Introduction
Glaucoma is the most common cause of irreversible
blindness worldwide. It is an optic neuropathy characterized by
structural damage to the optic nerve and the associated visual
dysfunction that may be caused by various pathological processes
(1). The most common form of
glaucoma is primary open-angle glaucoma (POAG), in which a high
intraocular pressure is the most critical risk factor in the
pathogenesis. The early onset of POAG is asymptomatic and the
majority of patients only seek medical treatment when visual
function has been damaged (2). The
treatment of POAG involves reducing the intraocular pressure to
protect the residual vision. Therefore, the elucidation of the
pathogenesis of primary open-angle glaucoma and the development of
effective treatments have become top priorities.
Rapid developments in cell and molecular biology in
recent years have revealed that the intraocular pressure in POAG is
caused by a resistance to the aqueous outflow, which comes
primarily from the trabecular meshwork (TM) (3). The form, number of changes, decline
in the phagocytic function, extracellular matrix deposits, abnormal
cell connections, actin cytoskeleton and cell shrinkage status of
the TM cells also affect the actin cytoskeleton (4). Increasing the crosslinking of the
actin cytoskeleton may reinforce the TM and increase the aqueous
outflow; however, this increased outflow may result in an
obstruction and increase the intraocular pressure, thereby damaging
the normal structure and function, and leading to the development
of POAG (5,6). Therefore, an investigation of the
characteristics of the TM cells in culture is important in the
study of the pathogenesis of glaucoma.
In recent years, the number of studies concerning
the ion channels formed in POAG, particularly the involvement of
chloride channels has increased. Chloride channels (CLCs) are
widely distributed in mammalian organs and cells, and function to
maintain the volume of cells, adjust the cell electrical activity
balance and mediate cell migration and other functions that impact
cell physiology and pathology (7,8).
Several chloride channels have been cloned, including CLC-1 to
CLC-7, CLC-ka and CLC-kb. TM cells express CLC-2, CLC-3 and CLC-5.
However, in TM cells, CLC-2 is the most prevalent channel of the
CLC family and is upregulated following cell swelling (9). This channel is activated by membrane
hyperpolarization in other cell types; due to the activation of
CLC-2 by cell swelling, this protein has been implicated in cell
volume regulation (10–12). In addition to cell swelling, CLC-2
may be involved in several cell mechanisms, including signal
transduction, transepithelial transport, phagocytosis and membrane
potential stabilization. In addition, CLC-2 may be important in
maintaining the volume of TM cells by adjusting the outflow of
aqueous solutions and maintaining the fluid balance (13). However, little is understood
concerning the functions of CLC-2 in the cytoskeleton in TM
cells.
In the present study, human TM cells (HTM) were
cultured to investigate the effects of CLC-2 on the function and
restructuring of the cytoskeleton to clarify the effects of CLC-2
on the pathogenesis of POAG.
Materials and methods
siRNA design
The candidate nucleotide sequence for the siRNA was
obtained by searching the nucleotide sequences of CLC-2 using the
Rational siRNA Design program (Qiagen, Hilden, German). A scrambled
siRNA with the same nucleotide composition as the siRNA, but
lacking significant sequence homology to the genome, was also
designed. These oligonucleotides were ligated into the siXpress
Human U6 PCR vector system (Mirus Bio, Madison, WI, USA) according
to the manufacturer’s instructions. The sequences of the siRNAs
targeting CLC-2 were as follows: Sense: 5′-TCCTGTGAGAAGCTTCTCAC-3′
and antisense: 5′-GTGAGAAGCTTCTCACAGGA-3′ for siRNA1; sense:
5′-CCATGCTTATGTCACCAG-3′ and antisense:
5′-GGTACGAAATCACAGAGTGGTC-3′ for siRNA2; and sense:
5′-CACTCTTCGAAGAGTGTCCT-3′ and antisense:
5′-GTGAGAAGCTTCTCACAGGA-3′ for scRNA.
Cell line and cell culture
HTM cells highly express CLC-2. The cell line was
purchased from ScienCell (ScienCell Research Laboratories, San
Diego, CA, USA). The cells were maintained and cultured in
Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10%
fetal bovine serum (ScienCell Research Laboratories), 100 IU/ml
penicillin, 100 IU/ml streptomycin and 2 mmol/l L-glutamine in a
humidified 5% CO2 atmosphere at 37°C (14). The cells were counted in
suspensions using a Cedex analyzer (Innovatis AG, Bielefeld,
Germany).
Transfection
The transfection was performed in a Lipofectamine
2000 system (Invitrogen Life Technologies, Grand Island, NY, USA),
according to the manufacturer’s instructions, using a 5 μl
expression vector in 250 μl serum-free medium. The concentration of
each siRNA used was 100 pmol in 250 μl medium (33 nM). After 6 h,
the medium was replaced with fresh serum-containing DMEM and
incubated for an additional 24 h.
Real time-PCR
Human CLC-2, actin, vinculin, β-catenin and β-actin
(housekeeping gene, control) PCR primers were designed using Primer
Express software (Perkin-Elmer Biosystems, Waltham, MA, USA) based
on their published sequences (Table
I). The total RNA of the HTM cells was isolated with TRIzol
reagent (Invitrogen Life Technologies, Grand Island, NY, USA)
according to the manufacturer’s instructions. To avoid DNA
contamination, the total RNA was treated with RNase-free DNase I
(Takara, Kyoto, Japan) for 1 h at 37°C and extracted again with
TRIzol reagent. The RNA purity was determined from the ratio of the
absorbances at 260:280 nm and the RNA integrity was assessed by
determining the intensity of the 28S and 18S rRNA bands following
formaldehyde agarose gel electrophoresis. A sample of the total RNA
(2 μg) was subjected to reverse transcription using the RevertAid™
First-Strand cDNA Synthesis kit (Fermentas Inc., Glen Burnie, MD,
USA) with a random hexamer primer and 2 μl cDNA solution was used
for real-time PCR. The genes were amplified in a 25 μl reaction
volume using SYBR-Green (Applied Biosystems, Forster City, CA, USA)
on a MiniOpticon™ Real-time PCR System (Bio-Rad, Hercules, CA,
USA). The temperature profile involved an initial denaturation step
at 95°C for 5 min, followed by 40 cycles at 95°C for 10 sec, 58°C
for 15 sec and 72°C for 10 sec, and a melting curve analysis was
performed. The specificity of the amplified products was evaluated
via agarose gel electrophoresis and was further verified with
automated cycle sequencing. To ensure consistency in the threshold
cycle (Ct) values, duplicate reactions were performed, and the mean
Ct values were used for calculating the relative expression levels
(14). The Ct values were analyzed
as described previously by Zhou et al(15), and the normalized Ct values for
each gene were subjected to Student’s t-test with a two-tailed
distribution to determine the statistical significance (95%
confidence interval). The reactions were conducted in triplicate
and the mean value was used. For standardization, β-actin was used
as an internal control for each sample.
 | Table IPCR primers. |
Table I
PCR primers.
| Gene | Primer sequences
(from 5′ to 3′) | Product size
(bp) |
|---|
| CLC-2 | Forward:
TCCTCACCCTGGTCATCTTC
Reverse: GCAGGTAGGGCAGTTTCTTG | 402 |
| Actin | Forward:
GGTGAAGGTCGGAGTCAAC
Reverse: CCATGGGTGGAATCATATTG | 153 |
| Vinculin | Forward:
AAGCTGTCAAGCCGTGTTC
Reverse: GCCTTCCGAGTCAGTTTCA | 401 |
| β-catenin | Forward:
AGGACTGGGTGCTGGTTATG
Reverse: CAGCAGCTAGTCTCGCATTG | 398 |
| β-actin | Forward:
ATCATGTTTGAGACCTTCAACA
Reverse: CATCTCTTGCTCGAAGTCCA | 318 |
Western blot analysis
Following transfection, the cells were washed twice
with pre-cooled phosphate-buffered saline (PBS) and 106 cells were
treated with RIPA buffer [50 mM Tris (pH 8.0), 150 mM NaCl, 0.1%
SDS, 1% NP40 and 0.5% sodium deoxycholate] containing a protease
inhibitor (1% cocktail and 1 mM PMSF). The total proteins were
separated on a 15% sodium dodecyl sulfate-polyacrylamide gel
electrophoresis gel and transferred onto polyvinylidene fluoride
(PVDF) membranes. The membrane was blocked with Tris-buffered
saline containing 0.1% Tween 20 (TBST, pH 7.6) for 1 h at room
temperature and the PVDF membrane was immunoblotted overnight at
4°C with a primary antibody solution (1:1000). Following two washes
with TBST, the membrane was incubated with horseradish
peroxidase-labeled secondary goat anti-mouse IgG2a-B antibody
(sc-2073) for 1 h at room temperature and washed three times with
TBST. The final detection was performed with enhanced
chemiluminescence (ECL) western blotting reagents (GE Healthcare,
Piscataway, NJ, USA) and the membranes were exposed to Lumi-Film
Chemiluminescent Detection Film (Roche Applied Science, Rotkreuz,
Switzerland) (16). The loading
differences were normalized using a monoclonal β-actin antibody.
The primary antibodies used in this study included anti-CLC-2
(SC-81871), anti-actin (SC-81760), anti-vinculin (SC-55465),
anti-β-catenin (SC-7963), β-actin (SC-130301), TGF-β (SC-166833)
and Smad2 (SC-101153). All antibodies were acquired from Santa Cruz
Biotechnology, Inc. (Santa Cruz, CA, USA).
Fluorescence microscopy
The cells were fixed and subjected to
immunofluorescent staining of the cytoskeletal proteins. Following
a brief rinse in PBS at 37°C, the cells were fixed with 3%
paraformaldehyde in PBS for 20–30 min at room temperature.
Subsequent to fixation, the cells were rinsed with PBS and
permeabilized with 0.5% Triton X-100 in PBS for 5 min. When a
higher degree of permeabilization was required (e.g., for myosin II
visualization in the HTM), the cells were initially fixed with a
mixture containing 0.5% Triton X-100 and 3% paraformaldehyde in PBS
for 2–3 min and then post-fixed with 3% paraformaldehyde for 20
min. To visualize β-actin, the cells were subsequently incubated
with 100 nM TRITC-phalloidin (Sigma-Aldrich, St. Louis, MO, USA).
For antibody staining, the cells were incubated with primary
antibodies diluted in PBS. The cells were then washed in PBS three
times and incubated with the secondary fluorochrome-conjugated
antibodies. The nuclei were stained with 2.5 mg/ml DAPI
(Sigma-Aldrich), which was added to the secondary antibody solution
(17). Following three final
washes, the coverslips were mounted in Elvanol (Mowiol®
4–88; Sigma-Aldrich, Hoechst, Germany). The primary antibodies used
for this method were the same as those used for the western blot
analysis.
Statistical analysis
All the measurements were performed in triplicate
and the results are expressed as the mean ± SD. The data were
obtained from at least three independent experiments. An analysis
of variance for multiple comparisons was conducted using
statistical analysis software (SPSS, Inc., Chicago, IL, USA).
P<0.05 was considered to indicate a statistically significant
difference (16).
Results
Inhibition of CLC-2 mRNA and protein
expression by siRNA
It was hypothesized that by preventing the
transcription of CLC-2 mRNA via siRNA, the CLC-2 protein levels are
significantly reduced. Real time-PCR and western blot analysis were
used to detect the effects of CLC-2 on target gene expression in
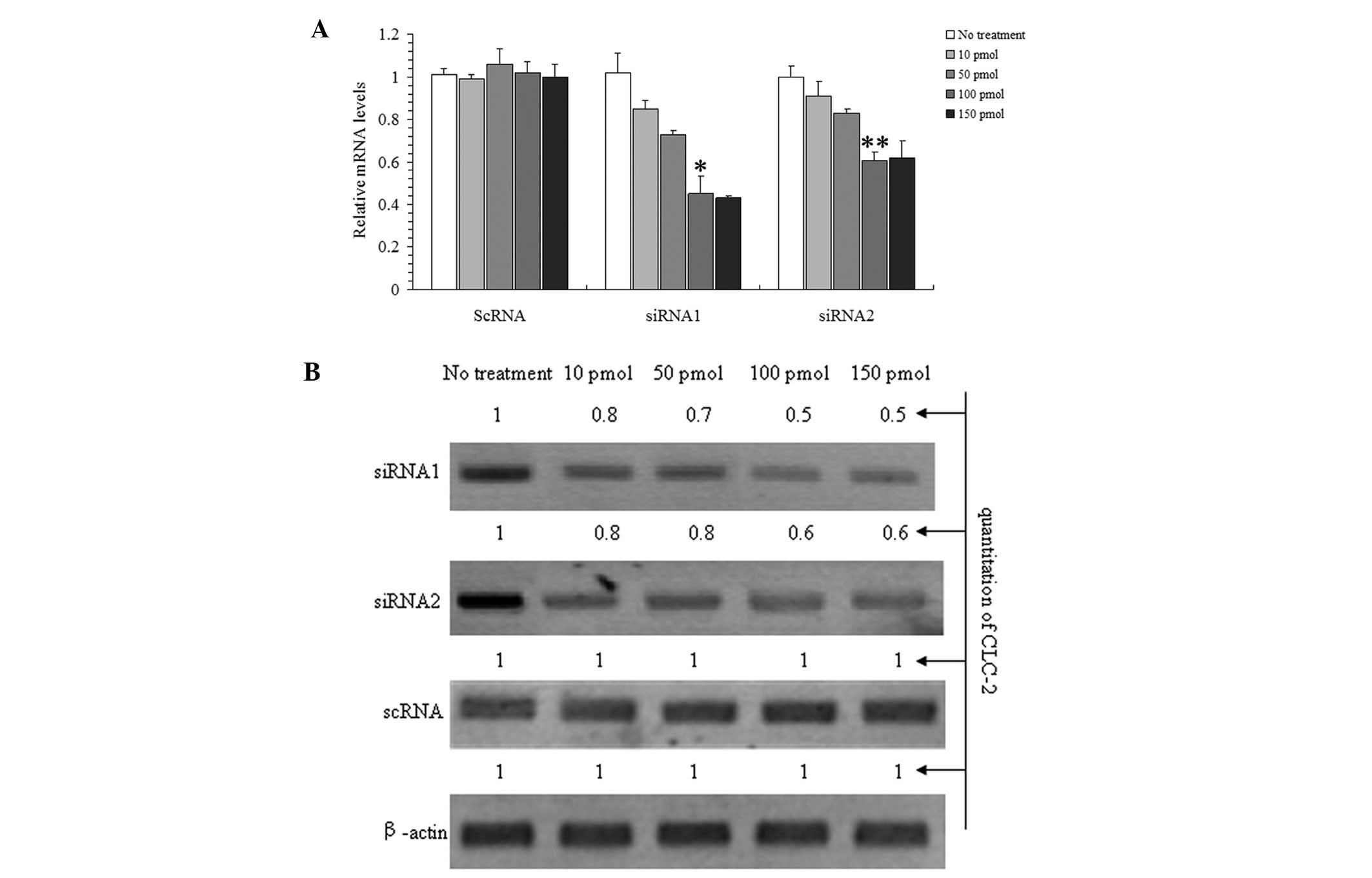
response to various concentrations (Fig. 1) to determine the optimal
incubation concentration and the effect of the treatment length
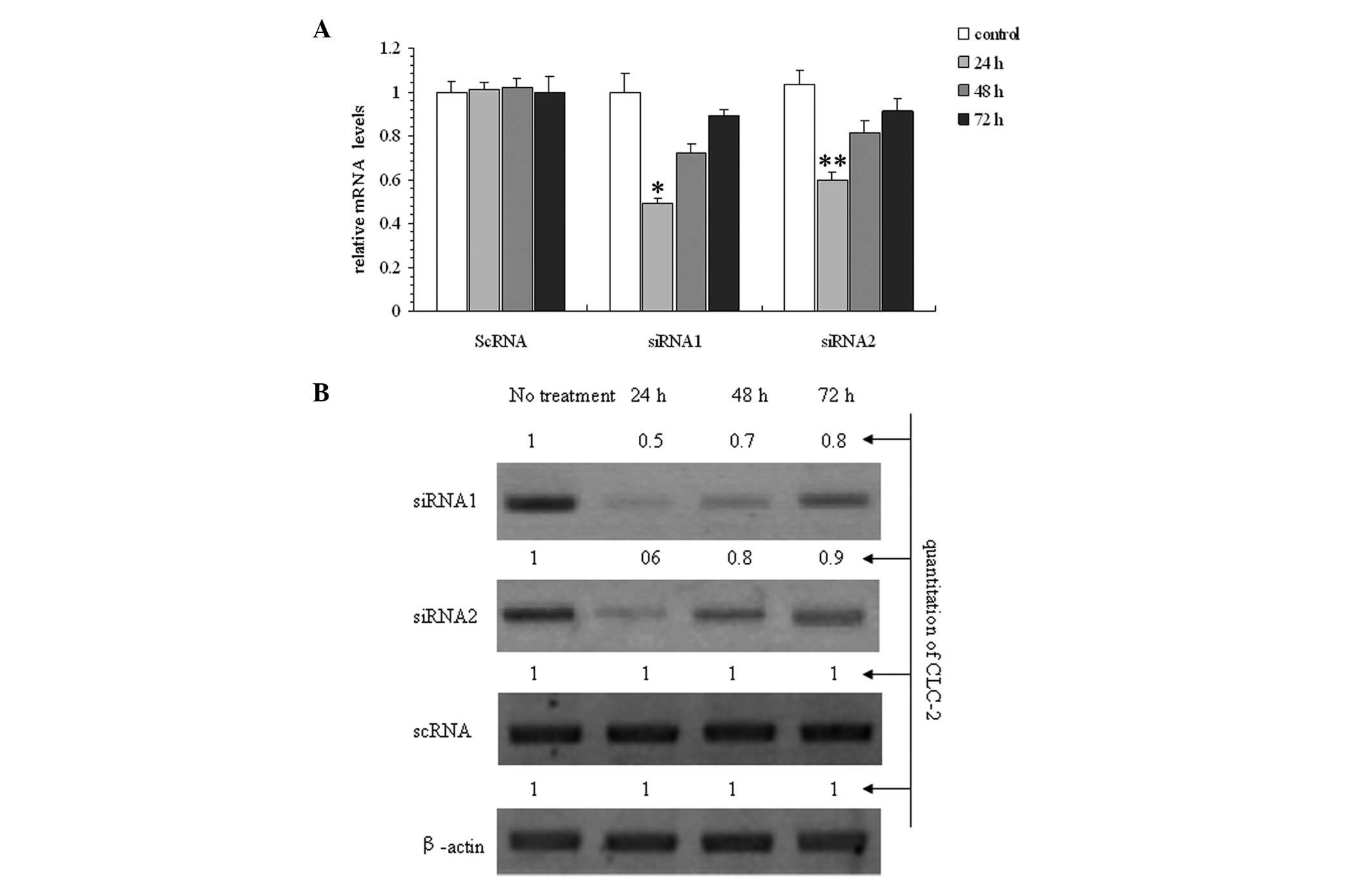
(Fig. 2).
Fig. 1A and B show
that siRNA1 and 2 were able to inhibit CLC-2 mRNA and protein
expression concomitantly. These effects were concentration
dependent as the CLC-2 mRNA and the protein expression levels
decreased with increasing concentrations of the siRNAs following 24
h of treatment. Furthermore, no significant additional effect was
observed at the highest concentration; therefore, 100 pmol siRNA
was determined to be the optimal concentration. Fig. 2A and B show a time-dependent
response to the incubation with 100 pmol siRNAs following 24, 48
and 72 h of treatment with CLC-2-siRNA. The optimal time was
determined to be 24 h. The results show that siRNA1 was the most
effective siRNA at reducing the CLC-2 mRNA and protein expression
levels.
CLC-2 siRNA increases the expression
levels of actin, vinculin and β-catenin
Actin, vinculin and β-catenin were observed in
normal HTM cell cultures and in transfected CLC-2-siRNA1 HTM cells
by fluorescence microscopy, real time-PCR and western blot
analysis. The predominant actin stress fibers were observed in
almost all cells and were primarily aligned along the longitudinal
axis of the cells (Fig. 3A). In
the siRNA1 treated cells, the stress fibers were observed in
certain cells; numerous cells contained a perinuclear, geodesic
dome-like pattern of crosslinked actin filaments (Fig. 3F), which have previously been
termed cross-linked actin networks (CLANs). Approximately half of
the cells contained a variety of CLANs, ranging from a small
perinuclear pattern to a network encompassing the entire cell
(Fig. 3F). The actin fiber bundles
were highly concentrated at the periphery of the cell, with a few
actin filaments detected in the central area (Fig. 3F). Increased vinculin staining was
also observed towards the cell periphery (Fig. 3G) compared with that in the
untreated cells (Fig. 3B and
C).
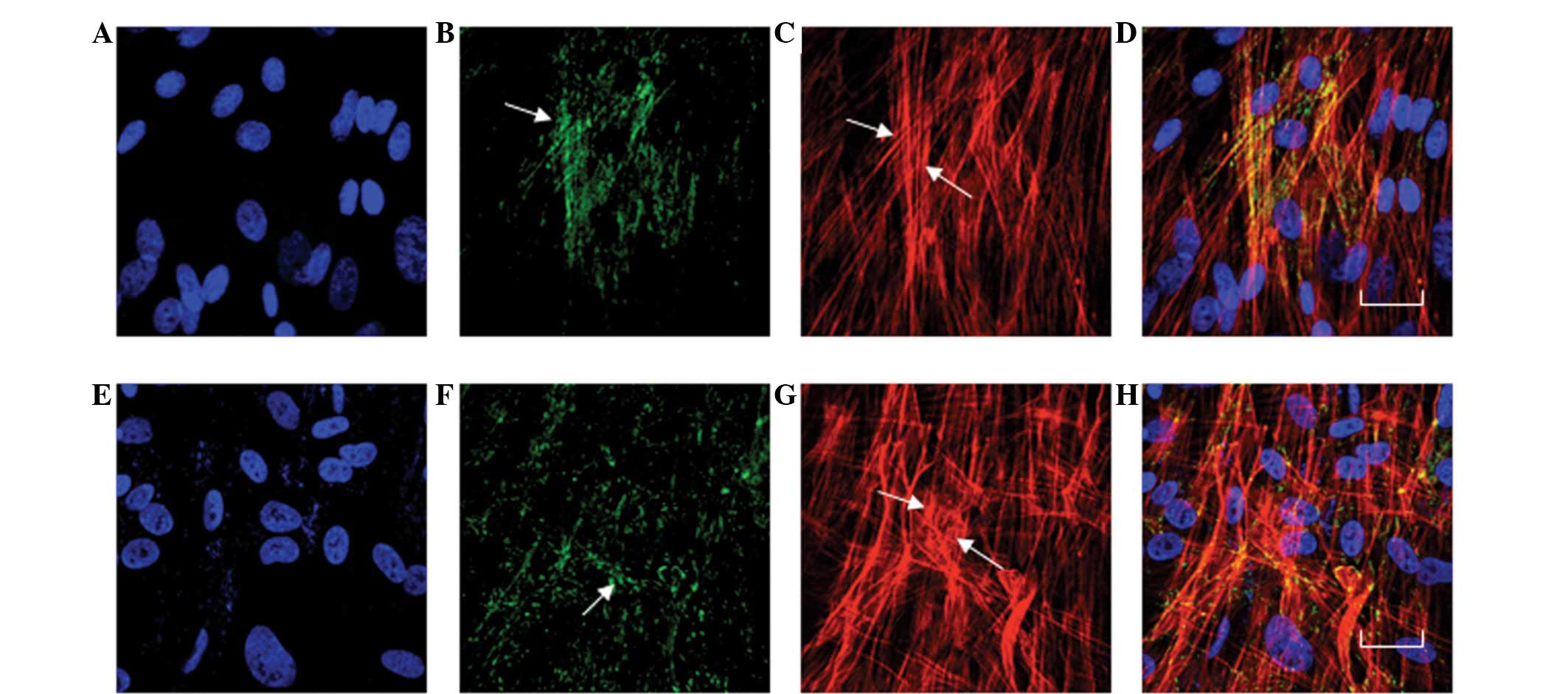 | Figure 3Effects of the CCL-2-siRNA on actin
and vinculin in cultured HTM cells. The HTM cells were treated with
(A–D) normal growth medium or (E–H) 100 pmol of the CLC-2-siRNA.
(C) In the cells treated with normal growth medium, actin stress
fibers were observed in almost all of the cells and were primarily
aligned along the longitudinal axis of the cells. (F) In the cells
treated with 100 pmol of the CLC-2-siRNA1 for 24 h, the vinculin
was also observed.(G) In the cells treated with 100 pmol of the
CLC-2-siRNA1 for 24 h, the actin fiber bundles were highly
concentrated at the periphery of the cell, while a few actin
filaments were detected in the central area. Increased vinculin
staining was also observed toward the cell periphery. (H)
CLC-2-A-siNRA treated HTM cells co-stained to visualize actin (red)
and vinculin (green). The siRNA1 induced changes are shown by
arrows. F and G, Bar=20 μm. A–D, No treatment; E–H, CLC-2 siRNA
treatment; A and E, DAPI staining; B and F, FITC staining; C and G,
TRITC staining; D and H, Co-staining; CLC-2, chloride channel
protein 2; HTM, human trabecular meshwork. |
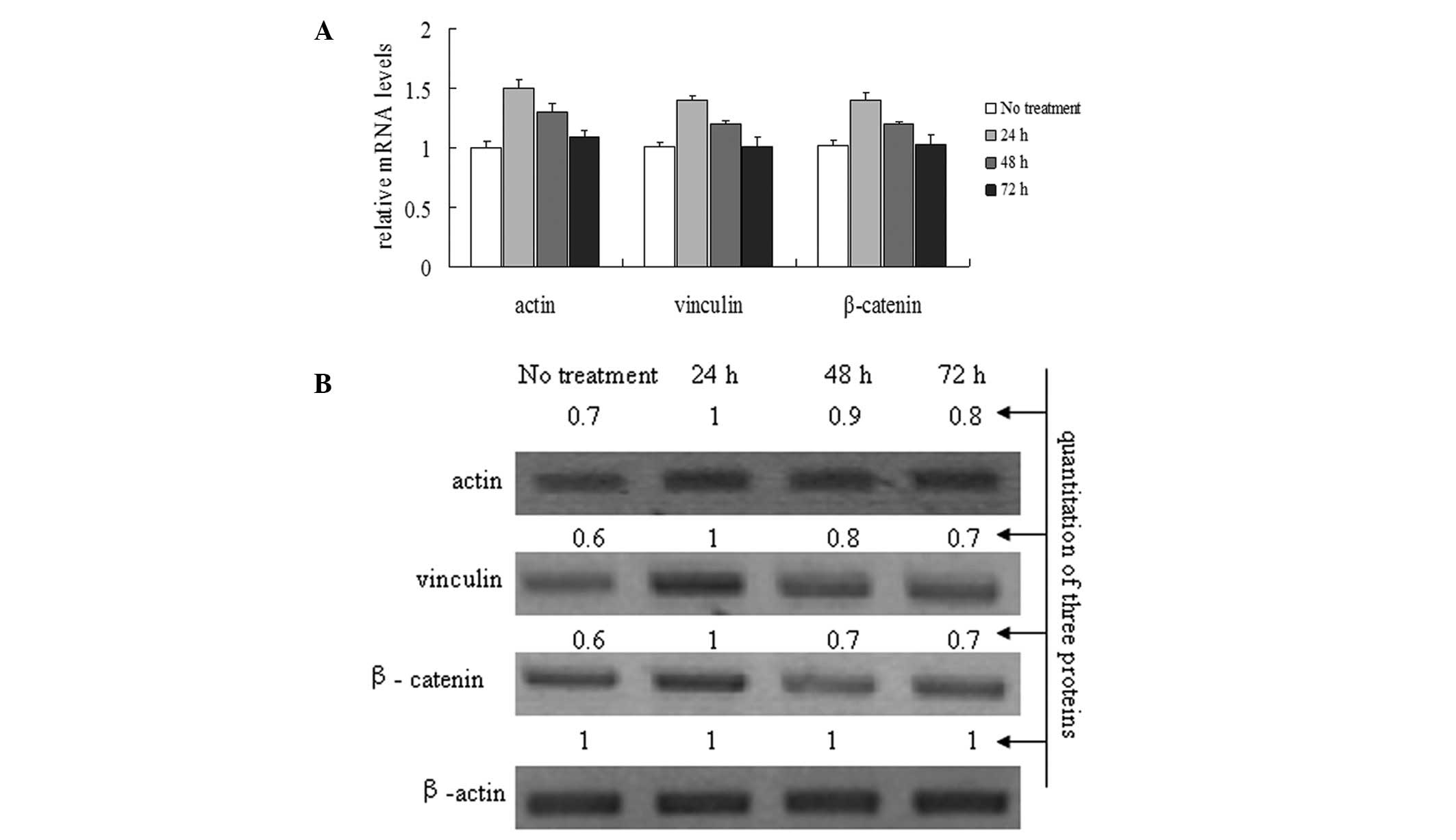
To investigate the effects of the CLC-2 siRNA1 on
actin, vinculin and β-catenin mRNA and protein expression levels in
the HTM cell line, HTM cells were treated with the CLC-2-siRNA1 for
24, 48 and 72 h. Actin, vinculin and β-catenin mRNA and protein
levels were analyzed using real time-PCR and western blot analysis
(Fig. 4). The relative
quantification results show that the HTM cells transfected with the
CLC-2 siRNA1 significantly induced the mRNA levels of actin,
vinculin and β-catenin compared with that in the control group
(Fig. 4A). Similar to the analysis
of RNA levels, the protein levels of actin, vinculin and β-catenin
were increased significantly in the siRNA1-treated cells compared
with the control group (Fig.
4B).
CLC-2 siRNA activates the TGF-β/Smad
signaling pathway in HTM cells
Transforming growth factor-β (TGF-β) is located in
increasing quantities in the aqueous humor (AH) and reactive optic
nerve astrocytes of patients with primary open-angle glaucoma
(POAG) (17). The available data
strongly indicate that TGF-β is important in contributing to the
structural changes characteristic of POAG in the extracellular
matrix of the TM and optic nerve head (18). In addition, CLC-2 may function to
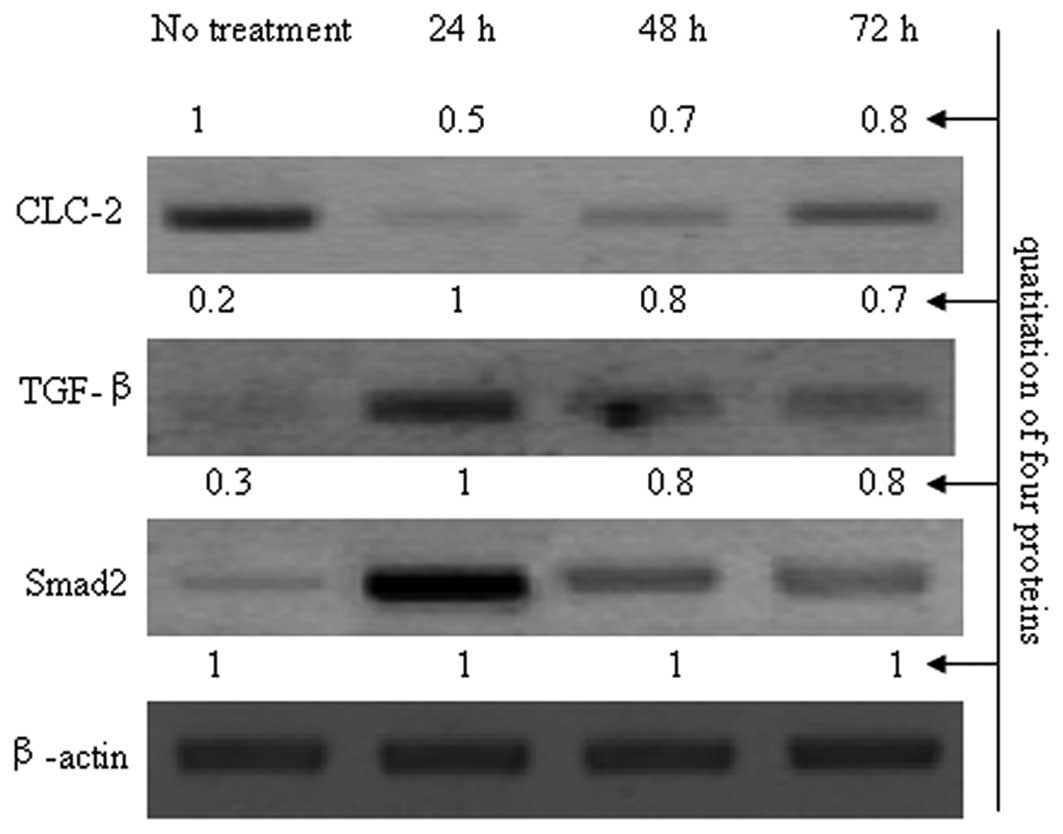
stabilize the cytoskeleton. CLC-2 siRNAs were used to inhibit CLC-2
expression and the variation in TGF-β levels was observed to
investigate the correlation between CLC-2 and TGF-β (Fig. 5). As shown in Fig. 5, compared with the control group,
the expression levels of TGF-β and Smad2 were increased when the
expression of CLC-2 was reduced by CLC-2 siRNA. Compared with the
24 h exposure period, as the inhibition effects of CLC-2 siRNA
declined, the expression levels of TGF-β and Smad2 gradually
reduced at 48 and 72 h; thus, these effects were dependent on CLC-2
expression levels. Based on these results, CLC-2 siRNA may
contribute to TM cytoskeleton disorders and may be related to the
TGF-β/Smad signaling pathway.
Discussion
In the eye, the predominant AH outflow pathway in
humans is through the TM and Schlemm’s canal, although ~20% of the
AH leaves the anterior chamber in between the ciliary muscle fibers
to reach the scleral tissue (18).
These sections of the eye express high levels of cytoskeletal
actin. The cytoskeleton is comprised of a complex network of fibers
that integrate various inclusions into the network to produce
coordination among cells. The cytoskeleton is important in
regulating cell morphology, extracellular matrix adhesion and cell
movement (19). Cells adapt to
changes in the surrounding environment by restructuring the actin
cytoskeleton. Increasing actin cytoskeleton crosslinking reinforces
the TM and increases the aqueous outflow; however, this increased
outflow may lead to an obstruction and increase the intraocular
pressure, thereby damaging the normal structure and function of the
eye and resulting in the development of POAG (20).
Voltage-gated chloride channels of the plasma
membrane are key in cell volume regulation, ionic homeostasis,
transepithelial transport and the regulation of electrical
excitability. Intracellular members of the chloride channel family
are also involved in organellar volume regulation and
electroneutrality maintenance. CLC-2 is a member of the large CLC
family of chloride-channel forming proteins and may be important in
TM cells (21–22).
In the present study, two siRNAs that target CLC-2
mRNA were designed as a potential novel genetic therapeutic
strategy to inhibit the expression of CLC-2 and affect the
cytoskeleton of HTM cells. The cytoskeleton was analyzed following
an siRNA-mediated CLC-2 knockdown. The results demonstrated that
siRNA1 and 2 were able to inhibit the expression of CLC-2. In
direct comparison, siRNA1 was shown to be more effective than
siRNA2 in downregulating the expression of CLC-2. The results also
showed that CLC-2 was related to the stability of the cytoskeleton,
as the cytoskeleton became disordered when the expression levels of
CLC were reduced in HTM cells. F-actin in vitro generally
contains two types of fiber: stress fibers that are distributed in
the parallel beam in the cells and fibers that are distributed
around the peripheral actin. Stress fibers arranged in the cell
stretch the direction of the cell and peripheral actin maintains
the form of cells and participates in the formation of the
connection between the cell and the extracellular matrix.
Fluorescence microscopy was used to observe the changes in actin
organization in the present study and the results showed that
siRNA1-mediated CLC-2 knockdown reconstructed the actin
cytoskeleton and formed CLAN structures. The reconstruction of the
actin cytoskeleton changed the zonula occludens and gap junction
protein expression levels and influenced the connection and
adhesion of the cells. These changes affected the TM structure and
function, and increased the TM aqueous outflow resistance.
TGF-β2 is the predominant TGF-β in the eye and is
located in large quantities in the aqueous humor of the anterior
eye and in the vitreous, neural retina and retinal pigmented
epithelium of the posterior eye. Only minor or non-detectable
TGF-β2 expression levels have been observed in studies of the
normal TM (17). In the aqueous
humor of patients with POAG, the quantities of TGF-β2 have been
observed to be significantly increased in several studies (19–20).
The increase in the quantity of the expression of TGF-β2 appears to
be a characteristic phenomenon in the eyes of patients with POAG
(18). In the present study, the
results demonstrated that siRNA1-mediated CLC-2 knockdown
reconstructed the TM cytoskeleton and that CLC-2 knockdown-related
cytoskeletal reconstruction may involve the TGF-β/Smad signaling
pathway. The initial analysis showed that the siRNA1-mediated CLC-2
knockdown significantly upregulated the expression levels of TGF-β
and Smad2. These results suggested that a CLC-2 knockdown may
promote TM cytoskeletal disorders and may be correlated with the
activation of the TGF-β/Smad signaling pathway.
In conclusion, the results demonstrated the
correlation between CLC-2 and the cytoskeleton in HTM cells. CLC-2
may be a promising potential novel therapeutic agent for combating
POAG, and further studies are required to confirm these
results.
Acknowledgements
This study was partially supported by the National
Natural Science Foundation of China (grant no. 81271002).
References
|
1
|
Thinda S, Melson MR and Kuchtey RW:
Worsening angle closure glaucoma and choroidal detachments
subsequent to closure of a carotid cavernous fistula. BMC
Ophthalmol. 12:282012. View Article : Google Scholar
|
|
2
|
Abdelrahman AM and Eltanamly RM: Selective
laser trabeculoplasty in Egyptian patients with primary open-angle
glaucoma. Middle East Afr J Ophthalmol. 19:299–303. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Roh M, Zhang Y, Murakami Y, et al:
Etanercept, a widely used inhibitor of tumor necrosis factor-α
(TNF-α), prevents retinal ganglion cell loss in a rat model of
glaucoma. PLoS One. 7:e400652012.
|
|
4
|
Grieshaber MC, Pienaar A, Olivier J and
Stegmann R: Clinical evaluation of the aqueous outflow system in
primary open-angle glaucoma for canaloplasty. Invest Ophthalmol Vis
Sci. 51:1498–1504. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kotliar KE, Kozlova TV and Lanzl IM:
Postoperative aqueous outflow in the human eye after glaucoma
filtration surgery: biofluidmechanical considerations. Biomed Tech
(Berl). 54:14–22. 2009. View Article : Google Scholar
|
|
6
|
Wang N, Chintala SK, Fini ME and Schuman
JS: Activation of a tissue-specific stress response in the aqueous
outflow pathway of the eye defines the glaucoma disease phenotype.
Nat Med. 7:304–309. 2001. View
Article : Google Scholar
|
|
7
|
Bomberger JM, Coutermarsh BA, Barnaby RL
and Stanton BA: Arsenic promotes ubiquitinylation and lysosomal
degradation of cystic fibrosis transmembrane conductance regulator
(CFTR) chloride channels in human airway epithelial cells. J Biol
Chem. 287:17130–17139. 2012. View Article : Google Scholar
|
|
8
|
Zhu L, Yang H, Zuo W, et al: Differential
expression and roles of volume-activated chloride channels in
control of growth of normal and cancerous nasopharyngeal epithelial
cells. Biochem Pharmacol. 83:324–334. 2012.
|
|
9
|
Comes N, Abad E, Morales M, Borrás T, Gual
A and Gasull X: Identification and functional characterization of
ClC-2 chloride channels in trabecular meshwork cells. Exp Eye Res.
83:877–889. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Földy C, Lee SH, Morgan RJ and Soltesz I:
Regulation of fast-spiking basket cell synapses by the chloride
channel ClC-2. Nat Neurosci. 13:1047–1049. 2010.PubMed/NCBI
|
|
11
|
Roman RM, Smith RL, Feranchak AP, Clayton
GH, Doctor RB and Fitz JG: ClC-2 chloride channels contribute to
HTC cell volume homeostasis. Am J Physiol Gastrointest Liver
Physiol. 280:G344–G353. 2001.PubMed/NCBI
|
|
12
|
Xiong H, Li C, Garami E, et al: ClC-2
activation modulates regulatory volume decrease. J Membr Biol.
167:215–221. 1999.PubMed/NCBI
|
|
13
|
Britton FC, Hatton WJ, Rossow CF, Duan D,
Hume JR and Horowitz B: Molecular distribution of volume-regulated
chloride channels (ClC-2 and ClC-3) in cardiac tissues. Am J
Physiol Heart Circ Physiol. 279:H2225–H2233. 2000.PubMed/NCBI
|
|
14
|
Thomasy SM, Wood JA, Kass PH, Murphy CJ
and Russell P: Substratum stiffness and latrunculin B regulate
matrix gene and protein expression in human trabecular meshwork
cells. Invest Ophthalmol Vis Sci. 53:952–958. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhou H, Tang Y, Liang X, Yang X, Yang J,
et al: RNAi targeting urokinase-type plasminogen activator receptor
inhibits metastasis and progression of oral squamous cell carcinoma
in vivo. Int J Cancer. 125:453–462. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Peng J, Lei CT, Hu JB and Fan YC: Effects
of travoprost on actin cytoskeleton and β-catenin in the human
trabecular meshwork cells treated with Dexamethasone. Zhonghua Yan
Ke Za Zhi. 47:336–341. 2011.(In Chinese).
|
|
17
|
Sethi A, Mao W, Wordinger RJ and Clark AF:
Transforming growth factor-beta induces extracellular matrix
protein cross-linking lysyl oxidase (LOX) genes in human trabecular
meshwork cells. Invest Ophthalmol Vis Sci. 52:5240–5250. 2011.
View Article : Google Scholar
|
|
18
|
Fuchshofer R and Tamm ER: The role of
TGF-β in the pathogenesis of primary open-angle glaucoma. Cell
Tissue Res. 347:279–290. 2012.
|
|
19
|
Kottler UB, Jünemann AG, Aigner T, Zenkel
M, Rummelt C and Schlötzer-Schrehardt U: Comparative effects of
TGF-beta and TGF-beta 2 on extracellular matrix production,
proliferation, migration, and collagen contraction of human Tenon’s
capsule fibroblasts in pseudoexfoliation and primary open-angle
glaucoma. Exp Eye Res. 80:121–134. 2005.
|
|
20
|
Cumurcu T, Bulut Y, Demir HD and
Yenisehirli G: Aqueous humor erythropoietin levels in patients with
primary open-angle glaucoma. J Glaucoma. 16:645–648. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Grosheva I, Vittitow JL, Goichberg P, et
al: Caldesmon effects on the actin cytoskeleton and cell adhesion
in cultured HTM cells. Exp Eye Res. 82:945–958. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Li A, Leung CT, Peterson-Yantorno K,
Stamer WD, Mitchell CH and Civan MM: Mechanisms of ATP release by
human trabecular meshwork cells, the enabling step in purinergic
regulation of aqueous humor outflow. J Cell Physiol. 227:172–182.
2012. View Article : Google Scholar : PubMed/NCBI
|