Introduction
Dendritic cells (DCs), one of the most potent
antigen-presenting cells, are important for the initiation of the
primary immune response of both helper and cytotoxic T lymphocytes
(1–4). After antigen capture, the DC
precursors migrate to T cell regions of draining lymph nodes where
they mature into functional DCs. The functional DCs further
stimulate naive T cells by triggering the signaling pathway
involving both major histocompatibility complex molecules
presenting antigen-peptides and costimulatory molecules (5–6). DCs
are also highly responsive to inflammatory cytokines, such as tumor
necrosis factor (TNF)-α and lipopolysaccharide (LPS), which induce
a series of phenotypic and functional changes in DCs (7–8).
Similar changes indicative of maturation have also been reported
following infection with mycoplasma, viruses, intracellular
bacteria and parasites (9–11). In addition, certain plant
polysaccharides, including Astragalus mongholicus
polysaccharides, induce regulatory effects on the phenotypic and
functional maturation of DCs (12).
Toll-like receptors (TLRs), which are a family of
pattern recognition receptors (PRRs), play an essential role in the
recognition of microbial components in mammals. Of the 10 TLRs
discovered to date, TLR2 is activated primarily by lipoproteins and
glycolipids (13–16), and TLR4 is predominantly activated
by LPS and lipoteichoic acid (17–18).
Recent studies showed that TLR2 and TLR4, which are the signaling
components of LPS and consequently trigger its cellular
transduction, led to NF-κB activation and DC maturation (19–21).
The adjuvant activity of bacterial products is important not only
for antibacterial responses induced by peripheral DCs but also for
vaccine development. LPS is the main cause of septic shock in
humans due to its high toxicity; therefore, it is important to find
an alternative substance to LPS.
Lycium barbarum, a well-known Chinese
traditional medicine and also an edible food, plays multiple roles
in pharmacological and biological processes. One of its bioactive
components is Lycium barbarum polysaccharides (LBPs). LBPs
are effective in regulating phenotypic and functional maturation of
murine DCs (22). However, there
are no studies on the molecular mechanism by which LBPs induce
maturation of murine DCs.
Materials and methods
Source of mice
Male or female C57BL/6j (H-2b) and BALB/c
(H-2d) mice were purchased from the Department of
Experimental Animals, College of Medicine, Zhejiang University,
Hangzhou, Zhejiang, China. Mice were used at 4–6 weeks of age. The
study was approved by the Ethics Committee of the Children’s
Hospital of Zhejiang University School of Medicine, Hangzhou,
China.
Source of drugs
LBPs for clinical application with an endotoxin
content <0.1 Eu/mg were purchased from Pharmagenesis Inc.
(Newtown, PA, USA). The percentage of LBPs was 84.32% according to
the phenol-sulfuric acid colorimetric method. The molecular weights
of LBPs were estimated to be 31,000 Da according to high
performance gel filtration chromatography. The LBPs mainly consist
of mannose, glucose, galactose, arabinose, rhamnose and xylose.
Generation of bone marrow-derived murine
myeloid DCs
DCs were prepared as described previously in detail,
with minor modifications (23).
Briefly, bone marrow cells were flushed from the femur and tibiae
of C57BL/6j mice and depleted of red blood cells by hypotonic lysis
using Tris-NH4Cl (pH 7.2). Cells with a starting
counting number of 2×106 cells/ml were cultured in
RPMI-1640 medium in six-well flat bottom plates (Orange Scientific,
Braine-l’Alleud, Belgium) at 37°C, in 5% CO2,
supplemented with 10% fetal calf serum (FCS), 2 mmol/l L-glutamine,
100 U/ml penicillin G, 100 μg/ml streptomycin, 30 ng/ml rmGM-CSF
(Peprotech, Inc., Rocky Hill, NJ, USA) and 20 ng/ml rmIL-4
(Peprotech, Inc.). On day 3, the old medium was replaced with fresh
medium. On day 5, cells were purified by MACS columns (Miltenyi
Biotec, Auburn, CA, USA). CD11c+ DCs were acquired and
divided into 5 groups. In parallel, 5 groups of DCs were incubated
at a concentration of 5×105/ml with 100 μg/ml LBPs,
serum-free RPMI-1640 media, 100 ng/ml LPS (Sigma, St. Louis, MO,
USA) and 100 μg/ml LBPs preincubated with antibody (anti-TLR2 or
anti-TLR4; Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA) for
1 h, respectively. The serum-free RPMI-1640 media group and 100
ng/ml LPS group were used as controls. On day 7, cells and culture
supernatants were collected for further experiments and
analysis.
Flow cytometric analysis
Cell surface expression of I-A/I-E or CD11c was
determined by immunofluorescence staining. On day 7, cells were
harvested, washed twice with PBS, and resuspended in washing buffer
(PBS containing 2% FCS and 0.1% sodium azide). Cells were first
blocked with 20% mixed serum of mice and rat (Bohua Company,
Shanghai, China)for 15 min at 4°C to reduce the background and then
stained with R-PE conjugated anti-mouse I-A/I-E antibody (BD
Pharmingen, San Diego, CA, USA) and FITC-conjugated anti-mouse
CD11c antibody (BD Pharmingen) at 4°C in the dark. Thirty minutes
later, the antibody-treated cells were washed twice with washing
buffer. Cell surface co-expression of I-A/I-E and CD11c was
detected using a flow cytometer (Becton Dickinson, Franklin Lakes,
NJ, USA). Histogram and density plots were produced by the
CellQuest software package (Becton Dickinson).
Cytokine assay
On day 7, DC culture supernatants were collected and
then the concentration of mouse IL-12 p40 was determined by a
sandwich enzyme-linked immunosorbent assay (ELISA) kit (Biosource,
Bethesda, MD, USA) according to the manufacturer’s instructions.
Cytokine concentrations were determined according to absorbance
readings at 450 nm on a universal microplate reader (Bio-Tek
Instruments, Winooski, VT, USA).
Mixed lymphocyte reaction (MLR) induced
by DCs
Responder mononuclear lymphocytes from
H-2d BALB/c splenocytes were isolated by
Ficoll-Urografin density gradient. On day 7, mature DCs were
harvested as inducers, treated with 25 μg/ml mitomycin C
(AppliChem, Darmstadt, Germany) for 45 min, then 5×103
cells were added to allogeneic lymphocytes (1×105 cells
per well) in flat-bottom 96-well tissue culture plates for 120 h.
Cell proliferation was estimated according to the cellular
reduction of tetrazolium salt MTT (Sangon, Shanghai, China) by the
mitochondrial dehydrogenase of viable cells into a blue formazan
product that can be measured spectrophotometrically.
DC ultrastructure
On day 7, DCs were harvested and fixed in methanol
and air-dried, and then processed with scanning electron microscopy
(Cambridge Scientific Instruments Ltd., Cambridge, UK) according to
the manufacturer’s instructions.
Nuclear protein extraction and western
blot analysis
On day 7, DCs were harvested and the medium was
removed, 5 groups of DCs (5×106) were suspended in 1 ml
of ice-cold PBS (pH 7.2), centrifuged at 1,000 × g for 5 min,
resuspended in 400 μl of ice-cold hypotonic buffer (10 mM HEPES-KOH
pH 7.9, 2 mM MgCl2, 0.1 mM EDTA, 10 Mm KCl, 1 mM DTT, 1
μg/ml leupeptin and 1 mM PMSF), left on ice for 10 min, vortexed
and centrifuged at 15,000 × g (at 4°C for 30 sec). Pelleted nuclear
protein was resuspended in 50 μl of ice-cold saline buffer (50 mM
HEPES-KOH pH 7.9, 10% glycerol, 300 mM NaCl, 1.5 mM KCl, 0.1 mM
EDTA, 1 mM DTT, 1 μl/ml leupeptin and 1 mM PMSF), left on ice for
20 min, vortexed and centrifuged at 15,000 × g (at 4°C for 10 min).
The protein concentration was determined and aliquots were stored
at −70°C. Thirty micrograms of nuclear protein was separated by 10%
SDS-polyacrylamide gel electrophoresis (SDS-PAGE), and then was
transferred onto a nitrocellulose membrane. The membrane was
blocked with 5% skimmed milk in TBST for 1 h at room temperature
and then incubated with rabbit anti-murine NF-κB p65 antibody
(Rockland Immunochemicals, Gilbertsville, PA, USA) for 1 h. After
washing three times in TBST, the membrane was incubated with
HRP-conjugated goat anti-rabbit-IgG (Rockland Immunochemicals) for
1 h and the antibody-specific protein was visualized by an enhanced
chemiluminesence detection system.
Statistical analysis
The results were expressed as the means ± standard
deviation (SD) of the indicated number of experiments. The
statistical significance was estimated using a t-test for unpaired
observations. P<0.05 was considered to indicate a statistically
significant difference.
Results
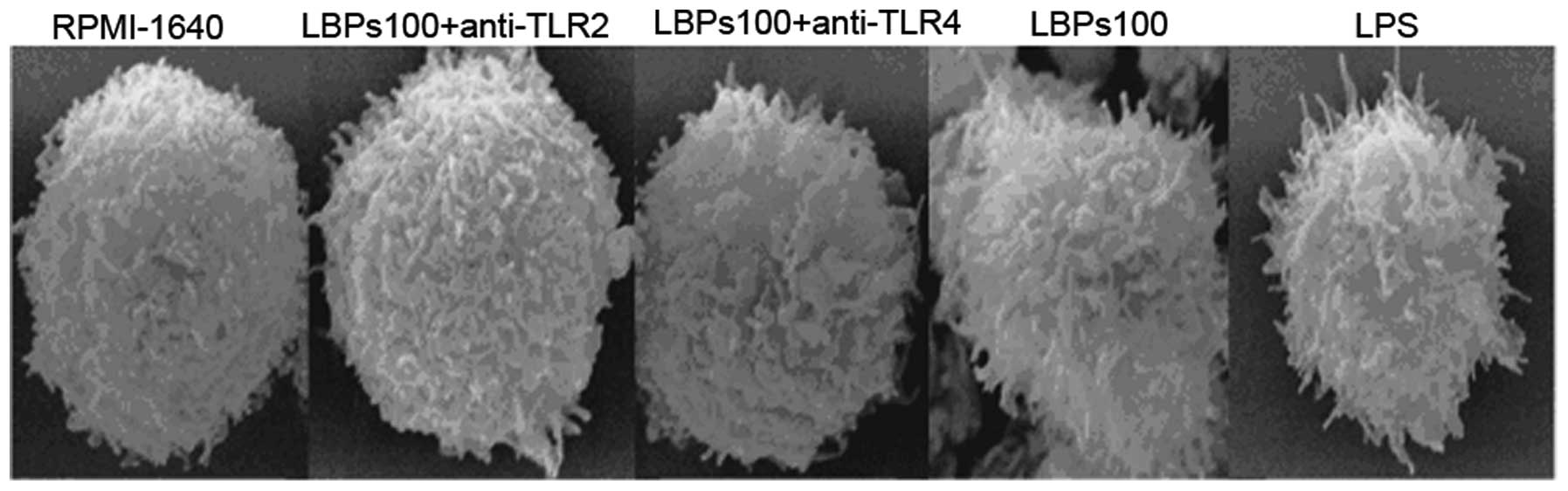
DCs treated by LBPs show a more mature
morphology in a TLR2- and 4-mediated manner
On day 7, DCs were harvested and processed for
scanning electron microscopy, as shown in Fig. 1. LBPs (100 μg/ml)- or LPS (100
ng/ml)-treated DCs showed a more mature morphology with long
protrusions. Untreated DCs, LBPs plus anti-TLR2 and LBPs plus
anti-TLR4-treated DCs had shorter protrusions than the LBPs- or
LPS-treated DCs.
LBPs upregulate the co-expression of
I-A/I-E and CD11c on the DC surface in a TLR2- and 4-mediated
manner
LBPs (100 μg/ml) or LPS (100 ng/ml)-treated DCs
showed an increased co-expression of I-A/I-E and CD11c on the DC
surface, and the double positive cell ratios were 57.4±7.2 or
65.2±9.0%, respectively, compared with 38.7±4.8% in the RPMI-1640
DCs. Additionally, LBPs (100 μg/ml) plus anti-TLR2 or LBPs (100
μg/ml) plus anti-TLR4 showed a decreased co-expression of I-A/I-E
and CD11c on the DC surface, and the double positive cell ratios
were 45.4±6.3 or 42.3±5.0%, respectively, compared with cells
treated with LBPs only. The difference was significant according to
the paired t-test analysis (n=5, P<0.01). One group of flow
cytometric analysis is shown (Fig.
2).
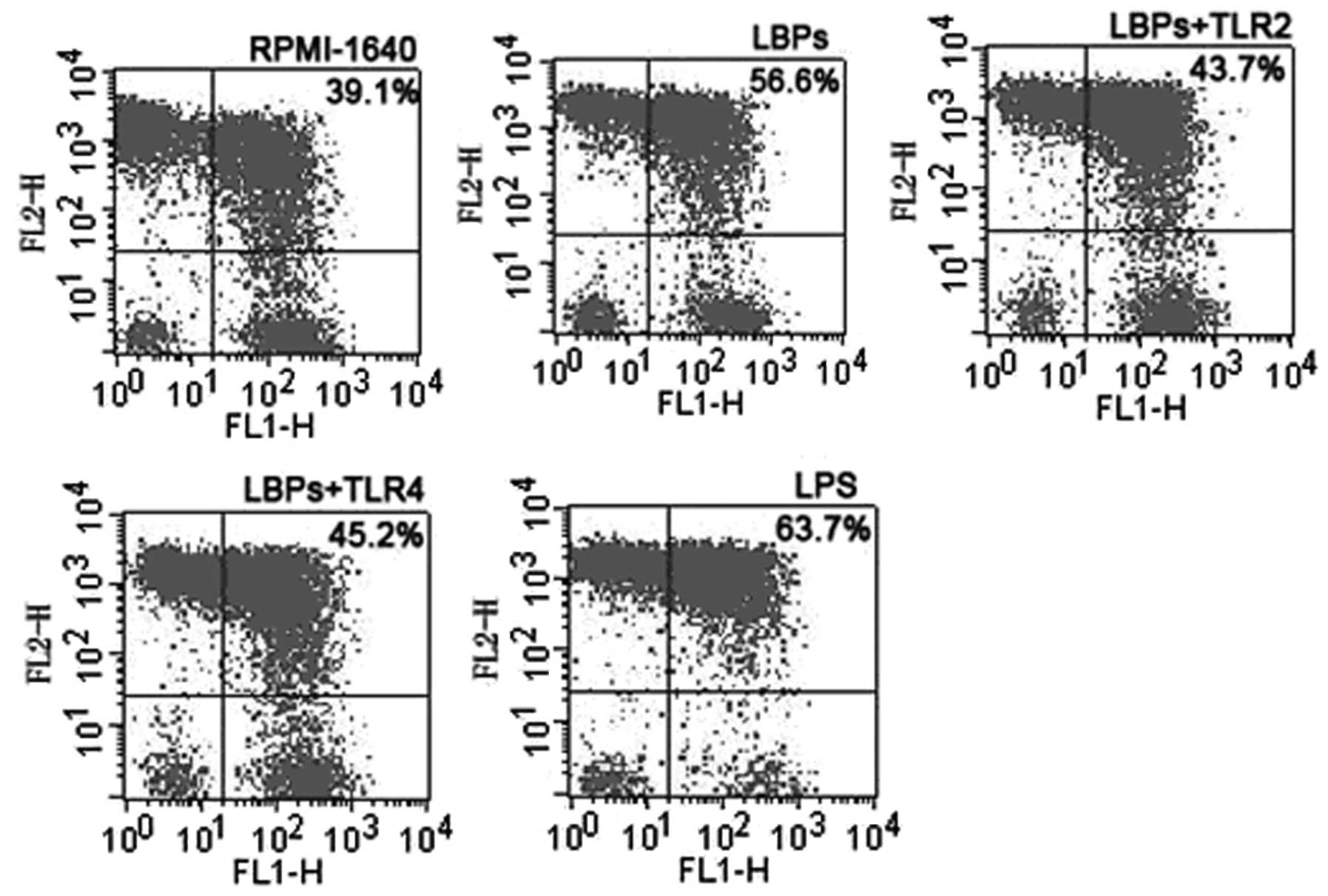 | Figure 2Representative flow cytometric
analysis of DCs treated with RPMI-1640, LBPs, LBPs plus anti-TLR2
or anti-TLR4 Ab, and LPS. On day 5 of DC culture, cells were
incubated at a concentration of 1×105 cells per well
with 100 ng/ml LPS, 100 μg/ml LBPs, LBPs plus anti-TLR2 and
anti-TLR4 Ab and RPMI-1640. On day 7, cells were stained with R-PE
conjugated anti-mouse antibody. DCs, dendritic cells; LBP,
Lycium barbarum polysaccharides; LPS,
lipopolysaccharide. |
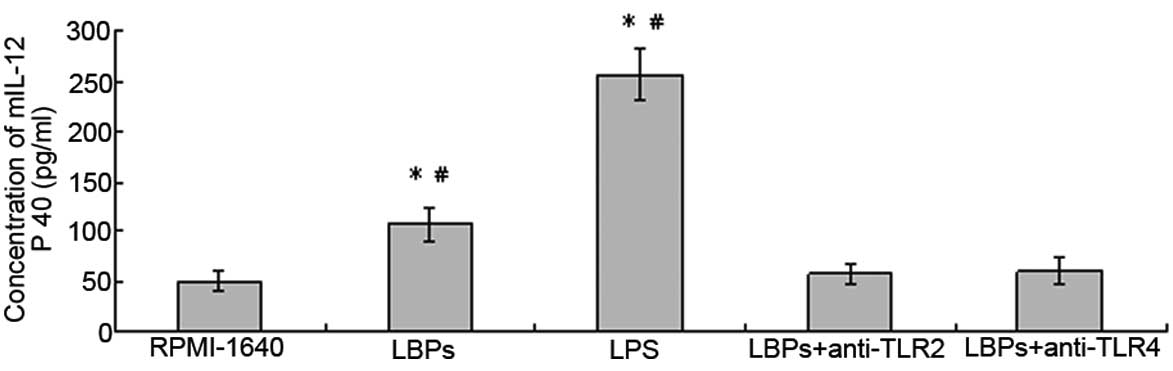
LBPs increase IL-12 p40 production of DCs
in a TLR2- and 4-mediated manner
LBPs (100 μg/ml) or LPS (100 ng/ml)-treated DCs
showed an increased production of IL-12 p40 in culture
supernatants, and the levels were 107.2±16.9 and 257.3±24.9 pg/ml,
respectively, compared with 49.6±10.0 pg/ml for RPMI-1640 control
DCs. Additionally, LBPs plus anti-TLR2- or LBPs plus
anti-TLR4-treated DCs did not show a marked difference in
production of IL-12 p40 in culture supernatants, and the levels
were 57.4±11.4 or 60.2±14.6 pg/ml, compared with the LBPs only
group (107.2±16.9 pg/ml). The difference was significant according
to the paired t-test analysis (n=5, P<0.05; Fig. 3).
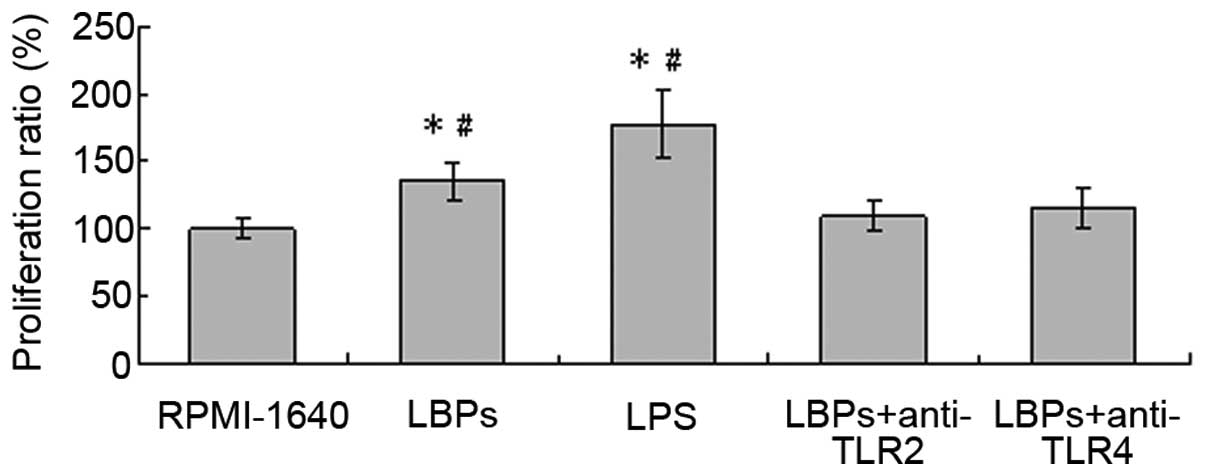
LBPs facilitate the allostimulatory
capacity of DCs in a TLR2- and 4-mediated manner
The effects of LBPs on the MLR induced by DCs were
illustrated in Fig. 4. LBPs (100
μg/ml)-treated DCs or LPS (100 ng/ml)-treated DCs stimulated
proliferative responses more effectively than the RPMI-1640-treated
DCs, and the proliferation ratio of the lymphocytes was 135.7±13.8
or 177.3±24.9%, respectively, compared with 100±7.0% for
RPMI-1640-treated cells only. Additionally, LBPs-treated DCs
stimulated proliferative responses more effectively than LBPs plus
anti-TLR2- or LBPs plus anti-TLR4-treated DCs, and the
proliferation ratio of the lymphocytes was 109.9±11.7 or
114.9±15.1% (Fig. 4). The
difference was significant according to the paired Student’s t-test
analysis (n=5, P<0.05). RPMI-1640 control served as 100%.
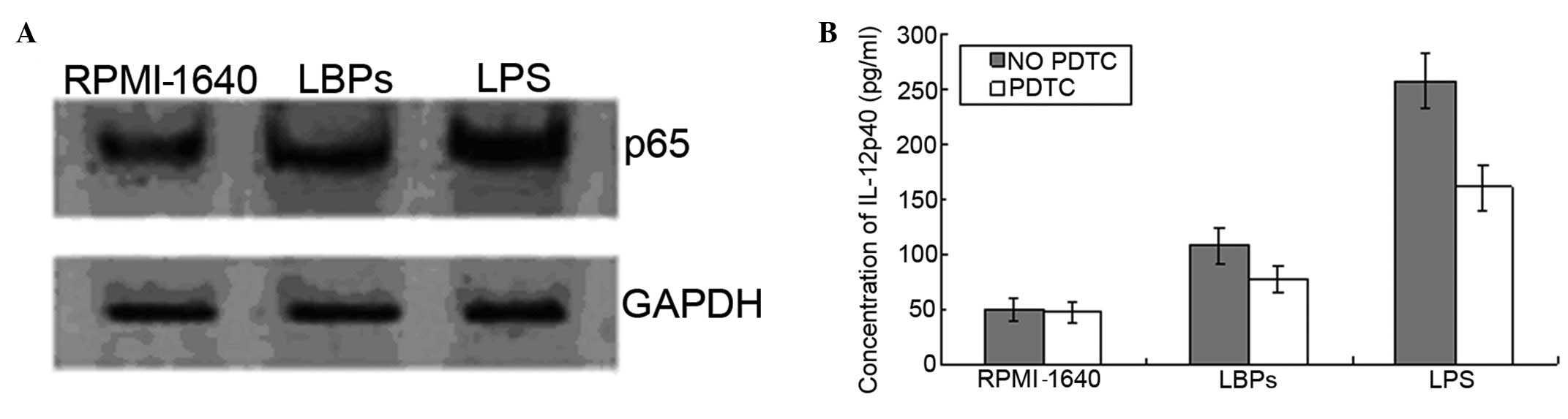
Activation of NF-κB in DCs treated with
LBPs
To elucidate whether the NF-κB pathway was activated
by LBPs in DCs, DCs were treated with LBPs (100 μg/ml) or LPS (100
ng/ml), and activation of the NF-κB pathway was measured as nuclear
translocation of the NF-κB p65 subunit by western blot analysis.
The content of p65 in the nuclear extract increased significantly
in response to LBPs (100 μg/ml) treatment as compared with that in
the nuclear extract of the untreated DCs (Fig. 5).
Discussion
It has been known for decades that LBPs are
biologically active components of Lycium barbarum that have
potential pharmacological and biological functions. DCs are potent
antigen-presenting cells that initiate primary immune responses and
antigen-specific adaptive immunity (1). DC immunogenicity correlates to its
functionally mature state. DCs can differentiate from immature to
mature stages by stimulation with microbial products (such as LPS)
or inflammatory cytokines (such as TNF) (24–26).
However, these stimulators are toxic and have limited applications.
It has been reported that a number of polysaccharides in plants,
including Ganoderma lucidum polysaccharides, Phellinus
linteus polysaccharides and Coriolus versicolor
polysaccharides, may stimulate the induction of DC maturation
(27–29).
In the present study, we have showed that LBPs are
capable of inducing the maturation of BM-derived DCs. LBPs
significantly increased membrane molecules, including CD11c and
I-A/I-E, and IL-12 in DCs. LBPs could also help DC to strengthen
the activation of the proliferation of allogenic lymphocytes and
make DC demonstrate a characteristic morphology. However, the
molecular basis of the signal transduction pathway activated by the
polysaccharides is not clearly understood. In particular, it is
unknown which cell surface receptors played a role in signal
transduction. TLRs are a class of proteins that play a key role in
the recognition of invading pathogens and activation of cytokine
production by DCs and macrophages. The members of the TLR family
play different roles in PRR signaling. Furthermore, it has been
suggested that different mechanisms may control IL-12 and TNF-α
production (30–32). The stimulation of TLRs leads to the
activation of several transcription factors, including NF-κB. In
this study, it was shown that the stimulation of murine DCs by LBPs
via TLR2 and/or TLR4 resulted in the activation of NF-κB. NF-κB
activation is necessary for the expression of a variety of
cytokines in the proinflammatory cytokine and LPS responses. At the
same time, we used PDTC, a potent NF-κB inhibitor, to inhibit
LBPs-induced NF-κB p65 subunit nuclear translocation, as well as to
cause the downregulation of IL-12 secretion. This suggested that
the NF-κB pathway is important in the LBPs-mediated TLR signaling
in DCs.
LBPs have been widely used as an injection in
clinical patients in China to improve immune functions. Our study
makes the mechanism involved in this process clearer than has
previously been known. According to the results of this study, LBPs
are effective in the induction of the phenotypic and functional
maturation of DCs, and are effective for anti-tumor DC-based
therapy.
References
|
1
|
Banchereau J, Briere F, Caux C, et al:
Immunobiology of dendritic cells. Annu Rev Immunol. 18:767–811.
2000. View Article : Google Scholar
|
|
2
|
Banchereau J and Steinman RM: Dendritic
cells and the control of immunity. Nature. 392:245–252. 1998.
View Article : Google Scholar
|
|
3
|
Lanzavecchia A and Sallusto F: Regulation
of T cell immunity by dendritic cells. Cell. 106:263–266. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Liu YJ: Dendritic cell subsets and
lineages, and their functions in innate and adaptive immunity.
Cell. 106:259–262. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Austyn JM, Kupiec-Weglinski JW, Hankins DF
and Morris PJ: Migration patterns of dendritic cells in the mouse.
Homing to T cell-dependent areas of spleen, and bingding within
marginal zone. J Exp Med. 167:646–651. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Rock KL: A new foreign policy: MHC class I
molecules monitor the outside world. Immunol Today. 17:131–137.
1996. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
De Smedt T, Pajak B, Muraille E, et al:
Regulation of dendritic cell numbers and maturation by
lipopolysaccharide in vivo. J Exp Med. 184:1413–1424.
1996.PubMed/NCBI
|
|
8
|
Cella M, Sallusto F and Lanzavecchia A:
Origin, maturation and antigen presenting function of dendritic
cells. Curr poin Immunol. 9:10–16. 1997. View Article : Google Scholar
|
|
9
|
Salio M, Cerundolo V and Lanzavecchia A:
Dendritic cell maturation is induced by mycoplasma infection but
not by necrotic cells. Eur J Immunol. 30:705–708. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kolb-Maurer A, Gentschev I, Fries HW, et
al: Listeria monocytogenes-infected human dendritic cells:
uptake and host cell response. Infect Immun. 68:3680–3686. 2000.
View Article : Google Scholar
|
|
11
|
Marovich MA, McDowell MA, Thomas EK and
Nutman TB: IL-12p70 production by Leishmania major-harboring human
dendritic cells is a CD40/CD40 ligand-dependent process. J Immunol.
164:5858–5865. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Shao P, Zhao LH, Zhi-Chen, et al:
Regulation on maturation and function of dendritic cells by
Astragalus mongholicus polysaccharides. Int Immunopharmacol.
6:1161–1166. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Aliprantis AO, Yang RB, Mark MR, et al:
Cell activation and apoptosis by bacterial lipoproteins through
toll-like receptor-2. Science. 285:736–739. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Brightbill HD, Libraty DH, Krutzik SR, et
al: Host defense mechanisms triggered by microbial lipoproteins
through toll-like receptors. Science. 285:732–736. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Hirschfeld M, Kirschning CJ, Schwandner R,
et al: Cutting edge: inflammatory signaling by Borrelia
burgdorferi lipoproteins is mediated by toll-like receptor 2. J
Immunol. 163:2382–2386. 1999.PubMed/NCBI
|
|
16
|
Takeuchi O, Kaufmann A, Grote K, et al:
Cutting edge: preferentially the R-stereoisomer of the mycoplasmal
lipopeptide macrophage-activating lipopeptide-2 activates immune
cells through a toll-like receptor 2- and MyD88-dependent signaling
pathway. J Immunol. 164:554–557. 2000. View Article : Google Scholar
|
|
17
|
Hoshino K, Takeuchi O, Kawai T, et al:
Cutting edge: toll-like receptor 4 (TLR4)-deficient mice are
hyporesponsive to lipopolysaccharide: evidence for TLR4 as the Lps
gene product. J Immunol. 162:3749–3752. 1999.PubMed/NCBI
|
|
18
|
Arbour NC, Lorenz E, Schutte BC, et al:
TLR4 mutations are associated with endotoxin hyporesponsiveness in
humans. Nat Genet. 25:187–191. 2000. View
Article : Google Scholar : PubMed/NCBI
|
|
19
|
Krieg AM and Waqner H: Causing a commotion
in the blood: immunotherapy progresses from bacteria to bacterial
DNA. Immunol Today. 21:521–526. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
May MJ and Ghosh S: Signal transduction
through NF-κB. Immunol Today. 19:80–88. 1998.
|
|
21
|
Yang RB, Mark MR, Gurney AL and Godowski
PJ: Signaling events induced by lipopolysaccharide-activated
toll-like receptor 2. Immunol. 163:639–643. 1999.PubMed/NCBI
|
|
22
|
Zhu J, Zhao LH, Zhao XP, et al: Lycium
barbarum polysaccharides regulate phenotypic and functional
maturation of murine dendritic cells. Cell Biol Int. 31:615–619.
2007. View Article : Google Scholar
|
|
23
|
Inaba K, Inaba M, Romani N, Aya H, et al:
Generation of large numbers of dendritic cells from mouse bone
marrow cultures supplemented with granulocyte/macrophage
colony-stimulating factor. J Exp Med. 176:1693–1702. 1992.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Sallusto F and Lanzavecchia A: Efficient
presentation of soluble antigen by cultured human dendritic cells
is maintained by granulocyte/macrophage colony-stimalating factor
plus interleukin 4 and downregulated by tumor necrosis factor α. J
Exp Med. 179:1109–1118. 1994.PubMed/NCBI
|
|
25
|
Winzler C, Rovere P, Resciqno M, et al:
Maturation stages of mouse dendritic cells in growth
factor-dependent long-term cultures. J Exp Med. 185:317–328. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Roake JA, Rao AS, Morris PJ, et al:
Dendritic cells loss from nonlymphoid tissues after systemic
administration of lipopolysaccharides, tumor necrosis factor, and
interleukin 1. J Exp Med. 181:2237–2247. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Cao LZ and Lin ZB: Regulation on
maturation and function of dendritic cells by Ganoderma lucidum
polysaccharides. Immunol Lett. 83:163–169. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Park SK, Kim GY, Lim JY, et al: Acidic
polysaccharides isolated from Phellinus linteus induce phenotypic
and functional maturation of murine dendritic cells. Biochem
Biophys Res Commun. 312:449–458. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kanazawa M, Mori Y, Yoshihara K, et al:
Effect of PSK on the maturation of dendritic cells derived from
human peripheral blood monocytes. Immunol Lett. 91:229–238. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Means TK, Pavlovich RP, Roca D, et al:
Activation of TNF-α transcription utilizes distinct MAP kinase
pathways in different macrophage populations. J Leukoc Biol.
67:885–893. 2000.
|
|
31
|
Martin M, Michalek SM and Katz J: Role of
innate immune factors in the adjuvant activity of monophosphoryl
lipid A. Infec Immun. 71:2498–2507. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Tapping RI, Akashi S, Miyake K, et al:
Toll-like receptor 4, but not toll-like receptor 2, is a signaling
receptor for Escherichia and Salmonella
lipopolysaccharides. Immunol. 165:5780–5787. 2000. View Article : Google Scholar : PubMed/NCBI
|