Introduction
Prostate cancer (PCa) is the most common malignancy
and the second leading cause of cancer-related mortality among
males in the United States (1).
The recommended treatment for patients with progressive carcinoma
of the prostate is hormone ablation achieved by surgical or
pharmacological castration (2).
Most patients initially respond well to this treatment; however,
they inevitably relapse and develop an incurable
castration-resistant condition within a few years (3). PCa involves highly complicated and
heterogeneous tumors resulting from the aberrant activation of
several important signaling pathways. Understanding the molecular
mechanisms underlying PCa is of utmost importance in the
investigation of novel therapeutic strategies.
The ILK gene is located on human chromosome 11
(4), encoding a protein which is
composed of three major domains: an N-terminal ankyrin repeat
domain, a middle pleckstrin homology (PH) domain and a C-terminal
kinase domain (5). This gene is
expressed as a serine/threonine (Ser/Thr) protein kinase, a binding
partner of β1 and β3 integrin subunit, which is a cytoplasmic
effector of integrin receptors, and constitutes a functional link
between them and the actin cytoskeleton (6). ILK expression and its oncogenic
potential have been studied in various malignancies including
ovarian, colon, hepatocellular, laryngeal and bladder cancer
(7–13). Previous studies have shown higher
ILK expression levels in primary PCa cells compared with adjacent
benign prostatic hyperplasia (BPH) cells (14). However, the functional
characterization of ILK in PCa and its potential in vivo
downstream effectors still remain to be elucidated.
The aim of the present study was to clarify the
functional characterization of ILK in PCa. Therefore, ILK-mediated
PCa tumorigenesis was investigated through the determination of ILK
expression in human PCa tumor samples, and its role in vitro
and in vivo was evaluated.
Materials and methods
Chemicals, reagents and plasmid
Tissue microarray, containing 63 PCa and 11 BPH
samples, was obtained from Ailina Biotechnology Co., Ltd. (Xian,
Shanxi, China). Antibodies against ILK and phosphorylated Akt
(Ser473) were obtained from Cell Signaling Technology, Inc.
(Beverly, MA, USA). RPMI-1640 medium, fetal bovine serum (FBS) and
other reagents were obtained from CWBIO Co., Ltd. (Beijing, China).
ILK short hairpin RNA (shRNA) was obtained from OriGene
Technologies (Beijing, China). The shRNA vector was cloned into a
retroviral silencing plasmid (pRS) under a U6 promoter for
mammalian cell expression. The disturbance sequence (sense,
5′-uggacaccgugauauugua-3′dTdT and antisense,
5′-accuguggcacuauaacau-3′dTdT) was designed and synthesized
according to the GenBank database.
Immunohistochemical analysis
Immunohistochemistry was performed using the
EnVision™ ABC kit according to the manufacturer’s instructions. The
concentration of primary antibody against ILK was 1:100. After
counterstaining with hematoxylin, the sections were dehydrated and
mounted. A previously established immunohistochemistry scoring
system for prostate carcinomas was used as a reference (15). The percentage of positively stained
cells was scored as 1, <25%; 2, 25–50%; and 3, >50%. The
staining intensity was also scored as follows: 0, absence of
signal; 1, low-intensity signal (light brown); 2, moderate
intensity (brown); and 3, high-intensity signal (dark brown). The
frequency and intensity scores were added to obtain the final score
for each case. The overall results were assigned a negative (−)
score when the sum was <4, and a positive (+) score when the sum
was ≥4.
Cell culture and transfection
DU145 human prostate cancer cells were obtained from
the Department of Pathology of Peking University (Beijing, China)
and maintained in RPMI-1640 medium supplemented with 10% FBS, 100
U/ml penicillin and 100 μg/ml streptomycin. The cells were cultured
in a 5% CO2 incubator (MCO-17AI; Sanyo Electric Co.,
Ltd., Osaka, Japan) at 37°C in a humidified atmosphere and
subcultured every 2–3 days. DU145 cells were seeded at a density of
10,000 cells/well in 96-well plates, and cultured in RPMI-1640
medium containing 10% FBS to 90% confluence. The medium was
replaced with serum/antibiotic-free RPMI-1640 medium prior to
transfection. Transfection was performed using Lipofectamine™ 2000
reagent according to the manufacturer’s instructions. The final
shRNA transfection concentration was 100 nM. Following
transfection, DU145 cells were allowed to recover in RPMI-1640
medium containing 10% FBS for 24 h prior to further examination.
The study was approved by the ethics committee of Peking University
Peoples’ Hospital, Beijing City, China
Protein extraction and western blot
analysis
Total cellular protein (30 μg) was fractionated on
sodium dodecyl sulfate-polyacrylamide gel (SDS-PAGE) and
transferred onto nitrocellulose membranes (Amersham Pharmacia
Biotech, Inc., Piscataway, NJ, USA). The blots were blocked and
incubated with the indicated specific primary antibody. After
incubation with the secondary antibody, the signal was visualized
using the ECL Western Blot kit and exposed to film (Kodak,
Rochester, NY, USA). β-actin was used as an internal control by
incubating a specific antibody against β-actin (Sigma, St. Louis,
MO, USA).
Proliferation assay
Cell viability was assessed using the MTT assay.
Briefly, transfected DU145 cells were plated at a density of
1×103 cells/ml in 96-well plates. After 48 h, 10 μl MTT
was added to each well. After 4 h of incubation at 37°C, the
solution was discarded and the produced formazan was solubilized in
100 μl of dimethyl sulfoxide (DMSO). Absorbance was measured at 570
nm using an automated microplate reader (Bio-Rad 550; Bio-Rad,
Hercules, CA, USA). All the experiments were performed in
triplicate.
Flow cytometric analysis of
apoptosis
The collected cells were washed twice with ice-cold
phosphate-buffered saline (PBS) and centrifuged at 150 × g for 5
min. Supernatants were discarded and the cells were resuspended in
300 μl binding buffer. Five microliters of FITC-conjugated Annexin
V (10 mg/ml) and 10 μl of propidium iodide (PI; 50 mg/ml) were
added and the mixtures were incubated for 15 min at room
temperature in the dark. The samples were then immediately analyzed
using fluorescence-activated cell sorting (FACS). The cells
(1×104) were collected and the percentage of apoptotic
cells was recorded.
Wound healing assay
Cell mobility was assessed using a scratch wound
assay. Transfected cells were cultured in a 6-well plate until
confluent. The cell layer was carefully wounded using sterile tips
and washed twice with fresh medium. Following incubation for 0, 12
and 24 h, the cells were imaged at low magnification (x40). The
experiments were performed in triplicate.
Xenograft assay in nude mice
To confirm the effect of downregulated ILK on
tumorigenicity in vivo, ILK shRNA- or vector-transfected
DU145 cells were resuspended in PBS (pH 7.4), mixed with 1X
Matrigel (BD Biosciences, Palo Alto, CA, USA), and subcutaneously
injected (5×106/injection) into the left flanks of male
athymic BALB/c nu/nu nude mice (4–6 weeks old; purchased from Vital
River Laboratories, Ltd., Beijing, China). The mice were sacrificed
4 weeks after the injection. Tumor volume was calculated using the
following formula: Volume = width2 × length × 0.5236.
Animal-based experiments were approved by the Peking University
Animal Care and Use Committee (Beijing, China) and performed in
accordance with accepted standards of humane animal care.
Statistical analysis
All statistical analysis was performed using the
Statistical Package of the Social Sciences (SPSS) software version
16.0. Data are shown as the mean ± SEM. Student’s t-test was used
to determine statistical differences and P<0.05 was considered
to indicate a statistically significant difference.
Results
ILK expression in PCa and BPH
tissues
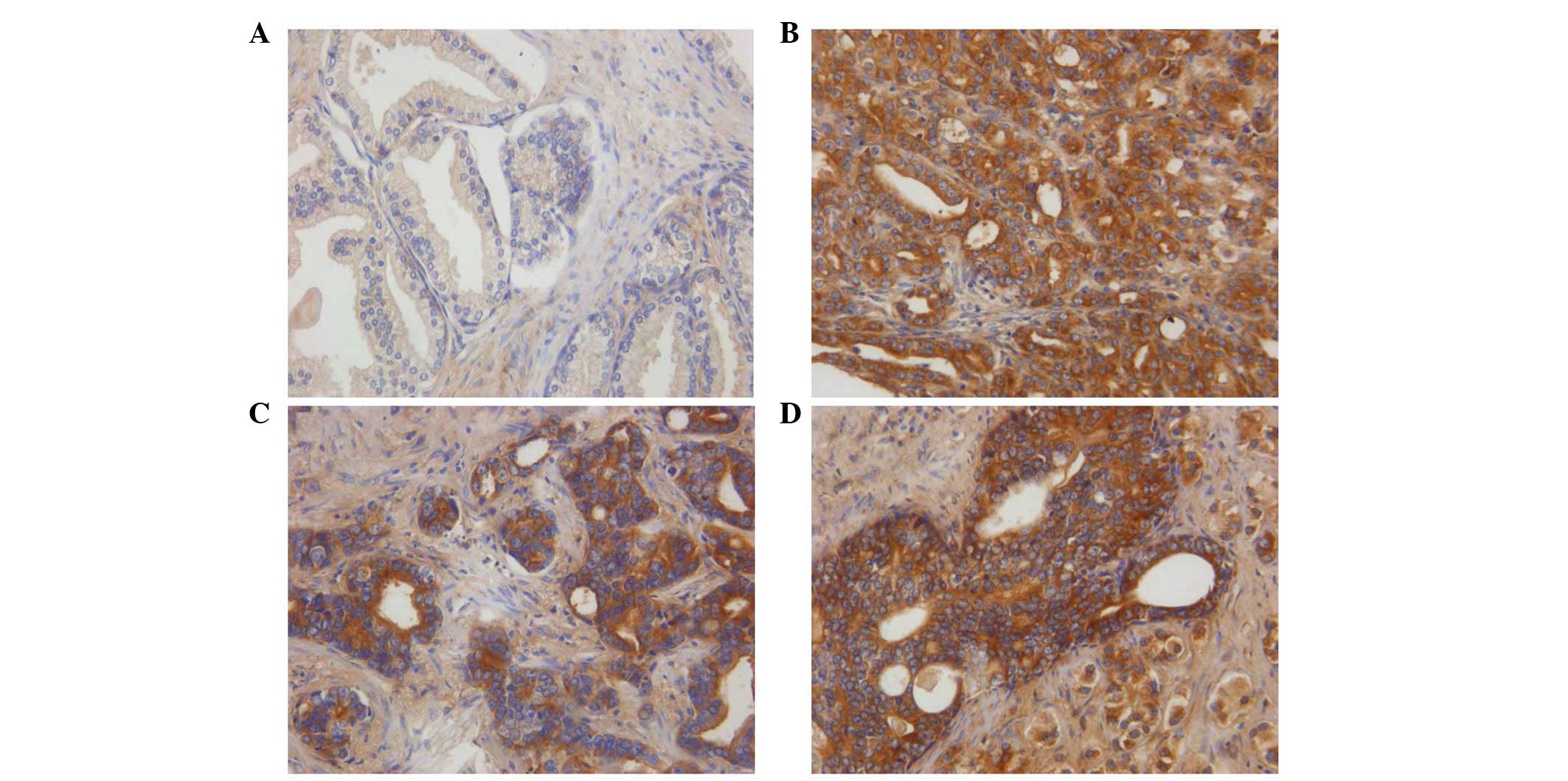
A total of 74 tissue samples were analyzed. ILK
expression was detected in the cytoplasm of PCa cells (Fig. 1). Similar to the results of a
previous study (14), ILK was
expressed in 57.1% (36/63) of the PCa samples and 18.2% (2/11) of
the BPH samples, indicating that ILK expression levels were
significantly higher in tumor tissues compared with BPH tissues
(P=0.017; Table I). However, in
contrast to previously reported results (14), ILK expression was not correlated
with the tumor grade of PCa, although there was a moderate
increasing percentage among the tissues with a different Gleason
score (Table I).
 | Table IExpression of ILK in PCa and BPH
tissues. |
Table I
Expression of ILK in PCa and BPH
tissues.
| Cell type | Cases, n | Negative staining, n
(%) | Positive staining, n
(%) | χ2 | P-value |
|---|
| BPH | 11 | 9 (81.8) | 2 (18.2) | 5.6904 | 0.017 |
| PCa | 63 | 27 (42.9) | 36 (57.1) | | |
| Gleason score |
| 2–4 | 17 | 8 (47.1) | 9 (52.9) | 0.7453 | 0.689 |
| 5–7 | 31 | 14 (45.2) | 17 (54.8) | | |
| 8–10 | 15 | 5 (33.3) | 10 (66.7) | | |
Effects of ILK knockdown on PCa cell
growth, migration and apoptosis
An effective way of elucidating the physiological
role of ILK in PCa cells is to inhibit the endogenous expression of
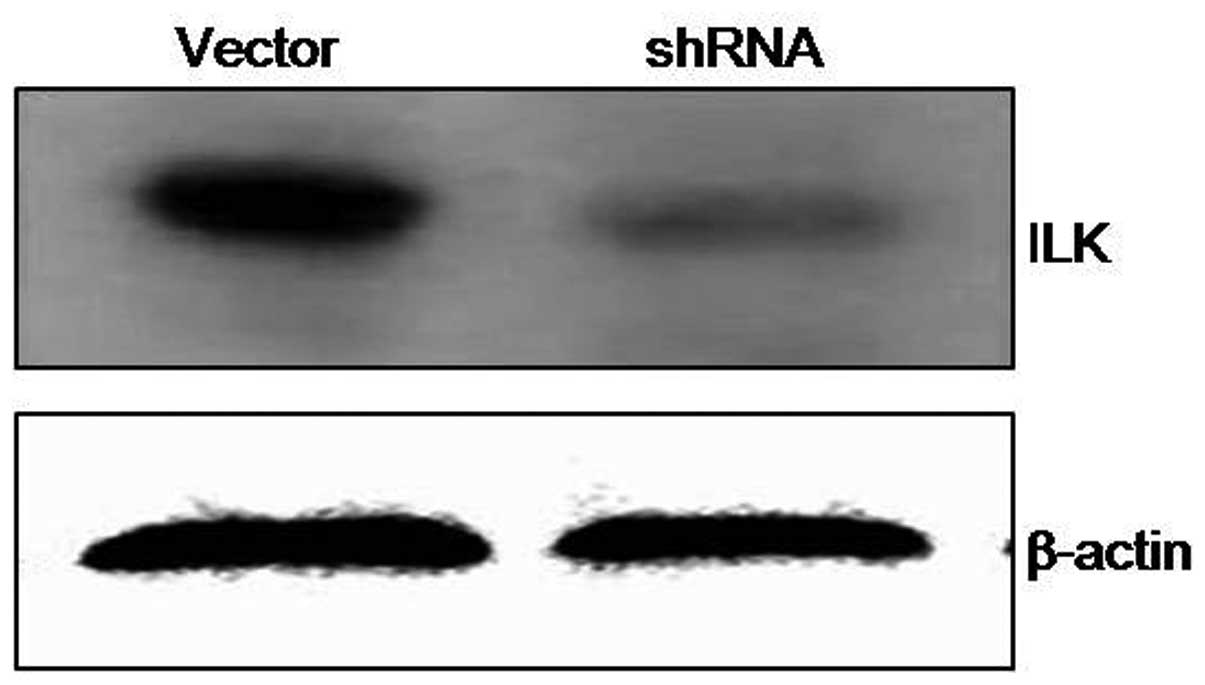
ILK. Efficient knockdown of ILK expression was achieved by the
successful delivery and expression of shRNA targeting ILK into
cells. Forty-eight hours after transfection with ILK shRNA, ILK
expression was significantly decreased in DU145 cells compared with
the control cells (Fig. 2). To
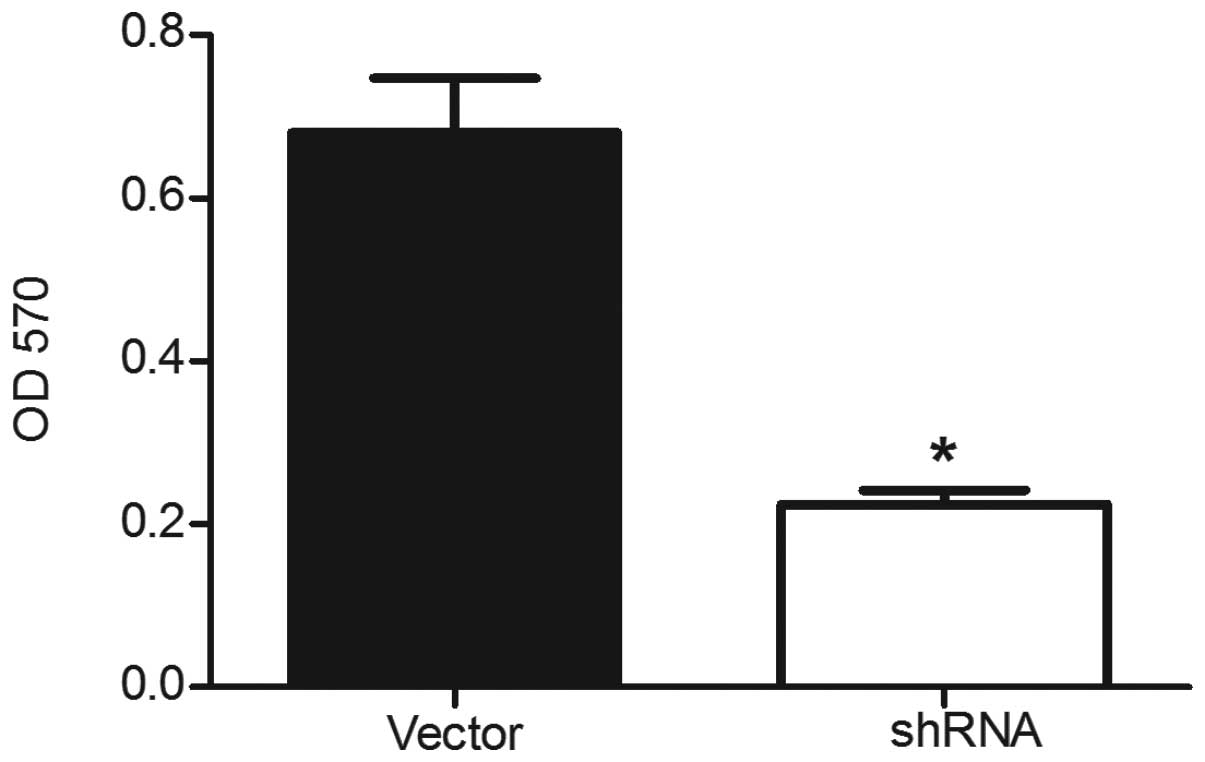
assess the effects of ILK silencing on the growth of DU145 cells,
cell viability was assessed using the MTT assay. ILK silencing was
found to have significant inhibitory effects on the proliferation
of ILK shRNA-transfected cells compared with the empty
vector-transfected cells (Fig. 3).
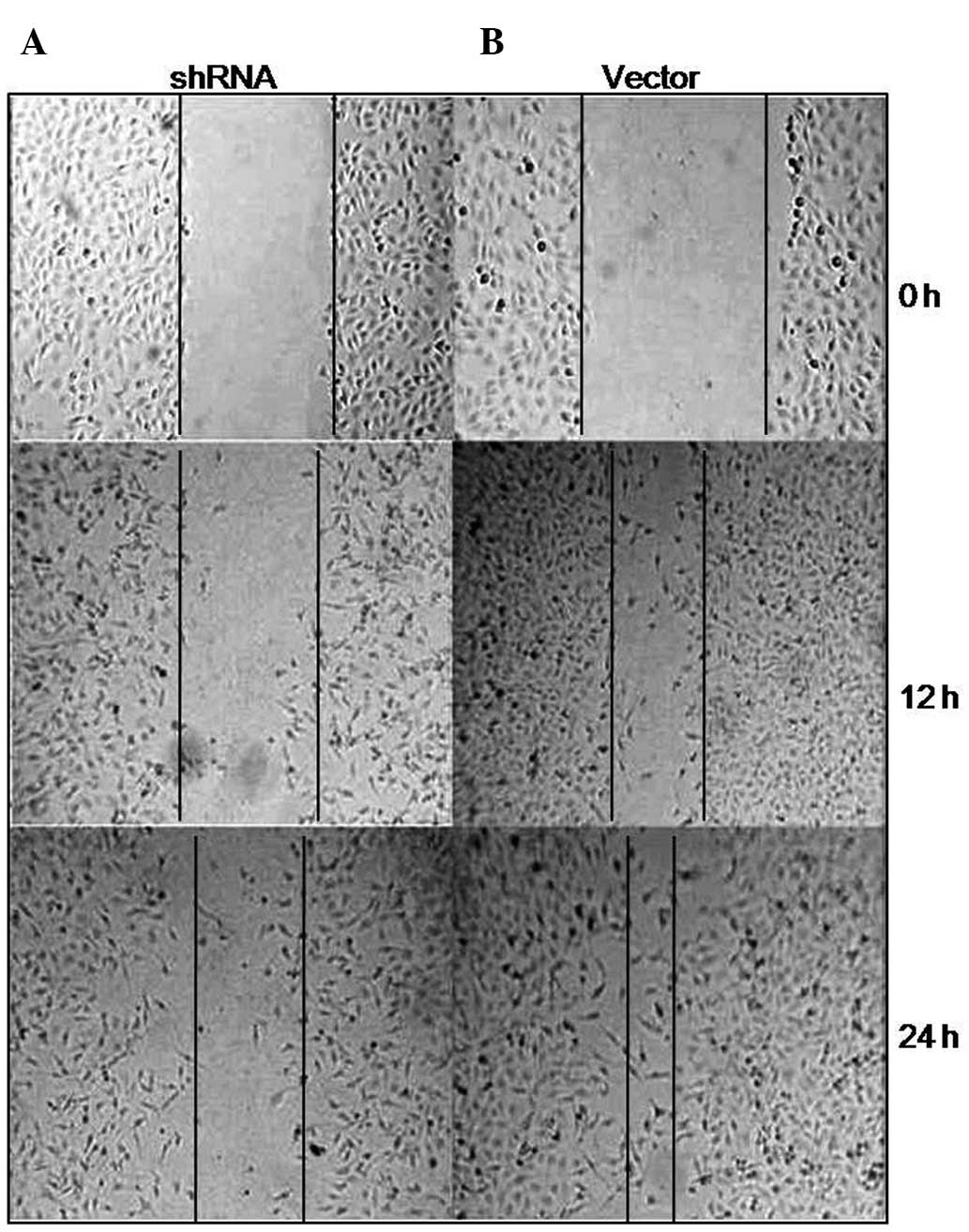
Additionally, wound healing assay was performed in the two groups
of cells. ILK-depleted cells spread along the wound edges were
characterized by slower migration compared with the
vector-transfected cells at 12 or 24 h, indicating that inhibition
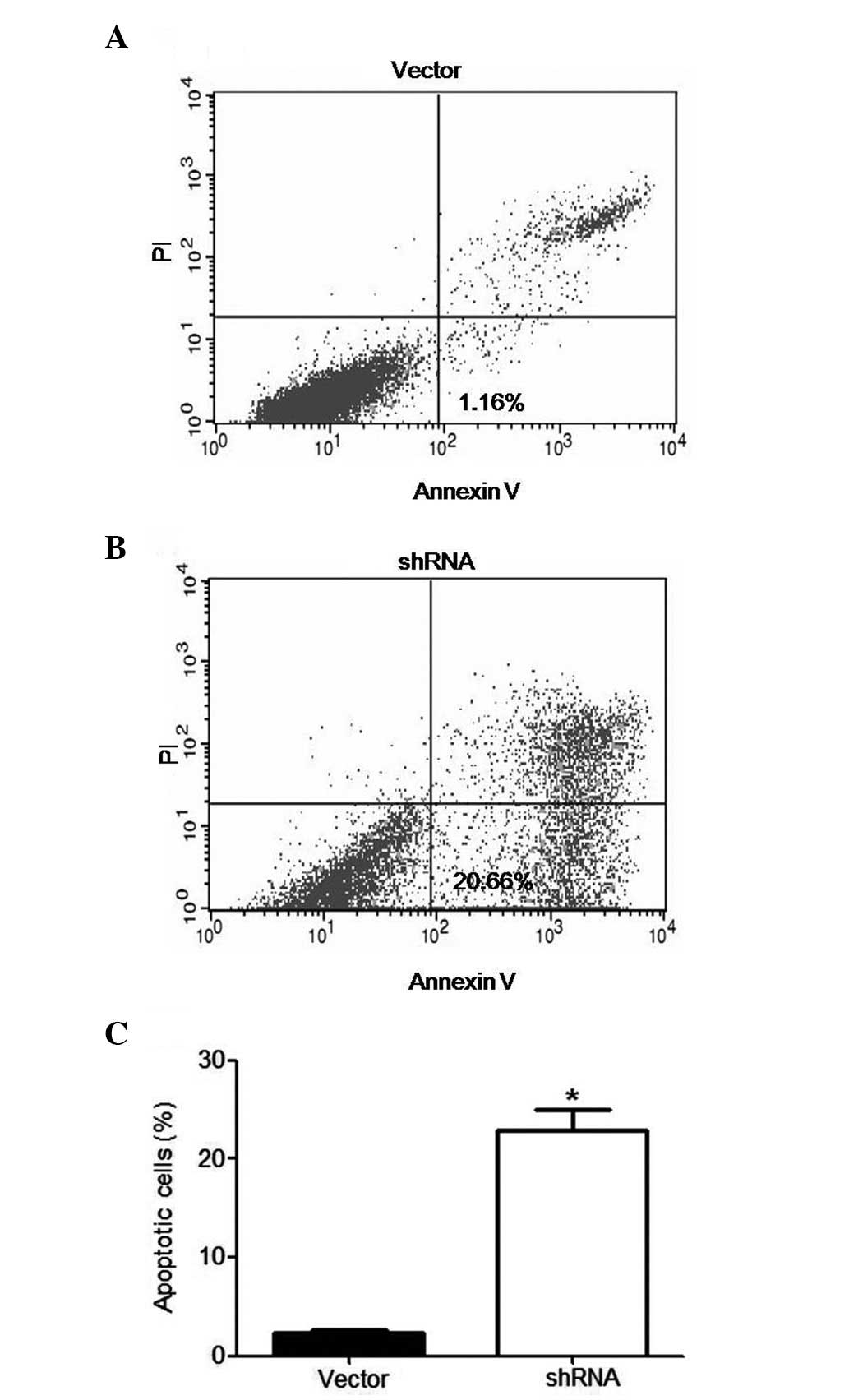
of ILK suppressed tumor cell migration (Fig. 4). Moreover, ILK-depleted cells and
control cells were double-stained with Annexin V and PI, followed
by flow cytometric analysis. An increased apoptosis rate was
observed in ILK shRNA-transfected DU145 cells compared with the
control cells, following RNA interference treatment for 48 h
(Fig. 5). Taken together, these
findings provide evidence that ILK silencing attenuates migration,
proliferation and survival of PCa cells.
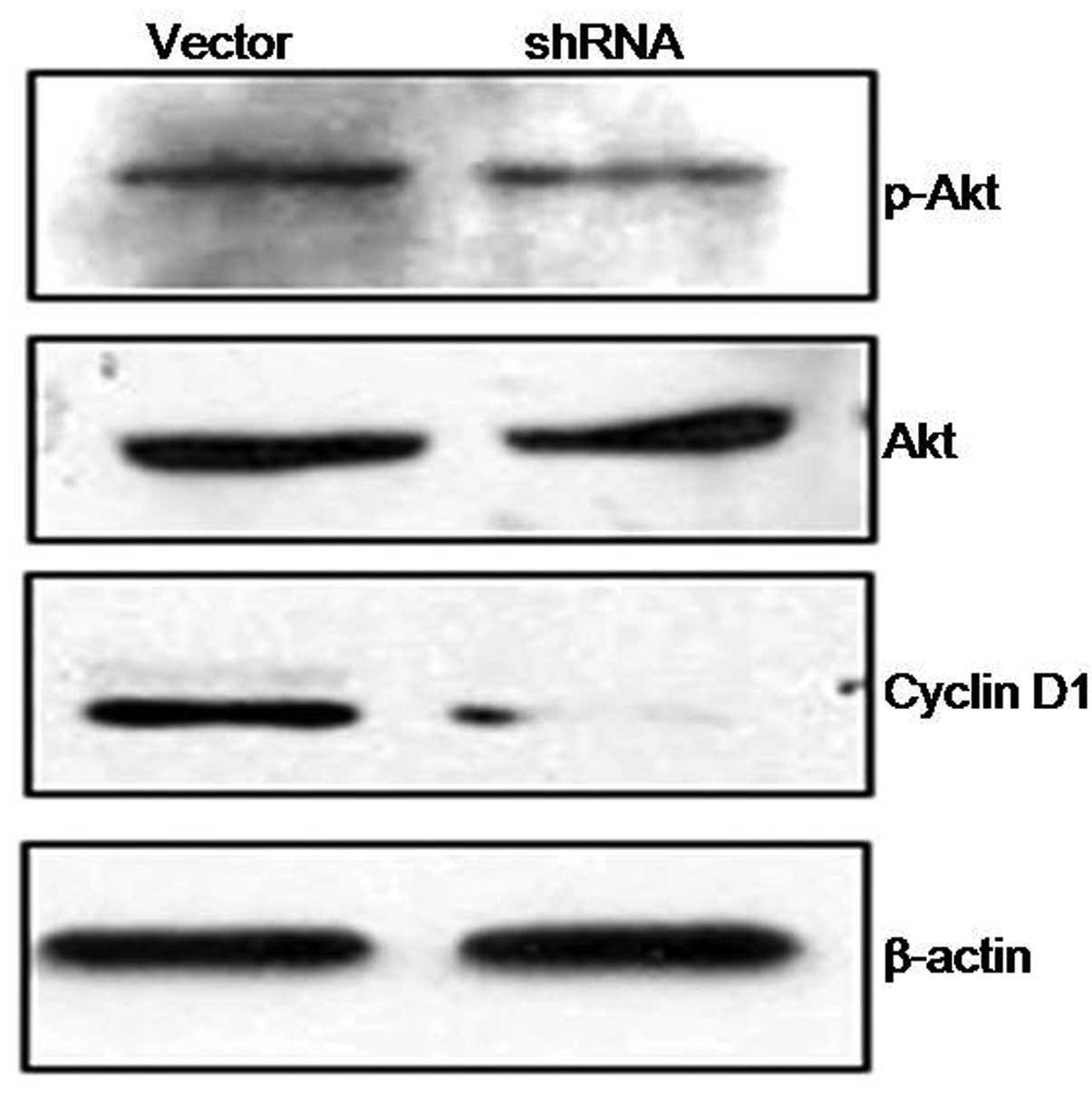
Effect of ILK knockdown on the downstream
Akt pathway
ILK is a multifunctional intracellular effector of
cell-matrix interactions, which is involved in cancer cell growth
and survival through modulation of downstream targets, notably Akt,
which plays a role in determining cell behavior with regards to
proliferation, migration and survival (7,16).
In order to determine whether Akt activity is decreased following
ILK suppression in PCa cells, the phosphorylated Akt and its
downstream molecule were detected by western blot analysis.
Phosphorylated Akt was found to be significantly decreased combined
with the downregulation of cyclin D1, which is one of the most
important proteins in tumor progression (Fig. 6).
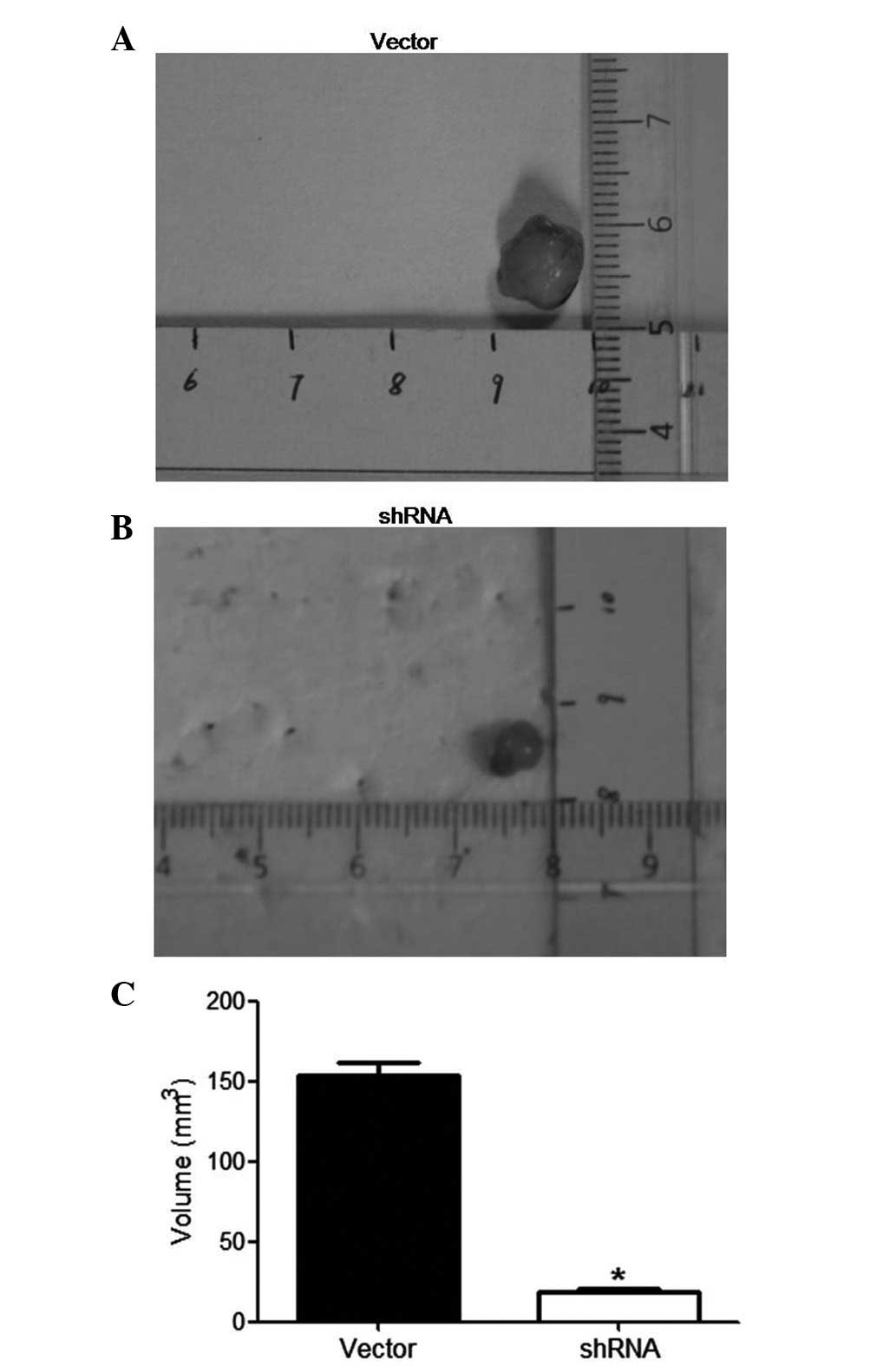
Effect of ILK knockdown on PCa growth in
vivo
The effects of ILK depletion on PCa xenograft growth
were assessed in vivo. ILK shRNA or vector were transfected
ex vivo into DU145 cells. The cells were subsequently
implanted in the subcutaneous layer of immunodeficient mice (n=10)
and tumor sizes were measured after 4 weeks. ILK shRNA-transfected
cells generated xenografts (19.0±1.1 mm3) that were
significantly smaller compared with control vector-transfected
xenografts (153.4±8.0 mm3; P<0.05; Fig. 7).
Discussion
Interaction between cell-cell and cell-extracellular
matrix, often triggered through the signals mediated by integrin
and growth factor receptors, controls the growth, differentiation,
migration and apoptosis of cells (6,17,18).
ILK is a cellular signaling protein with Ser/Thr protein kinase
activity, and is involved in the formation of the signaling
coupling complex of integrin and growth factor receptor (7). Due to its relevance to these
receptors, ILK participates in the regulation of basic cell
functions, such as the interaction between cell and extracellular
matrix, and intracellular proliferation signaling. Thus, minor
alterations in ILK expression or activity, could result in changes
of the corresponding signaling pathway and the subsequent cell
cycle, and may cause abnormal cell growth and proliferation,
leading to tumor formation (19,20).
ILK expression has been found to be increased in
many types of human cancer (11).
Previous studies have demonstrated that ILK expression is also
positively correlated with tumor grade and inversely correlated
with the 5-year patient survival rate (14). In the present study, 63 PCa and 11
BHP samples were subjected to immunohistochemical analysis to
assess the levels of ILK in tumorous and BPH tissues. ILK was found
to be overexpressed in >40% of tumorous prostate tissues
compared with BPH tissues. The positive rate of ILK expression in
PCa tissues increased with the increasing Gleason score, although
the difference was not statistically significant. These results
suggest that ILK plays a role in the development and progression of
PCa.
Despite the fact that the potential significance of
ILK in prostatic carcinogenesis has been previously reported, the
functional role of ILK in advanced PCa and the associated pathways
have yet to be fully elucidated. It has been demonstrated that
siRNA ILK and other small-molecule ILK inhibitors inhibit
proliferation and induce apoptosis in breast cancer cells, melanoma
and acute myeloid leukemia stem cells (12,13,21).
The effects of ILK knockdown on the growth, migration and apoptosis
of PCa cells should be further investigated. In the present study,
endogenous ILK expression was silenced in the PCa cell line DU145
using specific shRNA. The properties of the ILK-depleted cells were
then analyzed and compared with those of control vector-transfected
cells using various functional assays. ILK knockdown was found to
suppress cell proliferation and to induce PCa cell apoptosis. In
addition, cell motility was also impeded by ILK depletion. The
present study provides the first evidence regarding the functional
loss of ILK expression in vivo. PCa cells with suppressed
ILK expression displayed a reduced ability to form tumors in nude
mice. These observations provide direct evidence that ILK has a
critical role in the initiation phase of androgen-insensitive
prostate tumor induction.
The exact mechanism of ILK knockdown in inhibiting
PCa cell growth and inducing apoptosis has yet to be elucidated.
Akt, phosphorylation of which at the serine 473 position by ILK in
a phosphatidylinositol 3-kinase (PI3K)-dependent manner, has been
reported to be the main downstream player in ILK signaling pathway
(19), while persistent activation
of the Akt signaling cascade is prominent in various types of human
cancer. Thus, depletion of ILK expression or activity could slow
down the progression of several types of cancer, that may be
involved in the Akt pathway. A previous study has shown that
inhibition of ILK could also suppress the activation of Akt in
PTEN-mutant prostate cancer cells (20). Activated Akt phosphorylates or
regulates a number of cellular proteins that are implicated in
tumorigenesis and motility (22).
In the present study, we showed that blockade of the ILK pathway
attenuated tyrosine phosphorylation of the Akt pathway and its
downstream molecule cyclin D1, which significantly affected
malignant features of tumor cells. Thus, ILK-Akt signaling is
suggested to be an important regulatory pathway in advanced PCa.
Further studies are needed to investigate the exact mechanisms of
ILK involved in PCa.
In summary, the present study showed that ILK is
overexpressed in human PCa. ILK silencing significantly attenuates
PCa tumorigenicity as assessed by western blot analysis,
proliferation assay, flow cytometry and wound healing assay in
vitro, and using a xenograft model in vivo. ILK
knockdown is suggested to inhibit the biological behavior of
advanced PCa cells through attenuating Akt activity. The present
preliminary study provides the first direct evidence regarding a
potential association of ILK knockdown with antitumor progression.
Although further investigation and validation is needed concerning
the underlying mechanisms of ILK depletion affecting PCa
progression, ILK is suggested to be a potential therapeutic target
for PCa.
Acknowledgements
This study was supported by the National Natural
Science Foundation of China (81141029). The authors would like to
thank the Department of Pathology of Peking University for kindly
providing the DU145 human prostate cancer cell line and for the
technical support in immunohistochemical analysis.
References
|
1
|
Jemal A, Siegel R, Xu J and Ward E: Cancer
statistics, 2010. CA Cancer J Clin. 60:277–300. 2010. View Article : Google Scholar
|
|
2
|
Loblaw DA, Mendelson DS, Talcott JA, et
al: American Society of Clinical Oncology recommendations for the
initial hormonal management of androgen-sensitive metastatic,
recurrent, or progressive prostate cancer. J Clin Oncol.
22:2927–2941. 2004. View Article : Google Scholar
|
|
3
|
Feldman BJ and Feldman D: The development
of androgen-independent prostate cancer. Nat Rev Cancer. 1:34–45.
2001. View
Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hannigan GE, Bayani J, Weksberg R, et al:
Mapping of the gene encoding the integrin-linked kinase, ILK, to
human chromosome 11p15.5-p15.4. Genomics. 42:177–179. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hannigan G, Troussard AA and Dedhar S:
Integrin-linked kinase: a cancer therapeutic target unique among
its ILK. Nat Rev Cancer. 5:51–63. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Gil D, Ciolczyk-Wierzbicka D,
Dulinska-Litewka J, Zwawa K, McCubrey JA and Laidler P: The
mechanism of contribution of integrin linked kinase (ILK) to
epithelial-mesenchymal transition (EMT). Adv Enzyme Regul.
51:195–207. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Gao J, Zhu J, Li HY, Pan XY, Jiang R and
Chen JX: Small interfering RNA targeting integrin-linked kinase
inhibited the growth and induced apoptosis in human bladder cancer
cells. Int J Biochem Cell Biol. 43:1294–1304. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ahmed N, Riley C, Oliva K, Stutt E, Rice
GE and Quinn MA: Integrin-linked kinase expression increases with
ovarian tumour grade and is sustained by peritoneal tumour fluid. J
Pathol. 201:229–237. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Bravou V, Klironomos G, Papadaki E,
Taraviras S and Varakis J: ILK over-expression in human colon
cancer progression correlates with activation of beta-catenin,
down-regulation of E-cadherin and activation of the Akt-FKHR
pathway. J Pathol. 208:91–99. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Intaraprasong P, Assi K, Owen DA, et al:
Expression of integrin-linked kinase is not a useful prognostic
marker in resected hepatocellular cancer. Anticancer Res.
27:4371–4376. 2007.PubMed/NCBI
|
|
11
|
Goulioumis AK, Bravou V, Varakis J, Goumas
P and Papadaki H: Integrin-linked kinase cytoplasmic and nuclear
expression in laryngeal carcinomas. Virchows Arch. 453:511–519.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Muranyi AL, Dedhar S and Hogge DE:
Targeting integrin linked kinase and FMS-like tyrosine kinase-3 is
cytotoxic to acute myeloid leukemia stem cells but spares normal
progenitors. Leuk Res. 34:1358–1365. 2010. View Article : Google Scholar
|
|
13
|
Wong RP, Ng P, Dedhar S and Li G: The role
of integrin-linked kinase in melanoma cell migration, invasion, and
tumor growth. Mol Cancer Ther. 6:1692–1700. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Graff JR, Deddens JA, Konicek BW, et al:
Integrin-linked kinase expression increases with prostate tumor
grade. Clin Cancer Res. 7:1987–1991. 2001.PubMed/NCBI
|
|
15
|
Sasaki T, Ryo A, Uemura H, et al: An
immunohistochemical scoring system of prolyl isomerase Pin1 for
predicting relapse of prostate carcinoma after radical
prostatectomy. Pathol Res Pract. 202:357–364. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Hou Y, Mortimer L and Chadee K: Entamoeba
histolytica cysteine proteinase 5 binds integrin on colonic cells
and stimulates NFkappaB-mediated pro-inflammatory responses. J Biol
Chem. 285:35497–35504. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Buchheit CL, Rayavarapu RR and Schafer ZT:
The regulation of cancer cell death and metabolism by extracellular
matrix attachment. Semin Cell Dev Biol. 23:402–411. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Lu P, Takai K, Weaver VM and Werb Z:
Extracellular matrix degradation and remodeling in development and
disease. Cold Spring Harb Perspect Biol. 3:a0050582011.PubMed/NCBI
|
|
19
|
Delcommenne M, Tan C, Gray V, Rue L,
Woodgett J and Dedhar S: Phosphoinositide-3-OH kinase-dependent
regulation of glycogen synthase kinase 3 and protein kinase B/AKT
by the integrin-linked kinase. Proc Natl Acad Sci USA.
95:11211–11216. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Persad S, Attwell S, Gray V, et al:
Inhibition of integrin-linked kinase (ILK) suppresses activation of
protein kinase B/Akt and induces cell cycle arrest and apoptosis of
PTEN-mutant prostate cancer cells. Proc Natl Acad Sci USA.
97:3207–3212. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Troussard AA, McDonald PC, Wederell ED, et
al: Preferential dependence of breast cancer cells versus normal
cells on integrin-linked kinase for protein kinase B/Akt activation
and cell survival. Cancer Res. 66:393–403. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Hers I, Vincent EE and Tavare JM: Akt
signalling in health and disease. Cell Signal. 23:1515–1527. 2011.
View Article : Google Scholar : PubMed/NCBI
|