at 37°C with K-H buffer. The buffer was equilibrated
with 95% O2/5% CO2 (pH 7.4), and was composed
of (mmol/l): NaCl 118.0, KCl 4.7, CaCl2 1.25,
KH2PO4 1.2, NaHCO3 25.0 and
glucose 11.0. A latex, fluid-filled balloon was introduced into the
left ventricle through the left atrial appendage and the balloon
catheter was connected to a pressure transducer connected to a data
acquisition system (MedLab, Nanjing, China) to assess contractile
function. The left ventricular end diastolic pressure (LVEDP) was
adjusted to 4–8 mmHg. The cardiac parameters including heart rate
(HR), left ventricular developed pressure (LVDP, difference between
left ventricular end systolic pressure and end diastolic pressure)
and rate-pressure product (RPP = LVDP × HR) were continuously
monitored.
Detection of blood glucose and
glycosylated hemoglobin (HbA1c) levels
FBG and HbA1c levels were measured every 4 weeks
following the STZ injection.
Detection of plasma IL-1 and IL-4
levels
After the animals were anesthetized, 2 ml of blood
was drawn from the abdominal aorta, and plasma IL-1 and IL-4 levels
were measured using commercially available kits according to the
manufacturer’s instructions.
Detection of cardiac 4-HNE levels
At the end of the experimental period, 0.1 g of
heart tissue was homogenized in ice-cold phosphate-buffered saline
(PBS). 4-HNE was measured by commercially available kits according
to the manufacturer’s instructions.
Detection of Bax and Bcl-2 mRNA levels by
reverse transcriptase-polymerace chain reaction (RT-PCR)
RT-PCR was used to detect the mRNA levels of Bax and
Bcl-2 in the hearts of rats. Briefly, total RNA was extracted using
TRIzol reagent according to the manufacturer’s instructions. Total
RNA (2 μg)were reverse transcribed to cDNA, and PCR was performed
using a routine method. PCR products were analyzed on 1% agarose
gel. Densitometric analysis results of Bax and Bcl-2 genes were
compared to the corresponding β-actin levels in order to account
for loading differences.
Detection of ALDH2 activity
ALDH2 activity was measured using commercially
available kits according to the manufacturer’s instructions.
Statistical analysis
Values were expressed as the means ± standard error
of the mean (SEM). Statistical comparisons were performed using
one-way analysis of variance (ANOVA) and the Newman-Keuls test.
P<0.05 was indicated a statistically significant difference.
Results
Changes of ventricular hemodynamic
parameters
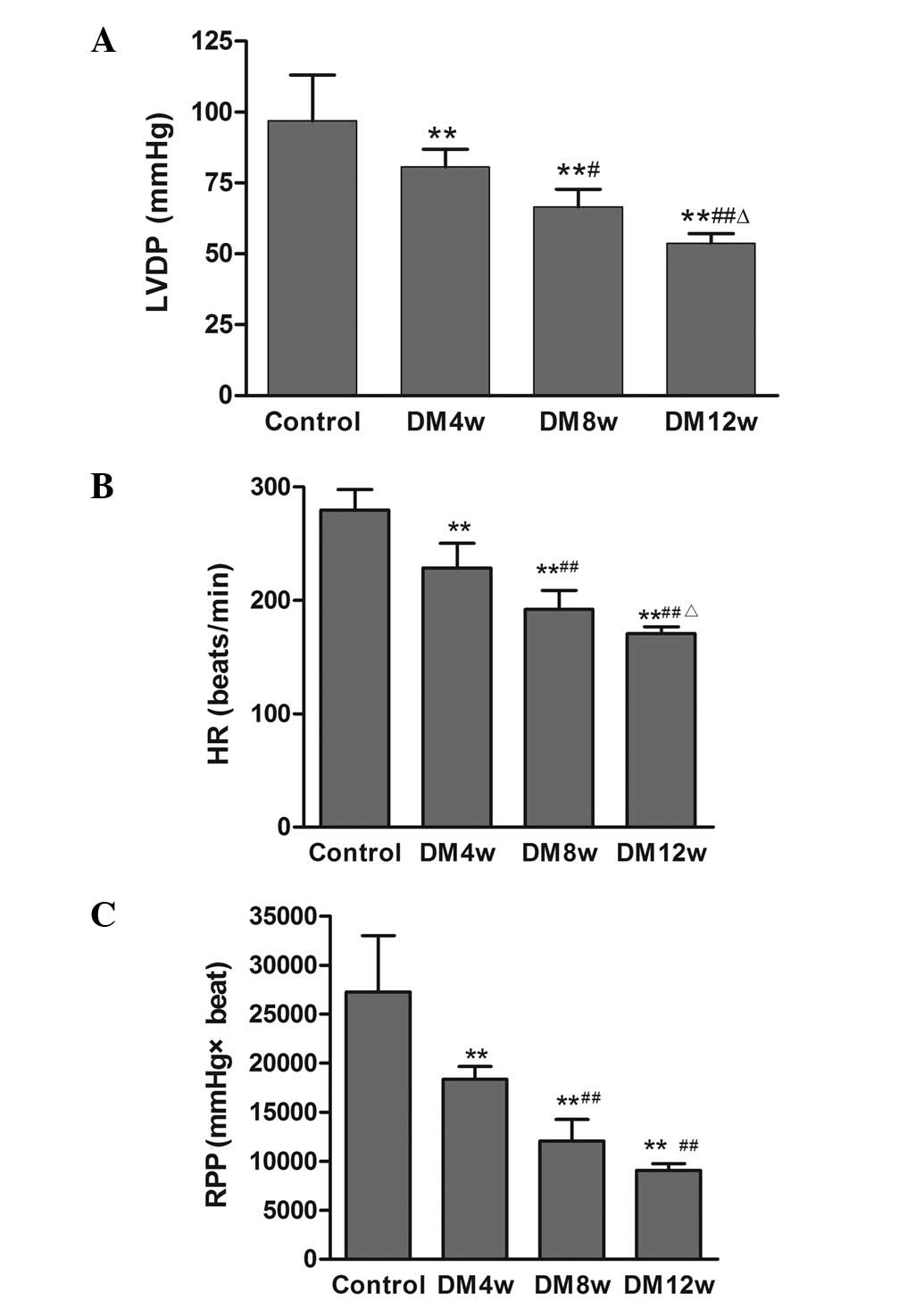
LVDP, HR and RPP in the DM groups were significantly
decreased compared to the control group (Fig. 1). LVDP, HR and RPP were also
statistically significant among the different stages of DM
(Fig. 1).
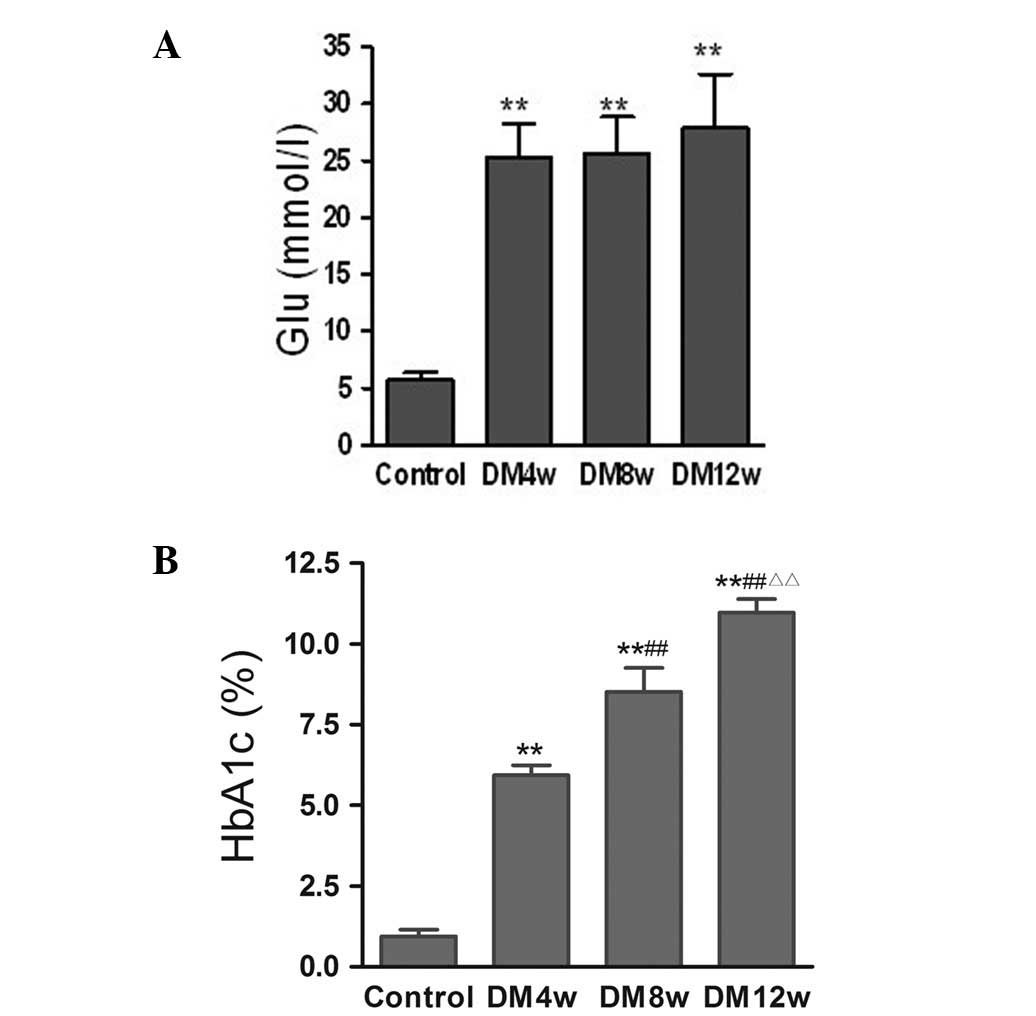
Changes of FBG and HbA1c levels
FBG levels were significantly increased in the DM
groups compared to the control group, while there were no
statistically significant differences among the different stages of
DM (Fig. 2A). HbA1c levels were
significantly increased in the DM4w, DM8w and DM12w groups compared
to the control group, and statistically significant differences
were observed among the different stages of DM (Fig. 2B).
Changes of plasma IL-1, IL-4 and cardiac
4-HNE levels
The changes of plasma IL-1, IL-4 and cardiac 4-HNE
in different stages of diabetes are shown in Table I. IL-1 and cardiac 4-HNE levels
were significantly increased, while IL-4 levels were decreased in
the DM groups compared to the control group with progression of
diabetes.
 | Table IChanges in plasma IL-1, IL-4 and
cardiac 4-HNE levels in the different groups of rats. |
Table I
Changes in plasma IL-1, IL-4 and
cardiac 4-HNE levels in the different groups of rats.
| Groups |
|---|
|
|
|---|
| Variable | Control | DM4w | DM8w | DM12w |
|---|
| IL-1 (pg/ml) | 29.60±9.64 | 99.22±24.04a | 249.03±36.17a,c | 437.73±67.08a,c,d |
| IL-4 (pg/ml) | 42.20±6.28 | 31.1±5.27a | 24.2±4.39a,b | 14.2±5.10a,c,d |
| 4-HNE (ng/l) | 5.65±0.51 | 28.16±7.73a | 45.16±11.2a,c | 57.80±6.06a,c,d |
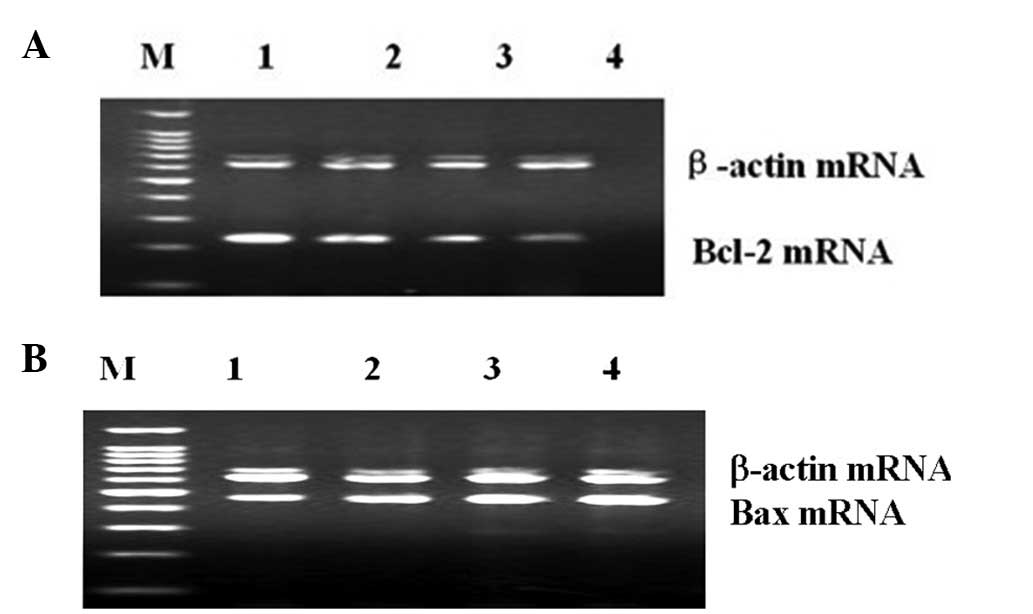
Changes of Bax and Bcl-2 mRNA levels in
hearts of rat
The changes of Bax and Bcl-2 mRNA levels in the
different stages of diabetes are shown in Table II. The mRNA expression levels of
cardiac Bax were increased, while Bcl-2 mRNA levels were decreased
in the DM groups compared to the control group. The Bcl-2 mRNA
levels were also decreased in the different stages of DM. The
Bcl-2/Bax mRNA ratios were further decreased with the progression
of diabetes (Fig. 3).
 | Table IIBcl-2 and Bax mRNA expression in the
heart tissue of rats. |
Table II
Bcl-2 and Bax mRNA expression in the
heart tissue of rats.
| Groups |
|---|
|
|
|---|
| Variable | Control | DM4w | DM8w | DM12w |
|---|
| Bax/β-actin | 0.55±0.05 | 0.67±0.09a | 0.84±0.06a,b | 0.92±0.04a–c |
| Bcl-2/β-actin | 0.75±0.09 | 0.66±0.04a | 0.49±0.03a,b | 0.37±0.04a–c |
| Bcl-2/Bax | 1.38±0.26 | 1.02±0.13a | 0.58±0.05a,b | 0.40±0.03a–c |
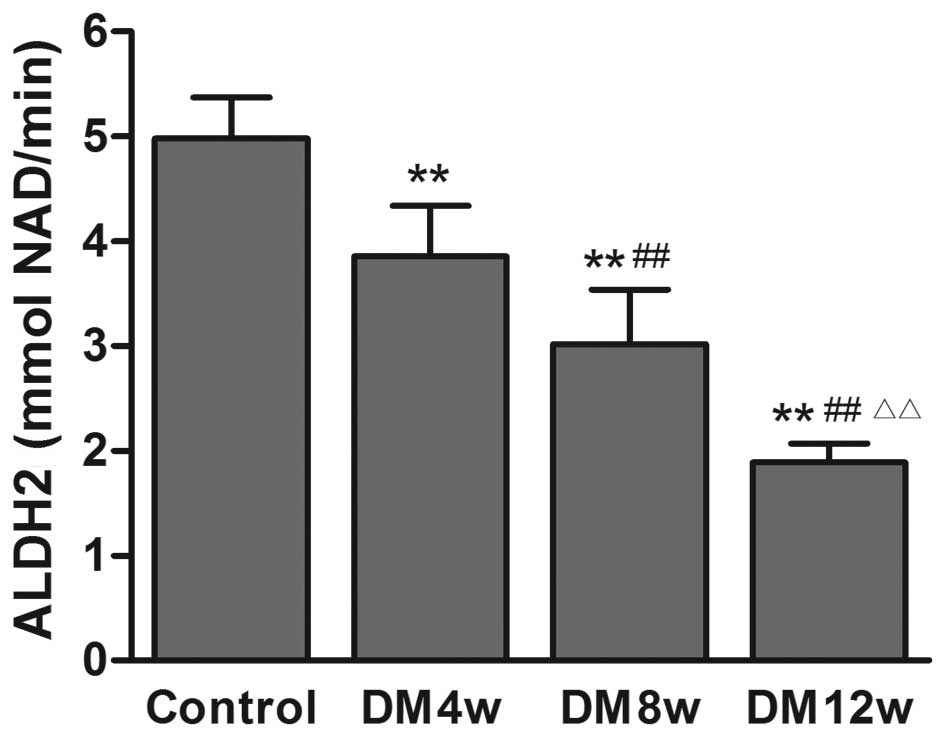
Changes of ALDH2 activity in the heart
tissue of rats
ALDH2 activity was decreased in the DM groups
compared to the control group, with the progression of DM (Fig. 4).
Discussion
In the present study, FBG and HbA1c levels were
found to be increased, ventricular function was decreased, plasma
IL-1 levels were increased, while IL-4 levels were decreased with
progression of DM. Cardiac 4-HNE levels were increased and ALDH2
activity was decreased, suggesting that inflammatory injury and
oxidative stress were aggravated in diabetes accompanied by a
decrease in ventricular function, which may be related to the
decrease in ALDH2 activity.
Inflammation plays a key role in inflammatory
diseases including diabetes, heart disease, Alzheimer’s and
Parkinson’s disease. Inflammation is the activation of the immune
system in response to infection, injury or irritation. ROS, pro-
and anti-inflammatory cytokines are often involved in the
pathogenesis of inflammation. ROS trigger a cascade of events,
including the upregulation of pro-inflammatory cytokines that
activate immune responses and determine occurrence of inflammation
(20). Pro-inflammatory cytokines,
such as IL-1, IL-2 and interferon (IFN)-γ, are secreted by Th1
cells. Th1 subsets promote cell immune response, cause the release
of pro-inflammatory cytokines, and subsequently induce an increase
in free radical production and the destruction of β-cells (21). However, anti-inflammatory
cytokines, such as IL-4, are also secreted by Th2 cells. The
proliferation and differentiation of β-cells is stimulated by IL-4,
which also promotes the growth of thymus cells with pro-activated T
cells, inhibits the precursor of the helper T cell (Th-p) shift to
Th1 cells, and induces the inhibition of monocyte/macrophage cells
to produce INF-α and IL-1. IL-4 is important in immune regulation
and inflammatory injury (22). In
the present study, the results showed that plasma IL-1 levels were
increased and that IL-4 levels were decreased in DM rats,
suggesting that inflammtory injury was aggravated with the
progression of diabetes.
4-HNE is the main product of oxidative stress. Under
pathological conditions, these detoxification pathways fail to
trigger the accumulation of oxidized lipids that damage key
proteins in the mitochondrial respiratory chain. 4-HNE reacts with
protein at the imidazole(s) of histidine, the sulfhydryl group of
cysteine, and/or the ɛ-amine of lysine and induces enzyme
inactivation (23). Therefore, the
generation of lipid peroxidation products capable of reacting with
cellular nucleophiles may be primary instigators of tissue injury
(24). In the present study, we
observed that cardiac 4-HNE levels were increased with the
progression of diabetes, suggesting that oxidative stress was
aggravated in the hearts of DM rats.
ALDH2 is a tetrameric enzyme that is present in
organs which require high mitochondrial capacity of oxidative ATP
generation, such as the heart and brain. In addition to its
dehydrogenase activity, ALDH2 functions as an esterase and
reductase, depending on the substrates. ALDH2 activation
facilitates the removal of cytotoxic aldehydes, such as 4-HNE,
reduces myocardial injury, and ultimately is involved in
anti-oxidative stress. It has been reported that ALDH2 protects
against ethanol toxicity through altered Akt and AMPK signaling
pathways, increases its enzymatic activity, diminishes the
pro-apoptotic signaling activity of JNK1/2 and reduces 4-HNE
protein adduct formation (25,26).
In the present study, 4-HNE content was increased when ALDH2
activity was decreased with the progression of diabetes, suggesting
that the decrease in ALDH2 activity leads to the metabolic
enhancement of acetaldehyde activity and the aggravation of cardiac
injury.
Apoptosis occurs in diabetic cardiomyopathy. It is
believed that the ultimate vulnerability of cells to various
apoptotic stimuli is determined by the relative ratio of
pro-apoptotic and anti-apoptotic members of the Bcl-2 family.
Accumulating evidence has proven that the overproduction of ROS
triggers myocyte apoptosis by upregulating pro-apoptotic members of
the Bcl-2 family, such as Bax, Bak and Bid. Bax plays a dominant
role in initiating cell death, thus disrupting the integrity of
mitochondrial membrane, and hastening the release of cytochrome
c from mitochondria, leading to DNA fragmentation and
caspase-3 activation. However, Bcl-2 exerts an opposite effect. In
the present study, Bax and Bcl-2 mRNA levels were detected. The
result showed that the mRNA expression of cardiac Bcl-2 was
decreased, Bax at mRNA levels was increased, and Bcl-2/Bax mRNA
ratios were decreased with progression of DM. ALDH2 activity was
also found to be decreased. These results suggest that the
increased apoptosis of myocardial cells is associated with the
decrease in ALDH2 activity.
In conclusion, with the development of diabetes,
which is accompanied by the decrease of cardiac ALDH2 activity,
inflammatory injury, oxidative stress and apoptosis were aggravated
in the hearts of DM rats. This finding suggests that ALDH2 is an
important regulator of diabetic cardiomyopathy. Additional studies
are needed to confirm this potential role of ALDH2.
Acknowledgements
This study was supported by research grants from the
National Natural Science Foundation of China (nos. 81000074 and
81170046) and the Anhui Province Natural Science Foundation of
China (no. 1208085MH131).
References
|
1
|
Adeghate E, Schattner P and Dunn E: An
update on the etiology and epidemiology of diabetes mellitus. Ann N
Y Acad Sci. 1084:1–29. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Gremizzi C, Vergani A, Paloschi V and
Secchi A: Impact of pancreas transplantation on type 1
diabetes-related complications. Curr Opin Organ Transplant.
15:119–123. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Boudina S and Abel ED: Diabetic
cardiomyopathy revisited. Circulation. 115:3213–3223. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Zgibor JC, Ruppert K, Orchard TJ,
Soedamah-Muthu SS, Fuller J, Chaturvedi N and Roberts MS:
Development of a coronary heart disease risk prediction model for
type 1 diabetes: the Pittsburgh CHD in Type 1 diabetes risk model.
Diabetes Res Clin Pract. 88:314–321. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kaneto H, Katakami N, Matsuhisa M and
Matsuoka TA: Role of reactive oxygen species in the progression of
type 2 diabetes and atherosclerosis. Mediators Inflamm.
2010:4538922010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Penckofer S, Schwertz D and Florczak K:
Oxidative stress and cardiovascular disease in type 2 diabetes: the
role of antioxidants and pro-oxidants. J Cardiovasc Nurs. 16:68–85.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ren J: Acetaldehyde and alcoholic
cardiomyopathy: lessons from the ADH and ALDH2 transgenic models.
Novartis Found Symp. 285:69–79. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Budas GR, Disatnik MH and Mochly-Rosen D:
Aldehyde dehydrogenase 2 in cardiac protection: a new therapeutic
target? Trends Cardiovasc Med. 19:158–164. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Forman HJ, Fukuto JM, Miller T, Zhang H,
Rinna A and Levy S: The chemistry of cell signaling by reactive
oxygen and nitrogen species and 4-hydroxynonenal. Arch Biochem
Biophys. 477:183–195. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Petersen DR and Doorn JA: Reactions of
4-hydroxynonenal with proteins and cellular targets. Free Radic
Biol Med. 37:937–945. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ma H, Li J, Gao F and Ren J: Aldehyde
dehydrogenase 2 ameliorates acute cardiac toxicity of ethanol: role
of protein phosphatase and forkhead transcription factor. J Am Coll
Cardiol. 54:2187–2196. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Doser TA, Turdi S, Thomasm DP, Epstein PN,
Li SY and Ren J: Transgenic overexpression of aldehyde
dehydrogenase-2 rescues chronic alcohol intake-induced myocardial
hypertrophy and contractile dysfunction. Circulation.
119:1941–1949. 2009. View Article : Google Scholar
|
|
13
|
Ge W, Guo R and Ren J: AMP-dependent
kinase and autophagic flux are involved in aldehyde
dehydrogenase-2-induced protection against cardiac toxicity of
ethanol. Free Radic Biol Med. 51:1736–1748. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Churchill EN, Disatnik MH and Mochly-Rosen
D: Time-dependent and ethanol-induced cardiac protection from
ischemia mediated by mitochondrial translocation of varepsilonPKC
and activation of aldehyde dehydrogenase 2. J Mol Cell Cardiol.
46:278–284. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Chen CH, Budas GR, Churchill EN, Disatnik
MH, Hurley TD and Mochly-Rosen D: Activation of aldehyde
dehydrogenase-2 reduces ischemic damage to the heart. Science.
321:1493–1495. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Budas GR, Disatnik MH, Chen CH and
Mochly-Rosen D: Activation of aldehyde dehydrogenase 2 (ALDH2)
confers cardioprotection in protein kinase C epsilon
(PKCvarepsilon) knockout mice. J Mol Cell Cardiol. 48:757–764.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wang J, Wang H, Hao P, Xue P, Wei S, Zhang
Y and Chen Y: Inhibition of aldehyde dehydrogenase 2 by oxidative
stress is associated with cardiac dysfunction in diabetic rats. Mol
Med. 17:172–179. 2011.PubMed/NCBI
|
|
18
|
Larsen CM, Faulenbach M, Vaag A, Ehses JA,
Donath MY and Mandrup-Poulsen T: Sustained effects of interleukin-1
receptor antagonist treatment in type 2 diabetes. Diabetes Care.
32:1663–1668. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zaitsev SV, Appelskog IB, Kapelioukh IL,
Yang SN, Köhler M, Efendic S and Berggren PO: Imidazoline compounds
protect against interleukin 1 beta-induced beta-cell apoptosis.
Diabetes. 50:70–76. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Tas SW, Remans PH, Reedguist KA and Tak
PP: Signal transduction pathways and transcription factors as
therapeutic targets in inflammatory disease: towards innovative
antirheumatic therapy. Curr Pharm Des. 11:581–611. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Lafaille JJ: The role of helper T cell
subsets in autoimmune diseases. Cytokine Growth Factor Rev.
9:139–151. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Hart PH, Jones CA and Finlay-Jones JJ:
Interleukin-4 suppression of monocyte tumour necrosis factor-alpha
production. Dependence on protein synthesis but not on cyclic AMP
production. Immunology. 76:560–565. 1992.PubMed/NCBI
|
|
23
|
Zhang M and Shah AM: Role of reactive
oxygen species in myocardial remodeling. Curr Heart Fail Rep.
4:26–30. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Lee Y and Gustafsson AB: Role of apoptosis
in cardiovascular disease. Apoptosis. 14:536–548. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Stewart MJ, Malek K and Crabb DW:
Distribution of messenger RNAs for aldehyde dehydrogenase 1,
aldehyde dehydrogenase 2, and aldehyde dehydrogenase 5 in human
tissues. J Investig Med. 44:42–46. 1996.PubMed/NCBI
|
|
26
|
Esterbauer H, Schaur RJ and Zollner H:
Chemistry and biochemistry of 4-hydroxynonenal, malonaldehyde and
related aldehydes. Free Radic Biol Med. 1:81–128. 1991. View Article : Google Scholar : PubMed/NCBI
|