Introduction
Yun Zhi (Coriolus versicolor), a Chinese
medicinal plant, is a macrofungi which belongs to the
Basidiomycetes class and Polyporaceae family of fungi (1). Extracts from Coriolus
versicolor have been previously demonstrated to represent a
valuable adjuvant for the treatment of various forms of cancer in
combination with chemotherapy or radiotherapy (2). Polysaccharopeptide (PSP) is extracted
from the Cov-1 strain of Coriolus versicolor and is the main
active ingredient, functioning as a biological response modifier
(3). PSP is a proteoglycan of ~100
kDa and contains a polysaccharide and polypeptide portion (4,5). PSP
is well known for its immunoregulatory, anticancer,
anti-inflammatory and antiviral effects, and is used widely as an
immune modifier in healthy and cancerous individuals in a number of
Asian countries (4,6–9). PSP
exerts its immunomodulatory actions by promoting the proliferation
of the activation of macrophages, T lymphocytes and natural killer
cells (10). However, the majority
of studies are in vitro and the effect of PSP on lymphocyte
proliferation, including T cells, is quite controversial (9,11).
The efficacy of PSP on immunological effector cells under an
immunosuppressive state in vivo is poorly understood.
Therefore, the present study was designed to elucidate the
immunomodulatory effects of PSP on immunological effector cells in
immunosuppressed mice induced by cyclophosphamide (Cy).
Numerous studies have begun to dissect the pathways
that may suppress immune responses, including effectors or
regulators of T cell exhaustion (12,13).
Three key negative regulatory pathways that have received
particular attention are forkhead/winged-helix transcription factor
box protein 3 (Foxp3)+ regulatory T cells (Tregs),
programmed death-1 (PD-1)/PD-L and interleukin (IL)-10/IL-10R
(12). In the present study, the
effect of PSP on the gene expression of negative immune regulators,
Foxp3, PD-1 and IL-10, was also investigated in immunosuppressed
mouse spleen tissues.
Materials and methods
Animals
Male Balb/c mice, obtained from the Animal Center of
Nanjing Medical University (Nanjing, Jiangsu, China), were
maintained in specific pathogen-free conditions and used at 6–8
weeks old. Mice were housed in an air-conditioned room at 25±2°C
with a relative humidity of 40–70% and a 12 h interval light/dark
cycle. The mice were fed with tap water and a standard laboratory
diet. All animal experiments were performed in accordance with
institutional guidelines and the study was approved by the ethics
committee of Nanjing Drum Tower Hospital, Nanjing University
Medical School, Nanjing, Jiangsu, China.
Drugs and chemicals
Pure polysaccharopeptide powder was provided by
Jiangsu Nanjing Lao Shan Pharmacy Co., Ltd. (Nanjing, Jiangsu,
China). Cyclophosphamide was provided by Jiangsu Hengrui Medicine
Co., Ltd. (Lianyungang, China). Alexa Fluor 647 rat anti-mouse
CD8a, PerCP-Cy 5.5 rat anti-mouse CD4, FITC hamster anti-mouse
CD3e, PE rat anti-mouse CD19 and RBC lysis buffer were all obtained
from BD Biosciences (Franklin Lakes, NJ, USA). TRIzol was purchased
from Invitrogen Life Technologies (Carlsbad, CA, USA); SYBR Premix
Ex Taq (Tli RNase H Plus) and PrimeScript RT Master Mix were
obtained from Takara Bio, Inc. (Shiga, Japan).
Experimental regimen
Balb/c mice were randomly assigned to four groups,
including normal control, Cy and two PSP groups. The mice in the
normal control and Cy groups were orally administrated with
physiological saline. The two PSP groups were orally administered
with PSP at a dose of 125 or 500 mg/kg/d body weight. All groups
were administrated once a day for 25 consecutive days. The Cy and
the two PSP groups were injected intraperitoneally with Cy at the
dose of 150 mg/kg/d body weight on day 17 and 21 to generate an
immunosuppressed animal model (14), while the mice in the normal control
group were injected intraperitoneally with physiological saline as
control. The solution of PSP and Cy was prepared by dissolving the
compounds in physiological saline.
Detection of WBCs
Peripheral blood was collected from the
retro-orbital plexus of each mouse on day 22 and 26 prior to being
sacrificed. The blood was placed in a sterile EDTA-anticoagulated
tube and WBCs were counted by a Sysmex XE-2100 hematology analyzer
(Sysmex Corporation, Kobe, Japan).
Flow cytometry of lymphocyte subsets
EDTA-anticoagulated whole blood was collected on day
22 and 26. Whole blood (50 μl) was gently mixed with anti-mouse
mAbs (Alexa Fluor 647 CD8a, PerCP-Cy 5.5 CD4, FITC CD3e and PE
CD19) and incubated for 20 min at room temperature in the dark.
After adding RBC lysis buffer for 10 min, the samples were
centrifuged at 392 × g at 25°C for 5 min and the supernatant was
discarded. The samples were washed with 1 ml phosphate-buffered
saline (PBS), centrifuged at 392 × g at 25°C for 5 min and the
supernatant was discarded. The cells were resuspended in PBS and
then analyzed on a BD FACSCanto flow cytometer (BD
Biosciences).
Calculation of absolute numbers of
CD3+CD4+ T cells,
CD3+CD8+ T cells and
CD3−CD19+ B cells
The absolute number of
CD3+CD4+ T cells,
CD3+CD8+ T cells and
CD3−CD19+ B cells in the peripheral blood in
each group was calculated by the percentage of each subset
multiplied by lymphocyte absolute count (15). Lymphocyte absolute count was
calculated by multiplying the proportion of lymphocytes by the WBC
absolute number which was counted using a hemacytometer. To
determine the proportion of lymphocytes, cells were treated with
Wright’s Giemsa stain and 200 cells/slide were manually counted
using an oil immersion microscope (magnification, ×100) (15).
Spleen and thymus indexes
On day 26, all mice were sacrificed. Prior to
sacrifice, the body weight of the mice was recorded. The spleens
and thymus glands were removed and the weights were recorded. The
organ indexes of spleens and thymus were calculated according to
the formula: organ index = weight of organ (mg)/body weight (10
g).
Total RNA isolation and real-time
quantitative PCR
The relative expression of IL-2, IL-4,
T-box-containing protein (T-bet), GATA binding protein 3 (GATA-3),
Foxp3, PD-1 and IL-10 mRNA in the spleen tissues of the normal
control, Cy and 125 mg/kg PSP groups was detected by real-time
quantitative PCR. Total RNA was extracted from spleen tissues using
TRIzol according to the manufacturer’s instructions (Invitrogen
Life Technologies). Concentration and quality of the extracted RNA
were determined by measuring light absorbance at 260 nm
(A260) and the ratio of A260/A280.
Reverse transcription reactions were performed using 0.5 μl RNA (1
μg/μl) in a volume of 10 μl according to the manufacturer’s
instructions. PCR was performed on an Applied Biosystems 7500
Real-Time PCR System (Applied Biosystems, Inc., Foster City, CA,
USA). PCR programs were optimized and the primers and PCR product
sizes are shown in Table I. The
mRNA levels of different groups were normalized against levels of
GAPDH. The relative differences in gene expression among study
groups were determined using the comparative Ct (ΔΔCt) method and
fold expression was calculated using the formula 2−ΔΔCt.
ΔΔCt represents ΔCt values normalized against the mean ΔCt of
control samples.
 | Table ISequences of primers used for
qPCR. |
Table I
Sequences of primers used for
qPCR.
| Gene | Direction | Primer sequence
(5′-3′) | Product size
(bp) |
|---|
| IL-2 | Forward |
CCCAAGCAGGCCACAGAATTGAAA | 81 |
| Reverse |
AGTCAAATCCAGAACATGCCGCAG | |
| IL-4 | Forward |
ATGGGTCTCAACCCCCAGCTAGT | 399 |
| Reverse |
GCTCTTTAGGCTTTCCAGGAAGTC | |
| T-bet | Forward |
AACCAGTATCCTGTTCCCAGC | 439 |
| Reverse |
TGTCGCCACTGGAAGGATAG | |
| GATA-3 | Forward |
GAAGGCATCCAGACCCGAAAC | 255 |
| Reverse |
ACCCATGGCGGTGACCATGC | |
| PD-1 | Forward |
TGAACATCCTTGACACACGGC | 170 |
| Reverse |
GCCTTCTGGTTTGGGCGA | |
| IL-10 | Forward |
CCAGTTTTACCTGGTAGAAGTGATG | 324 |
| Reverse |
TGTCTAGGTCCTGGAGTCCAGCAGACTCAA | |
| Foxp3 | Forward |
ATTTACTCAACCCAAACCCT | 156 |
| Reverse |
TGTGTGATAGTGCCCGT | |
| GAPDH | Forward |
CCCACAGTAAATTCAACGGCAC | 564 |
| Reverse |
CATTGGGGTTAGGAACACGGA | |
Statistical analysis
Data are presented as the mean ± SD. The results
were statistically analyzed using SPSS 13.0 software (SPSS, Inc.,
Chicago, IL, USA). Data were analyzed by one-way analysis of
variance. P<0.05 was considered to indicate a statistically
significant difference.
Results
General observations following Cy
injection
All mice injected with Cy showed lethargy, dull
pelage, fur piloerection and reduced food and water intake. In the
Cy and 125 mg/kg PSP group, one mouse died one day following the
first blood collection in each group.
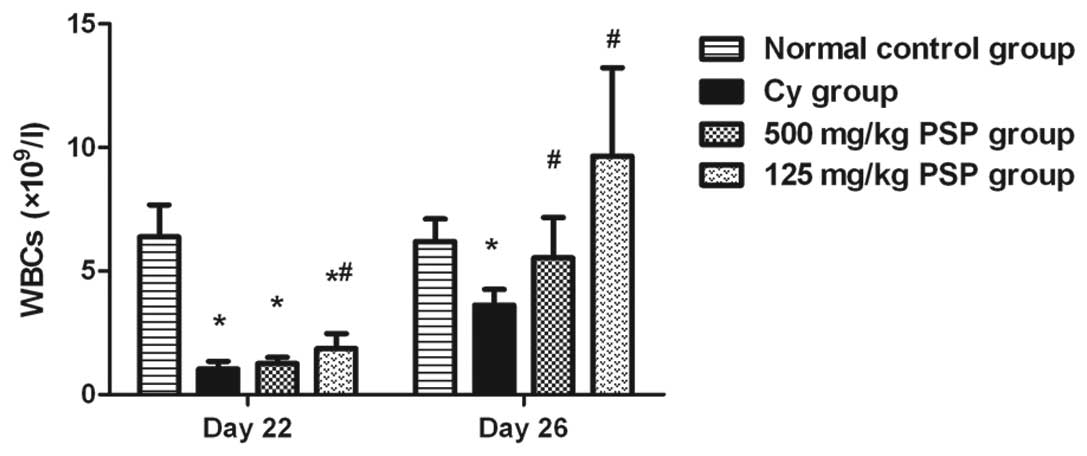
Effect of PSP on peripheral blood
WBCs
WBCs decreased on day 22 following the two Cy
injections and then began to increase on day 26 in all groups
except the normal control. In the two PSP groups, the peripheral
blood WBCs counts were significantly higher than the Cy group and
125 mg/kg PSP was higher than 500 mg/kg PSP (Fig. 1)
Effect of PSP on absolute number of
CD3+CD4+ T cells,
CD3+CD8+ T cells and
CD3−CD19+ B cells
On day 22, absolute numbers of
CD3+CD4+ T cells,
CD3+CD8+ T cells and
CD3−CD19+ B cells in the peripheral blood in
the Cy group were significantly decreased compared with the normal
control group. The reduction in the absolute number of
CD3−CD19+ B cells was the most marked. The
two PSP groups had significantly higher
CD3+CD4+ T cell,
CD3+CD8+ T cell,
CD3−CD19+ B cell absolute numbers compared
with the Cy group. The reduction in the 125 mg/kg PSP group was
smaller than the 500 mg/kg PSP group (Table II). On day 26, the absolute
numbers of CD3+CD4+ T cells,
CD3+CD8+ T cells and
CD3−CD19+ B cells in the Cy group, and the
two PSP groups recovered significantly, but in the Cy group the
absolute number of CD3+CD8+ T cells and
CD3−CD19+ B cells was still lower than the
normal control group. The absolute number of
CD3+CD4+ T cells and
CD3+CD8+ T cells in the 125 mg/kg PSP groups
was superior to those in the 500 mg/kg PSP, Cy and the normal
control groups. In addition, the absolute number of
CD3−CD19+ B cells in the two PSP groups was
higher than the Cy group, but still significantly lower than the
normal control group (Table
III).
 | Table IIAbsolute number of
CD3+CD4+ T cells,
CD3+CD8+ T cells and
CD3−CD19+ B cells on day 22. |
Table II
Absolute number of
CD3+CD4+ T cells,
CD3+CD8+ T cells and
CD3−CD19+ B cells on day 22.
| Group | n |
CD3−CD19+ B cells
(x107/l) |
CD3+CD4+ T cells
(x109/l) |
CD3+CD8+ T cells
(x109/l) |
|---|
| Normal control | 8 | 148.03±36.39 | 0.86±0.24 | 0.24±0.08 |
| Cy | 8 | 0.67±0.40a | 0.29±0.13a | 0.06±0.03a |
| 500 mg/kg PSP | 8 | 1.97±0.79a,b | 0.49±0.13a,b | 0.10±0.03a,b |
| 125 mg/kg PSP | 8 | 2.19±2.05a,b | 0.86±0.29b | 0.18±0.08b |
 | Table IIIAbsolute number of
CD3+CD4+ T cells,
CD3+CD8+ T cells and
CD3−CD19+ B cells on day 26. |
Table III
Absolute number of
CD3+CD4+ T cells,
CD3+CD8+ T cells and
CD3−CD19+ B cells on day 26.
| Group | n |
CD3−CD19+ B cells
(x107/l) |
CD3+CD4+ T cells
(x109/l) |
CD3+CD8+ T cells
(x109/l) |
|---|
| Normal control | 8 | 137.25±44.78 | 0.94±0.16 | 0.23±0.04 |
| Cy | 7 | 1.89±0.79a | 0.89±0.19 | 0.17±0.04a |
| 500 mg/kg PSP | 8 | 5.38±2.63a,b | 1.60±0.88 | 0.35±0.17b |
| 125 mg/kg PSP | 7 | 7.68±3.70a,b | 3.06±1.27a,b | 0.64±0.22a,b |
Effect of PSP on spleen and thymus gland
indexes
The spleen index in the Cy group was lower than in
the normal control and the two PSP groups; however, this difference
was not found to be statistically significant. The thymus index in
the Cy group was significantly lower than the normal control group
(P<0.05; Table IV).
 | Table IVSpleen and thymus gland indexes. |
Table IV
Spleen and thymus gland indexes.
| Group | n | Spleen index (mg/10
g) | Thymus index (mg/10
g) |
|---|
| Normal control | 8 | 64.49±43.46 | 10.91±2.49 |
| Cy | 7 | 39.11±16.51 | 7.71±3.70a |
| 500 mg/kg PSP | 8 | 52.88±29.67 | 10.04±1.45 |
| 125 mg/kg PSP | 7 | 60.09±35.52 | 7.35±4.74 |
Effect of PSP on Th1/Th2 balance
No marked differences were observed in the relative
mRNA expression of IL-2 and T-bet in each group. However, IL-4 and
GATA-3 mRNA expression in the Cy group was significantly higher
than the normal control and 125 mg/kg PSP groups (both P<0.01
and P<0.05, respectively). There was no statistical difference
between the normal control and 125 mg/kg PSP groups in the ratios
of IL-2/IL-4 and T-bet/GATA-3; however, the ratio in the two groups
was significantly higher than the Cy group (P<0.01; Fig. 2).
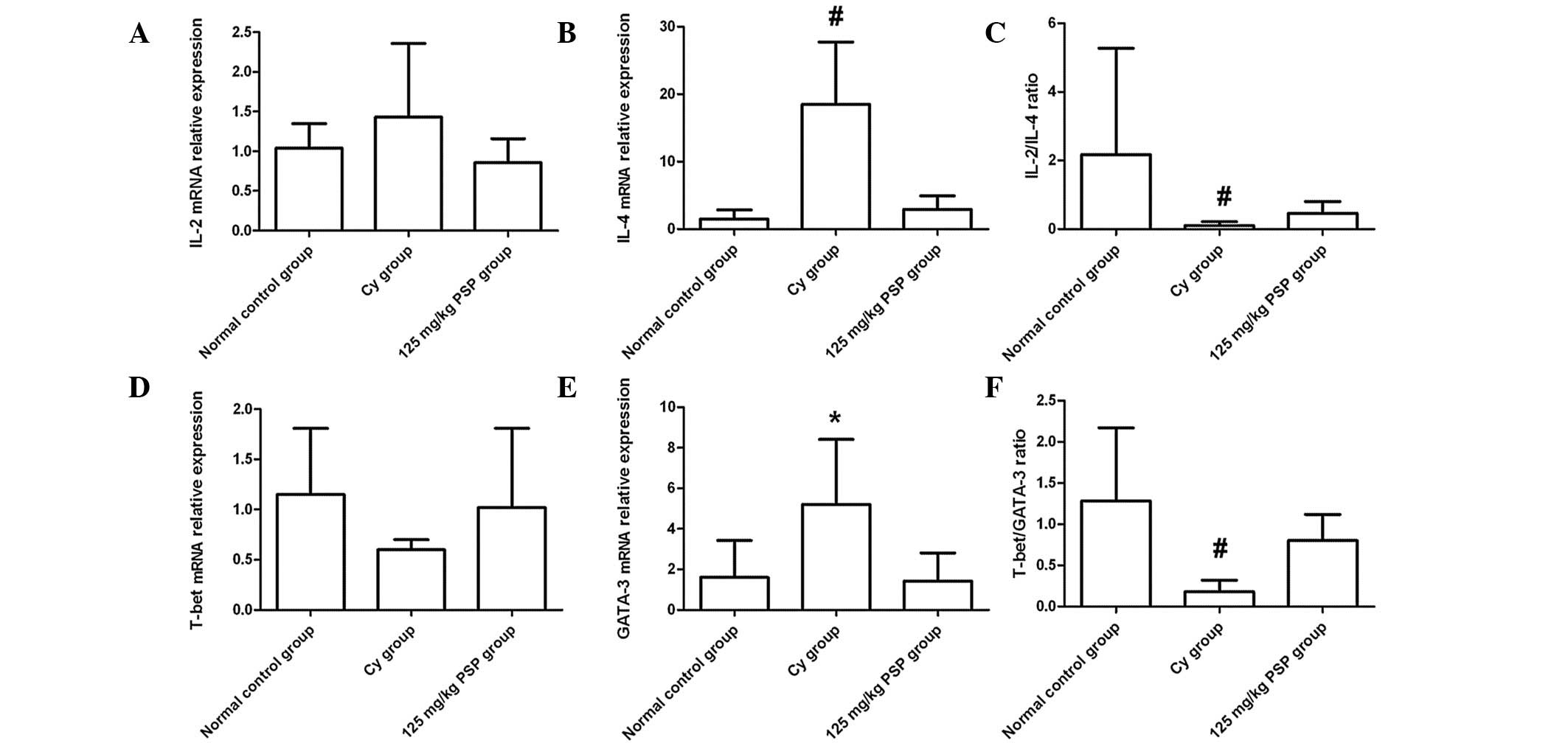 | Figure 2mRNA relative expression levels in
spleen tissues of (A) IL-2, (B) IL-4, (D) T-bet and (E) GATA-3, and
the ratios of (C) IL-2/IL-4 and (F) T-bet/GATA-3. mRNA levels were
normalized against GAPDH expression in each sample and values are
expressed as the mean ± SD fold increase over the normal control
group (n=6, *P<0.05, vs. normal control and 125 mg/kg
PSP; #P<0.01, vs. normal control and 125 mg/kg PSP).
T-bet, T-box-containing protein; GATA-3, GATA binding protein 3;
IL, interleukin; Cy, cyclophosphamide; PSP,
polysaccharopeptide. |
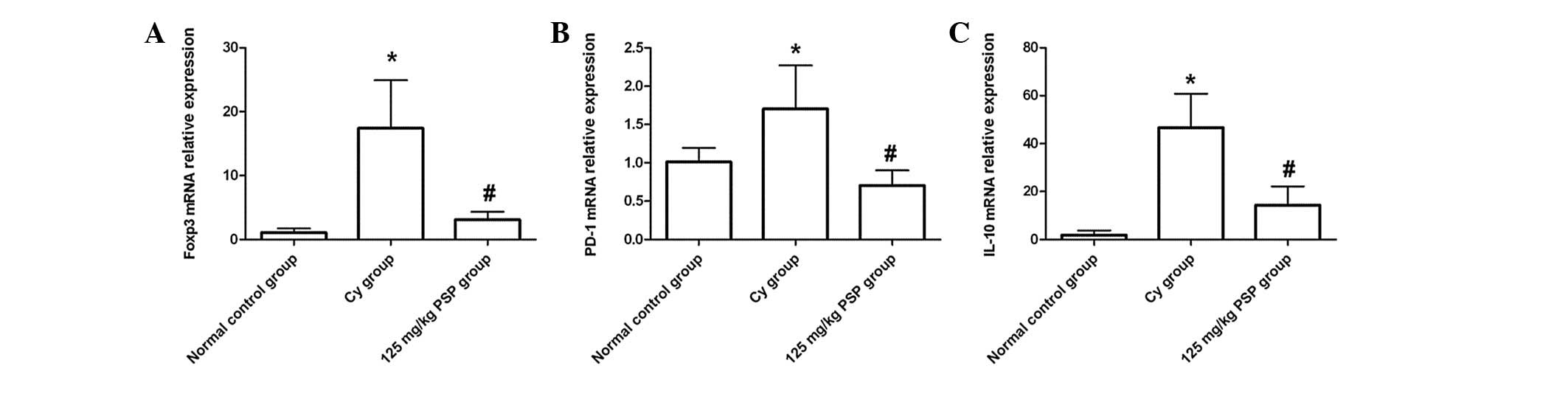
Effect of PSP on the mRNA expression of
negative immune regulators
The relative expression of Foxp3, PD-1 and IL-10
mRNA was significantly higher in the Cy group than the normal
control (all P<0.01), and in the 125 mg/kg PSP group the
relative expression of Foxp3 and IL-10 mRNA was lower than the Cy
group (all P<0.01), but still higher than the normal control
(P<0.01). PD-1 mRNA relative expression in the 125 mg/kg PSP
group was lower than the Cy and normal control groups (P<0.01;
Fig. 3).
Discussion
In the present study, the effects of PSP on the
immune response of immunosuppressed mice induced by Cy were
assessed by administering PSP to Balb/c mice for 25 days. The
immunostimulatory effects of a drug or nutritional supplement are
difficult to evaluate in healthy human individuals and animals
(16). Cy is one of the most
commonly used anticancer agents and immunodepressant drugs for
preventing graft rejection, treating specific chronic autoimmune
diseases and inducing experimental immunosuppression (17). Cy inhibits humoral and cellular
immunity, and is most toxic to rapidly proliferating tissue,
including the hematopoietic system, gastrointestinal epithelia,
hair follicles and genital glands (18). Therefore, in the present study, Cy
was selected as the immunosuppressive drug to induce the
immunosuppressed mice model. This model is often used to evaluate
the immunoregulatory effects of drugs (19–22).
In the Cy-induced murine model, WBC counts were significantly
decreased following Cy injection, then recovered spontaneously. In
leukopenic mice orally administered with PSP, the decline in the
WBC level was significantly alleviated and the WBC counts recovered
to normal levels more rapidly.
Thymus and spleens are the most important immune
organs. However, in this study, changes in spleen and thymus
indexes of mice were not found to be significantly different from
those of the two PSP groups and Cy group through the experimental
period. This indicated that PSP treatment did not affect the body
weight and spleen or thymus mass of mice.
T lymphocytes play a critical role in the
development of acquired immune responses and B lymphocytes are
important in the response to humoral immunity. CD4+ and
CD8+ T cells are the main T lymphocyte subsets. In the
present study, the proliferative responses to T- and B-cell
mitogens were reduced markedly in all Cy-treated groups. PSP
treatment promoted T- and B-cell proliferative responses and in
specific cases the response was higher than normal levels. Of note,
this response was higher in the 125 mg/kg PSP group compared with
500 mg/kg PSP. Previous studies have also indicated that PSP
significantly increases the percentage of CD4+ T
lymphocytes, the ratio of CD4+/CD8+ and the
quantity and percentage of the B lymphocytes, and enhances the
immune system of cancer patients (23,24).
However, in the present study, the absolute number of T lymphocyte
subsets and B lymphocytes was investigated which has not been
analyzed to date.
According to differences in corresponding cytokines,
helper T cells (Th) may be subdivided into two cell subsets, termed
Th1 and Th2. Th1 cytokines contribute to cell-mediated immunity
while Th2 cytokines are responsible for humoral immunity (25,26).
IL-2 is the main Th1 type cytokine and is important for promoting
T-cell proliferation, cytokine production and the functional
properties of B cells, macrophages and NK cells (27). IL-4 is a key regulator of the
immune response and promotes differentiation of naive T cells into
Th2 cells (28). T-bet and GATA-3
are specific transcription factors which have been hypothesized to
serve as master regulators of Th1 and Th2 differentiation,
respectively. Expression of T-bet and GATA-3 impose a complex
programme of lineage restriction that facilitates preferential
expression of the signature cytokines and, to varying degrees,
silencing of the opposite differentiation limb (29). Th1/Th2 balance is a prerequisite
for the functionality of immune system against infections. Ho et
al found that PSP promotes the proliferation of mouse splenic
lymphocytes and increases the expression of Th1 cell-associated
cytokines, including IL-2, IL-12, IFN-γ and IL-18, in mouse splenic
lymphocytes in vitro(30).
An additional study showed that PSP exhibited suppressive effects
on Th1 cytokines, including IL-2, but stimulated the production of
the Th2 cytokine, IL-10, to inhibit T-cell proliferation in human
PBMCs in vitro(31). The
effect of PSP on the Th1/Th2 balance is controversial. In the
present study, when the mice were treated with Cy, the relative
mRNA expression of the Th2 type cytokine, IL-4 and its specific
transcription factor, GATA-3, was significantly increased and the
ratios of IL-2/IL-4 and T-bet/GATA-3 were reduced. However, no
significant effect on Th1 type cytokines was found. These
observations indicate an imbalance of Th1/Th2 with Th2 shift in the
Cy model. PSP reduces the relative mRNA expression of Th2 type
cytokine, IL-4 and its specific transcription factor, GATA-3, to
moderate the Th1/Th2 balance.
More recently, research has begun to pay more
attention to the pathways that may suppress immune responses. One
key negative regulatory pathway is mediated by
CD4+CD25+ regulatory T cells. Another two
pathways that have also received particular focus are PD-1/PD-L and
IL-10/IL-10R.
Tregs employ several mechanisms to suppress immune
responses, including direct cell contact, indirectly by reducing
the antigen-presenting capacity of antigen-presenting cells
(32) or by suppressive cytokines,
such as inhibitory cytokines IL-10 and TGF-β (33,34). FOXP3
is considered to represent the most reliable marker of Treg
involvement in the formation and functioning of
CD4+CD25+ T lymphocytes (35–38)
and the level of FOXP3 expression has been shown to correlate with
suppressive activity (35,38).
PD-1, which belongs to the CD28 family is mainly
inducibly expressed on activated T cells, B cells, natural killer T
cells and myeloid cells (39–42).
PD-1 functions as an inhibitory co-signaling molecule and regulates
immunity (43–46). PD-1 decreases T-cell receptor
(TCR)-mediated cell proliferation, cytokine production and
cytolytic activity upon binding with its ligands, PD-L1 and PD-L2
(47–50). In addition, the PD-1 signaling
cascade may inhibit CD8+ T-cell effector function during
chronic murine viral infections (51).
IL-10 was originally identified by Fiorentino et
al(52,53). T-helper type 2 cells, subsets of
regulatory T cells designated Tr1, Th1 and Th17 cells, are the four
major T-cell sources of IL-10 (54). The main biological function of
IL-10 is exerted on dendritic cells (DCs) and macrophages, and is a
potent inhibitor of antigen presentation. IL-10 inhibits major
histocompatibility complex class II expression as well as the
upregulation of costimulatory molecules, CD80 and CD86, and
inhibits the differentiation and maturation of DCs (55). In addition, the IL-10/IL-10R
pathway plays a key role in the early events that determine whether
an infection is rapidly cleared or progresses to chronicity with
T-cell dysfunction (12).
The expression of negative immune regulators,
including Foxp3, PD-1 and IL-10, in immunosuppressed mice and the
effect of PSP on immunosuppressed mice is not clear.
In the present study, the expression of Foxp3, PD-1
and IL-10 mRNA was significantly higher in the Cy group, indicating
that Cy exerts its immunosuppressive effect by a negative
regulatory pathway. In the PSP group, the mRNA expression of Foxp3,
IL-10 and PD-1 was lower than the Cy group, indicating that PSP may
exert its immunomodulatory effects by downregulating the expression
of negative immune regulators, Foxp3, PD-1 and IL-10.
In summary, the results of the present study
demonstrate that PSP possesses immunoprotective effects and is
capable of restoring Cy-induced immunosuppression, including
depressed WBCs, CD4+ T lymphocytes, CD8+ T
lymphocytes and B lymphocytes, as well as reducing the expression
of the Th2 type cytokine, IL-4 and its specific transcription
factor, GATA-3 and negative immune regulators, such as Foxp3, PD-1
and IL-10. These observations show that PSP functions against the
immune inhibition induced by Cy, indicating that PSP is a potent
immunoenhancing and immunomodulating agent.
Acknowledgements
The present study was supported by the Medical
Science Intensive Developing Project of Nanjing Government (No.
ZKX10015), Jiangsu Province’s Outstanding Medical Academic Leader
Program (No. LJ201154) and Jiangsu Province’s Clinical Medicine and
Technology Special Program (No. BL2012034).
References
|
1
|
Hyde HA and Adams KF: Airborne allergens
at Cardiff 1942–59. Acta Allergol Suppl (Copenh). 7:159–169.
1960.PubMed/NCBI
|
|
2
|
Chu KK, Ho SS and Chow AH: Coriolus
versicolor: a medicinal mushroom with promising
immunotherapeutic values. J Clin Pharmacol. 42:976–984. 2002.
View Article : Google Scholar
|
|
3
|
Yang MM, Chen Z and Kwok JS: The
anti-tumor effect of a small polypeptide from Coriolus
versicolor (SPCV). Am J Chin Med. 20:221–232. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Cui J and Chisti Y: Polysaccharopeptides
of Coriolus versicolor: physiological activity, uses, and
production. Biotechnol Adv. 21:109–122. 2003.
|
|
5
|
Ng TB: A review of research on the
protein-bound polysaccharide (polysaccharopeptide, PSP) from the
mushroom Coriolus versicolor (Basidiomycetes: Polyporaceae).
Gen Pharmacol. 30:1–4. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kidd PM: The use of mushroom glucans and
proteoglycans in cancer treatment. Altern Med Rev. 5:4–27.
2000.PubMed/NCBI
|
|
7
|
Collins RA and Ng TB: Polysaccharopeptide
from Coriolus versicolor has potential for use against human
immunodeficiency virus type 1 infection. Life Sci. 60:PL383–PL387.
1997.PubMed/NCBI
|
|
8
|
Yang X, Sit WH, Chan DK and Wan JM: The
cell death process of the anticancer agent polysaccharide-peptide
(PSP) in human promyelocytic leukemic HL-60 cells. Oncol Rep.
13:1201–1210. 2005.PubMed/NCBI
|
|
9
|
Li J, Bao Y, Lam W, et al:
Immunoregulatory and anti-tumor effects of polysaccharopeptide and
Astragalus polysaccharides on tumor-bearing mice.
Immunopharmacol Immunotoxicol. 30:771–782. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Li W, Liu M, Lai S, Xu C, Lu F, Xiao X and
Bao Y: Immunomodulatory effects of polysaccharopeptide (PSP) in
human PBMC through regulation of TRAF6/TLR
immunosignal-transduction pathways. Immunopharmacol Immunotoxicol.
32:576–584. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lee CL, Jiang PP, Sit WH and Wan JM:
Proteome of human T lymphocytes with treatment of cyclosporine and
polysaccharopeptide: analysis of significant proteins that
manipulate T cells proliferation and immunosuppression. Int
Immunopharmacol. 7:1311–1324. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Shin H and Wherry EJ: CD8 T cell
dysfunction during chronic viral infection. Curr Opin Immunol.
19:408–415. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wherry EJ: T cell exhaustion. Nat Immunol.
12:492–499. 2011. View
Article : Google Scholar
|
|
14
|
Huyan XH, Lin YP, Gao T, Chen RY and Fan
YM: Immunosuppressive effect of cyclophosphamide on white blood
cells and lymphocyte subpopulations from peripheral blood of Balb/c
mice. Int Immunopharmacol. 11:1293–1297. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Tornatore KM, Reed K and Venuto R: 24-hour
immunologic assessment of CD4+ and CD8+
lymphocytes in renal transplant recipients receiving chronic
methylprednisolone. Clin Nephrol. 44:290–298. 1995.PubMed/NCBI
|
|
16
|
Huang GC, Wu LS, Chen LG, Yang LL and Wang
CC: Immuno-enhancement effects of Huang Qi Liu Yi Tang in a murine
model of cyclophosphamide-induced leucopenia. J Ethnopharmacol.
109:229–235. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
de Jonge ME, Huitema AD, Rodenhuis S and
Beijnen JH: Clinical pharmacokinetics of cyclophosphamide. Clin
Pharmacokinet. 44:1135–1164. 2005.
|
|
18
|
Schiavoni G, Mattei F, Di Pucchio T,
Santini SM, Bracci L, Belardelli F and Proietti E: Cyclophosphamide
induces type I interferon and augments the number of CD44(hi) T
lymphocytes in mice: implications for strategies of
chemoimmunotherapy of cancer. Blood. 95:2024–2030. 2000.PubMed/NCBI
|
|
19
|
Zhu XL, Chen AF and Lin ZB: Ganoderma
lucidum polysaccharides enhance the function of immunological
effector cells in immunosuppressed mice. J Ethnopharmacol.
111:219–226. 2007. View Article : Google Scholar
|
|
20
|
Bafna AR and Mishra SH: Immunostimulatory
effect of methanol extract of Curculigo orchioides on
immunosuppressed mice. J Ethnopharmacol. 104:1–4. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Bafna AR and Mishra SH: Protective effect
of bioactive fraction of Sphaeranthus indicus Linn. against
cyclophosphamide induced suppression of humoral immunity in mice. J
Ethnopharmacol. 104:426–429. 2006.PubMed/NCBI
|
|
22
|
Gergely P: Drug-induced lymphopenia: focus
on CD4+ and CD8+ cells. Drug Saf. 21:91–100.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Bao YX, Wong CK, Leung SF, et al: Clinical
studies of immunomodulatory activities of Yunzhi-Danshen in
patients with nasopharyngeal carcinoma. J Altern Complement Med.
12:771–776. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wong CK, Bao YX, Wong EL, Leung PC, Fung
KP and Lam CW: Immunomodulatory activities of Yunzhi and Danshen in
post-treatment breast cancer patients. Am J Chin Med. 33:381–395.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Abbas AK, Murphy KM and Sher A: Functional
diversity of helper T lymphocytes. Nature. 383:787–793. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Warren HS, Vogel FR and Chedid LA: Current
status of immunological adjuvants. Annu Rev Immunol. 4:369–388.
1986. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Blachère NE, Morris HK, Braun D, Saklani
H, Di Santo JP, Darnell RB and Albert ML: IL-2 is required for the
activation of memory CD8+ T cells via antigen
cross-presentation. J Immunol. 176:7288–7300. 2006.
|
|
28
|
Lafreniere JF, Mills P, Bouchentouf M and
Tremblay JP: Interleukin-4 improves the migration of human myogenic
precursor cells in vitro and in vivo. Exp Cell Res. 312:1127–1141.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Bowen H, Kelly A, Lee T and Lavender P:
Control of cytokine gene transcription in Th1 and Th2 cells. Clin
Exp Allergy. 38:1422–1431. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Ho CY, Lau CB, Kim CF, et al: Differential
effect of Coriolus versicolor (Yunzhi) extract on cytokine
production by murine lymphocytes in vitro. Int Immunopharmacol.
4:1549–1557. 2004.
|
|
31
|
Lee CL, Sit WH, Jiang PP, So IW and Wan
JM: Polysaccharopeptide mimics ciclosporin-mediated Th1/Th2
cytokine balance for suppression of activated human T cell
proliferation by MAPKp38 and STAT5 pathways. J Pharm Pharmacol.
60:1491–1499. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Baecher-Allan C, Brown JA, Freeman GJ and
Hafler DA: CD4+CD25high regulatory cells in human
peripheral blood. J Immunol. 167:1245–1253. 2001.PubMed/NCBI
|
|
33
|
Fowler S and Powrie F: Control of immune
pathology by IL-10-secreting regulatory T cells. Springer Semin
Immunopathol. 21:287–294. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Powrie F, Carlino J, Leach MW, Mauze S and
Coffman RL: A critical role for transforming growth factor-beta but
not interleukin 4 in the suppression of T helper type 1-mediated
colitis by CD45RB(low) CD4+ T cells. J Exp Med.
183:2669–2674. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Sakaguchi S, Ono M, Setoguchi R, et al:
Foxp3+ CD25+ CD4+ natural
regulatory T cells in dominant self-tolerance and autoimmune
disease. Immunol Rev. 212:8–27. 2006.
|
|
36
|
Hori S, Nomura T and Sakaguchi S: Control
of regulatory T cell development by the transcription factor Foxp3.
Science. 299:1057–1061. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Wan YY and Flavell RA: Regulatory T-cell
functions are subverted and converted owing to attenuated Foxp3
expression. Nature. 445:766–770. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Gavin MA, Rasmussen JP, Fontenot JD, Vasta
V, Manganiello VC, Beavo JA and Rudensky AY: Foxp3-dependent
programme of regulatory T-cell differentiation. Nature.
445:771–775. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Ishida Y, Agata Y, Shibahara K and Honjo
T: Induced expression of PD-1, a novel member of the immunoglobulin
gene superfamily, upon programmed cell death. EMBO J. 11:3887–3895.
1992.PubMed/NCBI
|
|
40
|
Okazaki T, Iwai Y and Honjo T: New
regulatory co-receptors: inducible co-stimulator and PD-1. Curr
Opin Immunol. 14:779–782. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Chen L: Co-inhibitory molecules of the
B7-CD28 family in the control of T-cell immunity. Nat Rev Immunol.
4:336–347. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Greenwald RJ, Freeman GJ and Sharpe AH:
The B7 family revisited. Annu Rev Immunol. 23:515–548. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Liang SC, Latchman YE, Buhlmann JE,
Tomczak MF, Horwitz BH, Freeman GJ and Sharpe AH: Regulation of
PD-1, PD-L1, and PD-L2 expression during normal and autoimmune
responses. Eur J Immunol. 33:2706–2716. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Rodig N, Ryan T, Allen JA, et al:
Endothelial expression of PD-L1 and PD-L2 down-regulates
CD8+ T cell activation and cytolysis. Eur J Immunol.
33:3117–3126. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Seo SK, Seo HM, Jeong HY, et al:
Co-inhibitory role of T-cell-associated B7-H1 and B7-DC in the
T-cell immune response. Immunol Lett. 102:222–228. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Ding H, Wu X and Gao W: PD-L1 is expressed
by human renal tubular epithelial cells and suppresses T cell
cytokine synthesis. Clin Immunol. 115:184–191. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Ishida M, Iwai Y, Tanaka Y, Okazaki T,
Freeman GJ, Minato N and Honjo T: Differential expression of PD-L1
and PD-L2, ligands for an inhibitory receptor PD-1, in the cells of
lymphohematopoietic tissues. Immunol Lett. 84:57–62. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Parry RV, Chemnitz JM, Frauwirth KA, et
al: CTLA-4 and PD-1 receptors inhibit T-cell activation by distinct
mechanisms. Mol Cell Biol. 25:9543–9553. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Chemnitz JM, Parry RV, Nichols KE, June CH
and Riley JL: SHP-1 and SHP-2 associate with immunoreceptor
tyrosine-based switch motif of programmed death 1 upon primary
human T cell stimulation, but only receptor ligation prevents T
cell activation. J Immunol. 173:945–954. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Kim HK, Guan H, Zu G, et al: High-level
expression of B7-H1 molecules by dendritic cells suppresses the
function of activated T cells and desensitizes allergen-primed
animals. J Leukoc Biol. 79:686–695. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Barber DL, Wherry EJ, Masopust D, et al:
Restoring function in exhausted CD8 T cells during chronic viral
infection. Nature. 439:682–687. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Fiorentino DF, Bond MW and Mosmann TR: Two
types of mouse T helper cell. IV Th2 clones secrete a factor that
inhibits cytokine production by Th1 clones. J Exp Med.
170:2081–2095. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Moore KW, Vieira P, Fiorentino DF,
Trounstine ML, Khan TA and Mosmann TR: Homology of cytokine
synthesis inhibitory factor (IL-10) to the Epstein-Barr virus gene
BCRFI. Science. 248:1230–1234. 1990. View Article : Google Scholar
|
|
54
|
Mosser DM and Zhang X: Interleukin-10: new
perspectives on an old cytokine. Immunol Rev. 226:205–218. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
55
|
O’Garra A and Vieira P: T(H)1 cells
control themselves by producing interleukin-10. Nat Rev Immunol.
7:425–428. 2007.PubMed/NCBI
|