Introduction
Phytochemicals, including polyphenolic compounds,
are well studied chemopreventive agents in the development of
cancer. These compounds, including flavonoids, occur ubiquitously
in foods of plant origin and are consumed daily in the majority of
Western diets (1). Flavonoids are
subcategorized into flavonols, flavones, catechins, flavanones,
chalcones, anthocyanidins and isoflavonoids. Flavonoids exhibit
antioxidant properties by inhibiting lipid peroxidation induced by
various pro-oxidants in liver homogenate, microsomes, mitochondria
and liposomes (2). Free
radical-mediated lipid peroxidation has been grossly implicated in
the pathogenesis of diseases, including cancer, atherosclerosis,
neurological disorders and toxic cell injury (3–5), as
radicals react with critical cellular components, such as DNA,
lipids and proteins. The antioxidant activities of polyphenols have
been observed to be associated with the ability of polyphenols to
chelate metal ions and to scavenge singlet oxygen, superoxide
anions, peroxyl radicals, hydroxyl radicals and peroxynitrite
(2,6), as well as inhibit lipid peroxidation
(7). Thus, polyphenols with
antioxidant activities may foster useful health maintenance
protection to cells against damage caused by reactive oxygen
species.
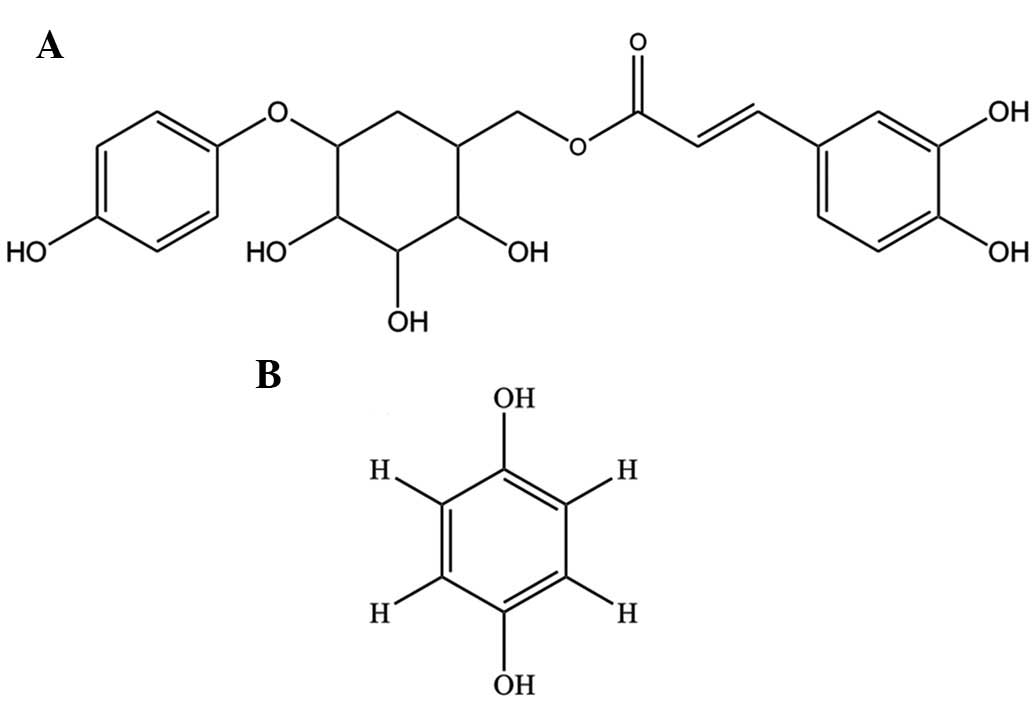
The phenolic compounds, robustaside B (6′-3″,
4″-dihydroxycinnamoyl), an arbutin derivative (8,9), and
para-hydroxyphenol (Fig.
1), have been isolated for the first time (10) from leaves of Cnestis
ferruginea (Connaraceae). This plant is commonly found in West
Africa and is known for its use as a laxative, antimicrobial agent
and treatment of tooth cavities (11). Arbutins are known for antibacterial
and diuretic activities as well as inhibition of melanin
biosynthesis and are therefore raw materials in cosmetic industries
(12). This class of compounds has
been reported in the leaves of Grevillea robusta(8). The chemical structure of robustaside
B and para-hydroxyphenol indicates the presence of phenolic
moieties which have been shown to scavenge DPPH radicals (10).
According to the WHO in 2009 (13), cancer was the third most common
cause of mortalities worldwide, particularly in developed
countries, with 12.7 million new cases diagnosed and 7.6 million
cancer-related mortalities occurring in 2008 (14). Numerous anticancer drugs used for
chemotherapy aim to prolong the life of the patient. A number of
these anticancer agents originate in plants, including
camptothecins, vincristine and irinotecan, and also in
microorganisms, such as doxorubicin, dactinomycin, mitomycin and
bleomycin (15). At present,
anticancer drugs are highly cytotoxic to normal cells causing
unpleasant side-effects to patients and eventually having reduced
therapeutic efficacy due to drug resistance (16). Therefore, there has been a shift in
research attention into the development of efficient, selective and
less toxic anticancer drugs.
The induction of apoptosis is an inexhaustible
mechanism for combating and gradually eliminating cancer cells.
Apoptosis may be mediated via the extrinsic death receptor and
intrinsic mitochondrial-mediated pathways (17). The mitochondrial-mediated pathway
involves the opening of a non-specific pore in the inner
mitochondrial membrane known as the mitochondrial permeability
transition pore (MPT). Mitochondrial permeability transition is a
phenomenon caused by calcium overload, oxidative stress or cellular
insult, which leads to massive swelling and depolarization of the
mitochondria, depletion of pyrimidine nucleotides, release of
cytochrome c and other apoptotic factors (18,19),
uncoupling of oxidative phosphorylation, hydrolysis of ATP by
mitochondrial F0F1 ATPases and cell death
(20,21). A number of phytochemicals,
including polyphenols, have been observed to function as
chemopreventive agents in the development of cancer and diseases
resulting from dysregulated apoptosis, including autoimmune
diseases and spreading of viral infections (22,23).
To investigate the incidence of cancer, these phytochemicals and
plant extracts are being targeted at mechanisms of apoptosis
involving regulatory pathways. Pathways, including the Bcl-2 family
of proteins, induction of mitochondrial swelling and dissipation of
membrane potential, modulation of caspase activation and
suppression, as well as induction of cytochrome c release
(24–26), are being examined. There is a lack
of information on the pharmacological importance of robustaside B
and para-hydroxyphenol beyond their radical scavenging
potential (10). Thus, it has
become imperative to establish the antioxidant properties of these
compounds, in particular, the anti-lipid peroxidative properties
and modulatory effects on membrane permeability transition pore
opening in rat liver mitochondria.
Materials and methods
Chemicals and reagents
Robustaside B and para-hydroxyphenol were
previously purified from the leaves of Cnestis ferruginea
and characterized in our laboratory. Quercetin, spermine, HEPES,
D-mannitol and thiobarbituric acid were purchased from
Sigma-Aldrich (St. Louis, MO, USA). Other reagents used were of
analytical grade.
Animals
Healthy male Wistar strain albino rats, weighing
between 150 and 200 g, were purchased from the Animal House of the
Department of Biochemistry, University of Ibadan (Ibadan, Nigeria).
Animals were handled according to the NIH regulations guiding
animal handling (ensured by the Department of Biochemistry
Postgraduate Programme Board of the University of Ibadan, Ibadan,
Nigeria) and were kept under standard conditions of light/dark
cycles and a temperature of 23±2°C. Animals were supplied with
water and fed ad libitum throughout the duration of the
experiment.
Preparation of low ionic strength
mitochondria
Rats were sacrificed by cervical dislocation and
livers were excised, rinsed with buffer C [210 mM mannitol, 70 mM
sucrose, 5 mM HEPES-KOH and EGTA (1mM, pH 7.4)], blotted, weighed,
minced and homogenized in a glass Teflon homogenizer to produce a
10% suspension. The homogenate was subjected to differential
centrifugation to prepare low ionic strength mitochondria according
to the method described by Johnson and Lardy (27). The homogenate was spun twice at 885
× g for 5 min and mitochondria were centrifuged at 5,000 × g for 20
min in an Angle 13 refrigerated centrifuge (MSE, London, UK).
Mitochondria were washed twice with buffer D [210 mM mannitol, 70
mM sucrose, 5% BSA (pH 7.4)]. Mitochondrial pellets were suspended
in MSH buffer [210 mM mannitol, 70 mM sucrose and 5 mM HEPES-KOH
(pH 7.4)] to produce a mitochondrial suspension (1 ml) that is the
equivalent to mitochondria isolated from 1 g tissue.
Determination of protein
concentration
Mitochondrial protein concentrations were determined
according to the method previously described by Lowry et
al(28) using BSA as the
standard.
Antioxidant studies
Inhibition of lipid peroxidation in mitochondria was
determined spectrophotometrically by measuring the intensity of the
pink color of thiobarbituric acid reactive substances (TBARS)
induced in the Fe2+/ascorbate system at 532 nm according
to the method described by Varshney and Kale (29). The reaction mixture contained
mitochondria (0.12 mg/ml) in Tris-HCl (30 mM), ferrous ammonium
sulphate (0.16 mM), ascorbic acid (0.06 mM) and various
concentrations of the compounds (0.05–1 mM) in a final reaction
volume of 0.5 ml, as previously described (30) and was incubated for 1 h at 37°C.
The resulting TBARS were measured spectrophotometrically. The
reaction mixture (0.4 ml) was mixed with 1.6 ml Tris-HCl buffer
(0.15 M) to which 0.5 ml trichloroacetic acid (30%) was added (to
terminate the reaction). Thereafter, 0.5 ml thiobarbituric acid
(0.75%) was added and placed in a water bath for 30 min at 95°C,
cooled on ice and centrifuged at room temperature for 10 min at
3,000 rpm in an SM902B benchtop centrifuge (Surgifriend Medicals,
Middlesex, UK). Absorbance of the clear pink supernatant was
measured against a reference blank of distilled water at 532 nm
using a Camspec 106 spectrophotometer (Spectronic Camspec Ltd.,
Garforth, UK).
Determination of mitochondrial
swelling
The extent of mitochondrial swelling was utilized as
a measure of MPT pore opening in the presence or absence of
calcium, the triggering agent, according to the method described by
Lapidus and Sokolove (31).
Briefly, mitochondria (0.12 mg/ml) were preincubated with 0.8 μM
rotenone in MSH buffer [210 mM mannitol, 70 mM sucrose, 5 mM
HEPES-KOH (pH 7.4)] for 3.5 min, following which, 5 mM succinate
was added to energize the reaction in a total reaction volume of
1.25 ml in the absence of calcium chloride. For estimation of
calcium-induced MPT pore opening, mitochondria (0.12 mg/ml) were
incubated for 3 min with rotenone in MSH buffer and 300 nmol
CaCl2.2H2O was added immediately to trigger
swelling. In addition, 5 mM succinate was added 30 sec later. To
assess the effects of spermine (0.1 mM), robustaside B or
para-hydroxyphenol (0.1–1 mM), mitochondria were
pre-incubated with rotenone, spermine or the compounds for 3 min in
MSH buffer. Calcium chloride was added to the reaction medium at 3
min and 30 seconds later, sodium succinate was added to energize
the reaction. To determine the effects of the compounds alone on
mitochondrial swelling, mitochondria were pre-incubated with
rotenone and the pure compounds for 3.5 min in MSH buffer. During
the incubation, sodium succinate was added at 3.5 min and the rate
of mitochondrial swelling was estimated spectrophotometrically as a
decrease in absorbance at 540 nm and was measured every 30 sec for
12 min.
Statistical analysis
Data were analyzed using ANOVA and are expressed as
the mean ± SD. P<0.05 was considered to indicate a statistically
significant difference.
Results
Antioxidant activity of Robustaside B and
para-hydroxyphenol
Comparative effects of robustaside B,
para-hydroxyphenol and quercetin on
Fe2+/ascorbate-induced mitochondrial membrane lipid
peroxidation are presented in Fig.
2. Robustaside B decreased the amount of TBARS produced during
lipid peroxidation induced by the Fe2+/ascorbate system
in rat liver mitochondria in vitro in a
concentration-dependent manner. Varying concentrations of
robustaside B (0.05, 0.1, 0.2, 0.25, 0.5, 0.75 and 1 mM)
significantly (P<0.05) reduced the amount of TBARS generated by
85.3, 86.4, 86.0, 86.1, 86.0, 86.0 and 86.0%, respectively.
Similarly the same concentrations of para-hydroxyphenol
significantly (P<0.05) decreased the amount of TBARS by 86.7,
81.3, 81.3, 80, 80, 82.6 and 83.1%, respectively. In addition,
similar concentrations of quercetin, an antioxidant used as the
standard, significantly (P<0.05) reduced the amount of TBARS
produced by 86.6, 84.8, 83.8, 83.0, 84.2, 84.8 and 84.2%,
respectively (Fig. 2). The
observations indicated that there was no significant difference
(P>0.05) in the degree of inhibition of membrane lipid
peroxidation by all three compounds. All compounds elicited at
least an 80% reduction in the amount of TBARS and the same
IC50 value of 0.025 mM.
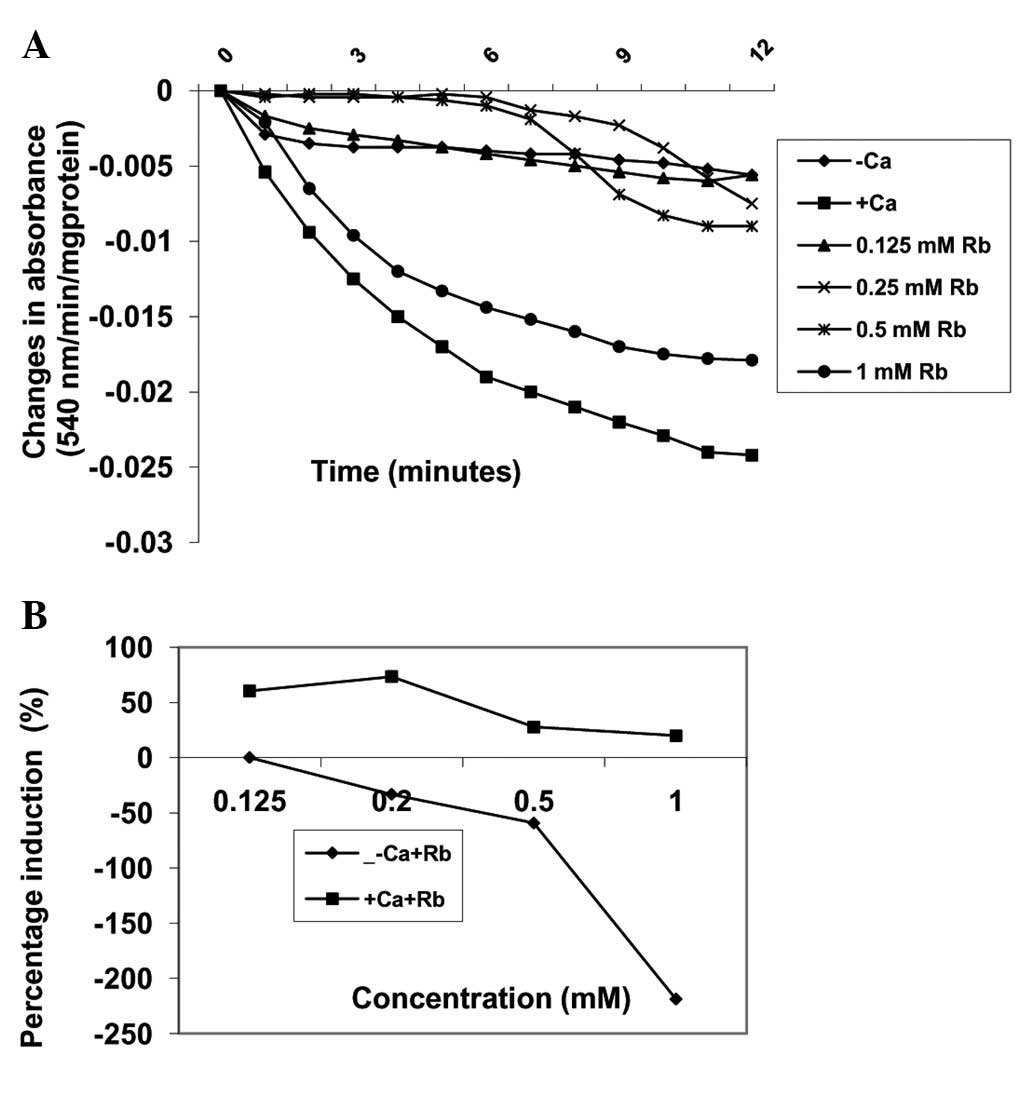
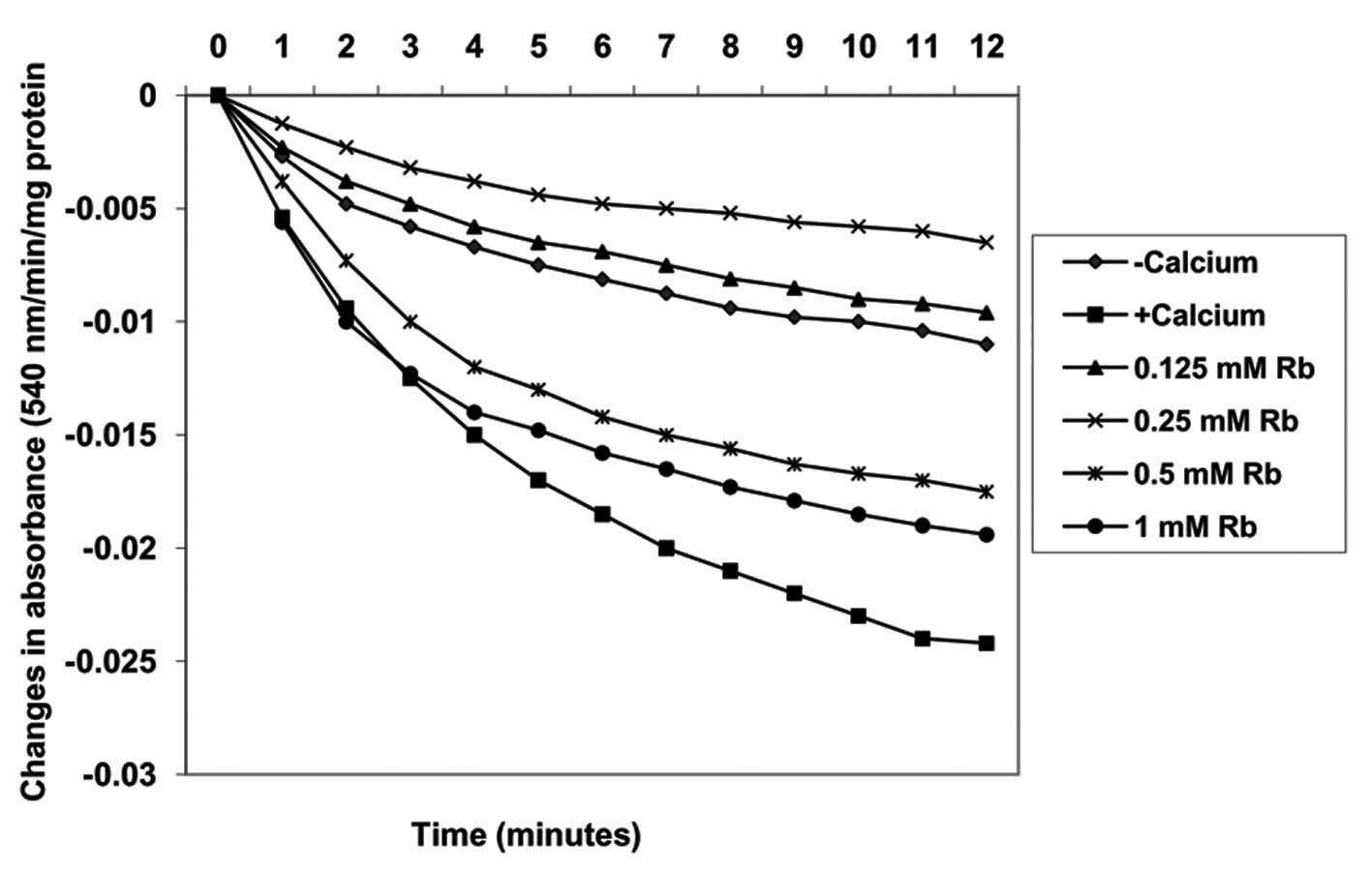
Effects of Robustaside B on MPT pore
opening with or without CaCl2
Pre-incubation of varying concentrations of
robustaside B (0.125, 0.25, 0.5 and 1 mM) with mitochondria
energized by succinate induced MPT pore opening in a
concentration-dependent manner by 0, −33.3, −59.3 and −218.5%,
respectively, as compared with control untreated mitochondria in
the absence of calcium (Fig. 3).
In the presence of calcium, similar concentrations of robustaside B
decreased the extent of MPT pore opening in a
concentration-dependent manner but in the reverse order. For
example, 0.125, 0.25, 0.5 and 1.0 mM robustaside B protected the
mitochondrial membrane from calcium-induced MPT by 60.3, 73.3, 27.6
and 19.8%, respectively (Fig. 4).
The maximum inhibitory concentration of robustaside B obtained was
0.25 mM following which the extent of inhibition decreased as the
concentration increased. There was a negative correlation observed
between the inhibition of lipid peroxidation and induction of
mitochondrial membrane permeability transition (MMPT) pore opening
in the presence or absence of robustaside B (absence of calcium,
r=−0.3; presence of calcium, r=−0.834).
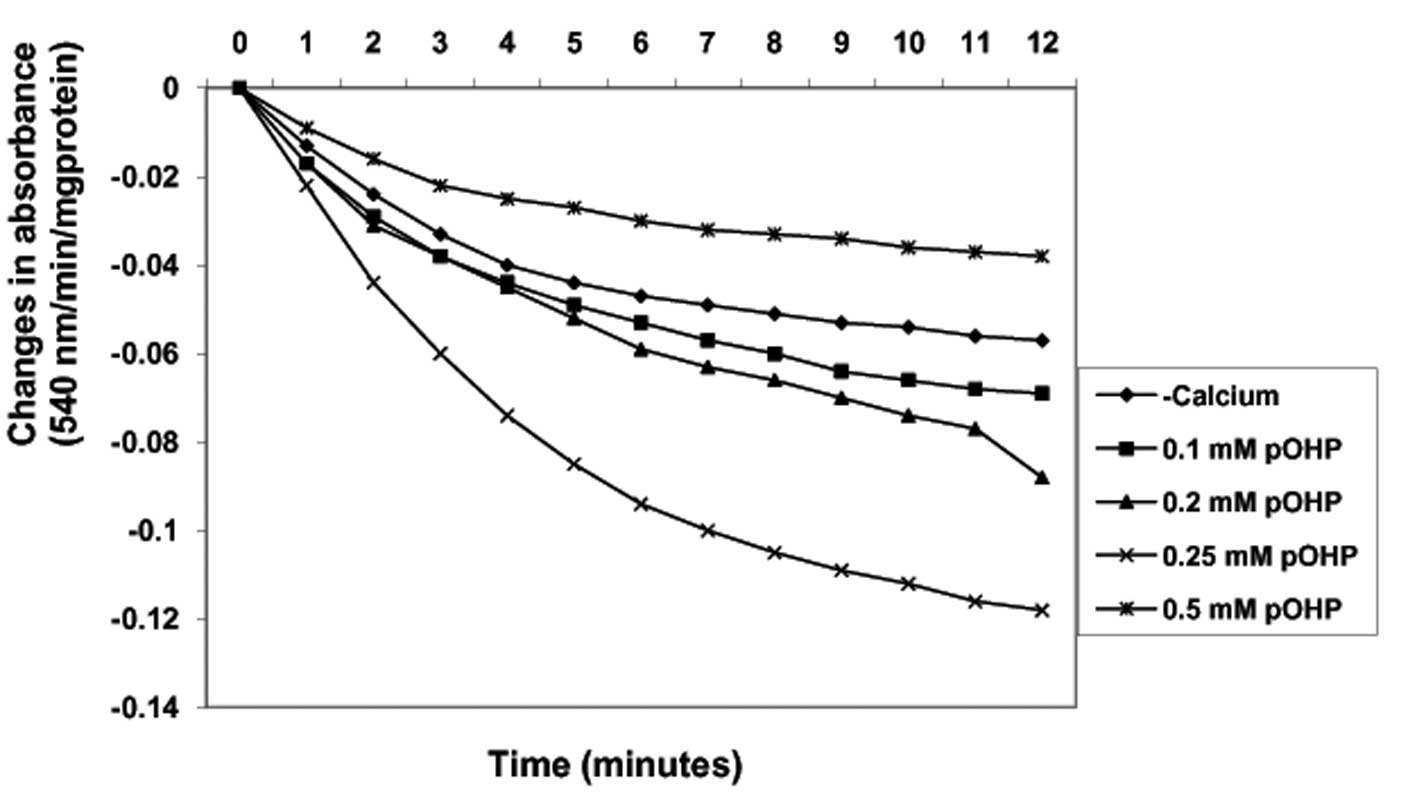
Induction of MPT pore opening with or
without CaCl2 by para-hydroxyphenol
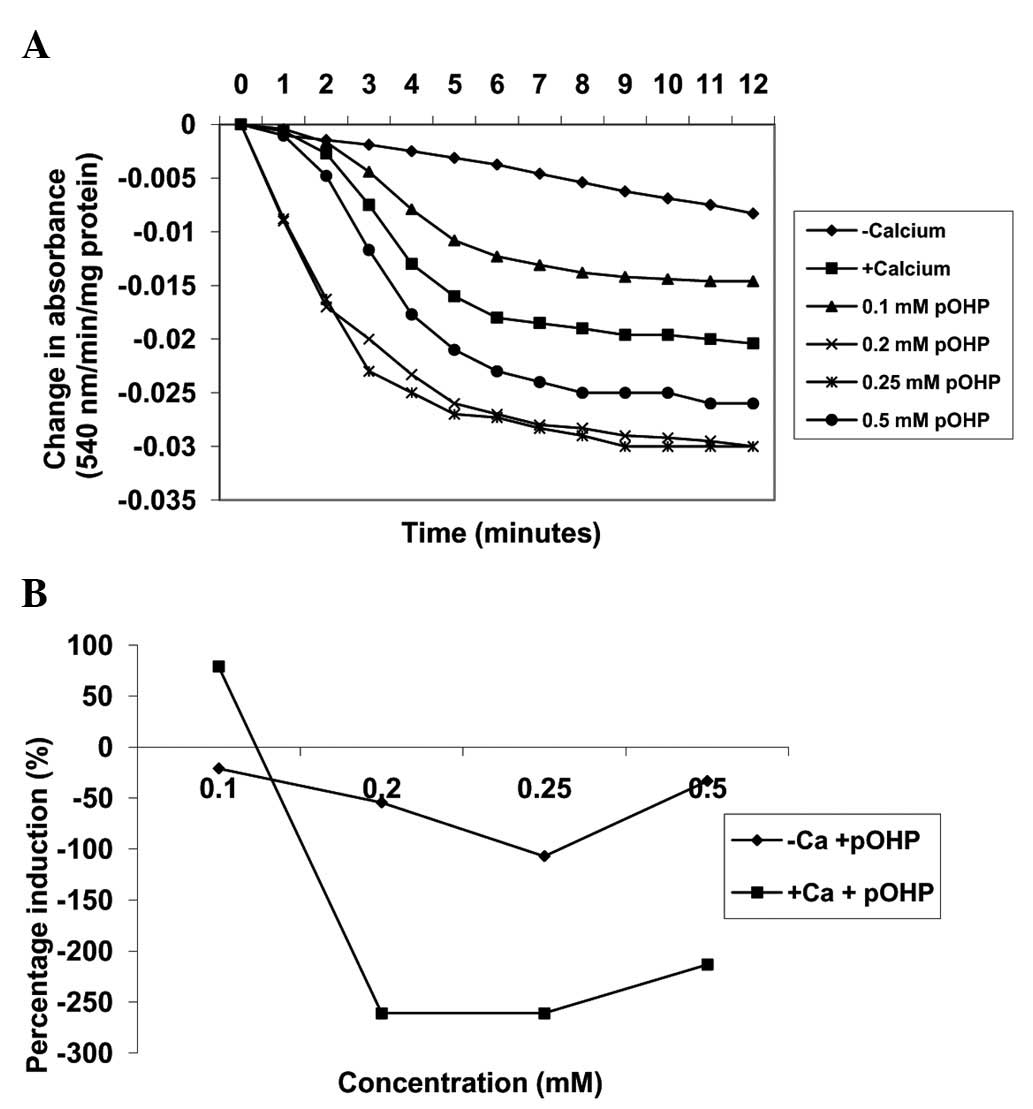
By contrast, in the absence of calcium, varying
concentrations of para-hydroxyphenol (0.1, 0.2, 0.25 and 0.5 mM)
induced MPT pore opening in a concentration-dependent manner up to
0.25 mM by −21, −54.4 and −107.0%, respectively (Fig. 5). Similarly, in the presence of
calcium, the same concentrations of para-hydroxyphenol
further induced MPT pore opening by 78.9, −261, −261 and −213.3%,
respectively, when compared with induction by calcium alone. As the
concentration of the compound increased, the extent of pore opening
also increased (Fig. 6). In
addition, there was a positive correlation observed between the
inhibition of lipid peroxidation and the induction of MMPT pore
opening in the presence or absence of calcium by
para-hydroxyphenol (absence of calcium, r=0.437; presence of
calcium, r=0.408).
Effects of quercetin on MPT pore opening
with or without CaCl2
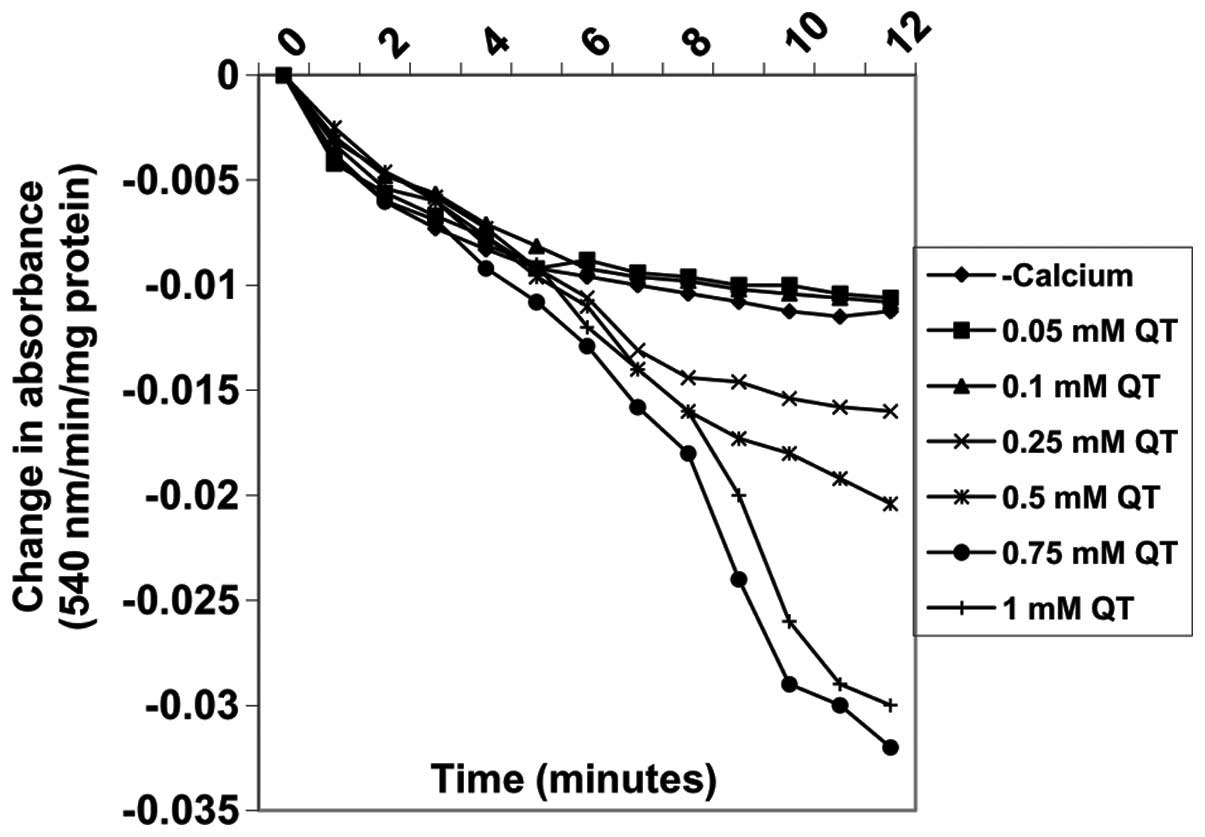
Varying concentrations of quercetin (0.05, 0.1,
0.25, 0.5, 0.75 and 1 mM) also induced MPT pore opening in the
absence of calcium in a concentration-dependent manner by 5, 3.7,
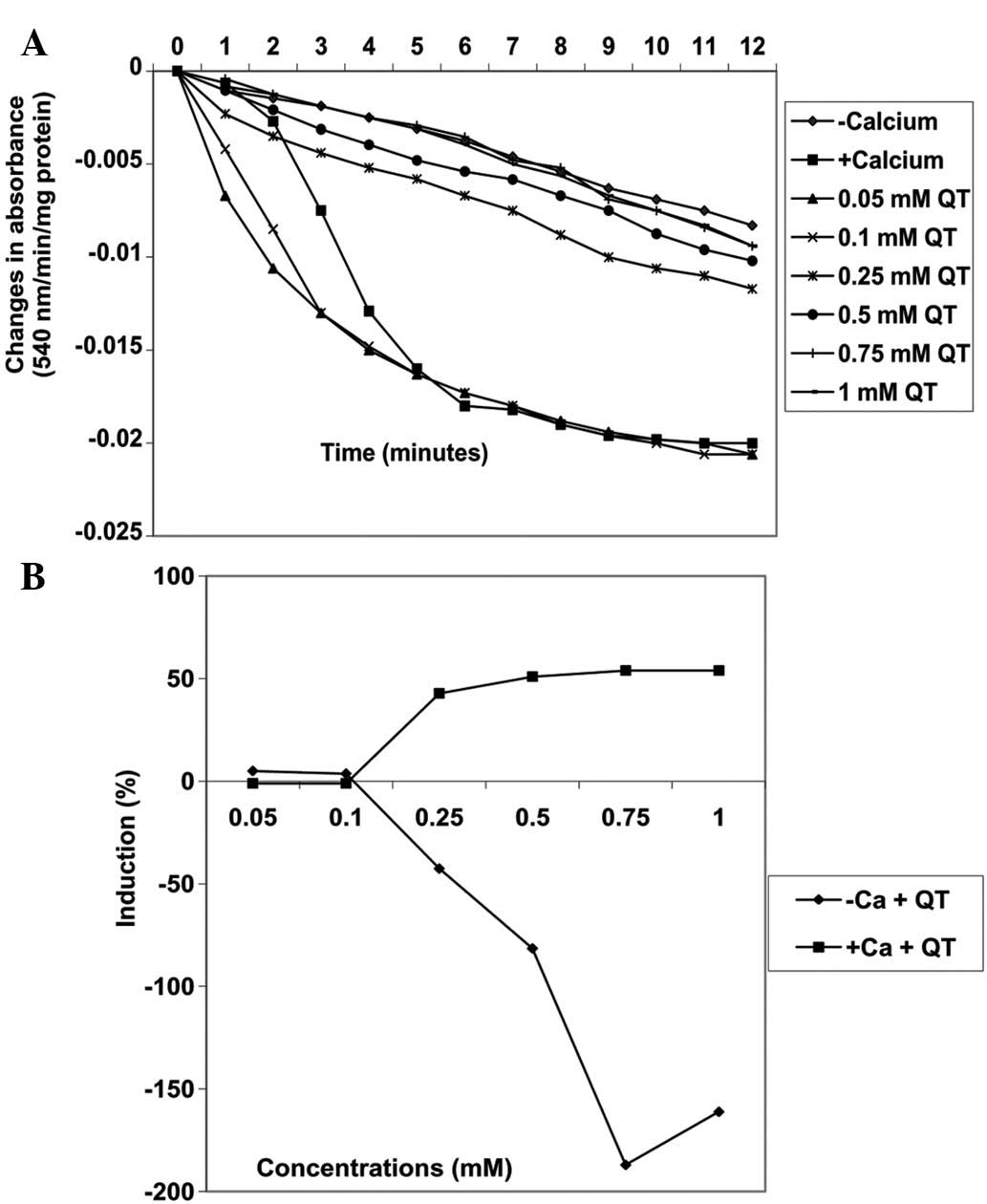
−42.6, −81.5, −187 and −161.1%, respectively (Fig. 7). By contrast, the same
concentrations of quercetin decreased MPT pore opening triggered by
calcium by −1.0, −1.0, 42.9, 51.0, 54 and 54%, respectively
(Fig. 8A). There was a positive
correlation between the inhibition of lipid peroxidation and
induction of mitochondrial permeability transition pore opening by
quercetin. By contrast, the inhibition of lipid peroxidation was
negatively correlated with inhibition of mitochondrial permeability
transition pore opening by quercetin.
Discussion
Research is currently directed towards the search
for novel anticancer agents from natural sources which are
physiologically non-toxic and inert to normal cells. Numerous
traditionally used herbal remedies are being subjected to modern
purification processes for the characterization of active
components for possible therapeutic purposes against human cancers
(32–34). Robustaside B and
para-hydroxyphenol were purified for the first time from the
leaves of Cnestis ferruginea and identified as radical
scavengers (10). In the current
study, the anti-lipid peroxidation activity of these compounds and
their modulatory activity on mitochondrial membrane permeability
pore opening in rat liver was reported.
Robustaside B (0.1 mM) and para-hydroxyphenol
(0.05 mM) significantly (P<0.05) inhibited mitochondrial lipid
peroxidation induced by the Fe2+/ascorbate system by
causing a significant decrease (86.4 and 86.7%), respectively, in
the amount of TBARS released by the system. There was no
significant difference (P>0.05) observed between the percentage
inhibitions by these compounds and 0.05 mM quercetin (86.6%). In
addition, the IC50 values (0.025 mM) for robustaside B,
para-hydroxyphenol and quercetin also clearly revealed the
similarity in their potential to inhibit lipid peroxidation
stimulated in mitochondrial membranes by the
Fe2+/ascorbate system. The current observations indicate
that robustaside B and para-hydroxyphenol have strong and
similar antioxidant potentials comparable to that of quercetin,
attributable to the presence of phenolic moieties involved in the
Fenton and Haber-Weiss reactions (35) of the lipid peroxidation system.
These results further support our earlier study on the strong
radical scavenging properties of the compounds (10).
MMPT, measurable by mitochondrial swelling, is one
of the indices of evaluating apoptosis induced through the
mitochondrial pathway (36). The
present observations indicated that robustaside B, pre-incubated
with isolated mitochondria from rat liver, induced MPT pore opening
in a concentration-dependent manner in the absence of calcium.
However, in the presence of calcium, robustaside B inhibited
calcium-induced MPT pore opening with a maximum inhibitory
concentration of 0.25 mM. At higher concentrations, inhibition was
decreased in a concentration-dependent manner. Notably, this dual
activity of robustaside B at different concentrations may be
beneficial, in that high concentrations of robustaside B (0.5–1 mM)
may be targeted at the induction of apoptosis in proliferating
cells. Following a curative period or effect, the concentration of
the compound may be gradually decreased, to below 0.5 mM, before
total drug withdrawal from the patient. This effect at low
concentrations may also be a useful chemotherapy for patients
following surgery. In addition, patients presenting with
pathological conditions resulting from excessive apoptosis,
including sarcopenia and HIV, may find robustaside B effective at
low doses to gradually reduce apoptosis and replenish cell
populations (26).
The inhibitory activity of robustaside B in the
presence of calcium may be due to the compound mopping up calcium
ions by coordination of the calcium ions to oxygen atoms in its
structure. This reaction, if sustained, reduces the concentration
of free calcium ions available for induction of pore opening and
thus, encourages pore closure as observed at low (0.125–0.25 mM)
concentrations of robustaside B. At higher concentrations of
robustaside B, once the internal milieu calcium ion concentration
is almost completely consumed, the excess robustaside B may
intercalate between the fatty acid phospholipids in the
mitochondrial membrane bilayer. This interaction may lead to
alterations in membrane fluidity, a leaky membrane with dissipation
of mitochondrial membrane potential and uncoupling of oxidative
phosphorylation, consequently leading to apoptosis.
In the presence and absence of calcium,
para-hydroxyphenol significantly induced mitochondrial
permeability transition pore opening in a concentration-dependent
manner. In the absence of calcium, the induction of pore opening by
robustaside B is greater than that of quercetin and
para-hydroxyphenol. The induction of pore opening by these
compounds in the presence of calcium decreases. The presence of the
polyphenolic group in the structure of robustaside B may confer
polarity on the membrane structure thereby rendering it leaky. This
event may encourage the association of VDAC, ANT and cyclophilin D,
leading to the formation of the MPT pore. The differences in the
structure of the compounds, particularly in the phenol ring
structures present in robustaside B, indicate a structure-function
correlation accounting for the strong inductive power of
robustaside B compared with para-hydroxyphenol. In addition,
the similarity in the pattern of induction of MPT pore opening by
robustaside B and quercetin may be accounted for by the presence of
polyhydroxyl groups in the core ring structures of the compounds.
Similar to quercetin, robustaside B and para-hydroxyphenol
may be of potential use in the treatment of diseases involving the
induction of apoptosis, including cancer.
In conclusion, to the best of our knowledge,
robustaside B and para-hydroxyphenol have been demonstrated
for the first time to possess powerful antioxidant properties since
they inhibit membrane lipid peroxidation stimulated by the
Fe2+/ascorbate system in a similar manner to that of
quercetin, a known antioxidant. In addition, the compounds induced
MPT pore opening in rat liver mitochondria and protected the MPT
pore from calcium-induced opening in vitro. By contrast,
these compounds also interact with the intact mitochondria to
induce pore opening in the absence of calcium. Thus, robustaside B
and para-hydroxyphenol may be useful pharmaceutical
applications for the design of drugs for the treatment of cancer,
cardiovascular diseases and muscle wasting, along with
stabilization of canned food against oxidative deterioration and
amelioration of oxidatively induced disorders leading to cell
death. Further in depth studies targeting the compounds against
cancer cell lines are required to substantiate the anticancer
activity and mechanism of induction of apoptosis in cancer
cells.
References
|
1
|
Critchfield JW, Welsh CJ, Phang JM and Yeh
GC: Modulation of adriamycin accumulation and efflux by flavonoids
in HCT-15 colon cells. Activation of P-glycoprotein as a putative
mechanism. Biochem Pharmacol. 48:1437–1445. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Briviba K and Sies H: Nonenzymatic
antioxidant defense systems. Natural Antioxidants in Human Health
and Disease. Frei B: Academic Press; San Diego, CA: pp. 107–128.
1994, View Article : Google Scholar
|
|
3
|
Halliwell B and Gutteridge JM: Role of
free radicals and catalytic metal ions in human disease: an
overview. Methods Enzymol. 186:1–85. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Dargel R: Lipid peroxidation - a common
pathogenetic mechanism? Exp Toxicol Pathol. 44:169–181. 1992.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kehrer JP and Smith CV: Free radicals in
biology: sources, reactivities, and roles in the etiology of human
diseases. Natural Antioxidants in Human Health and Disease. Frei B:
Academic Press; San Diego, CA: pp. 25–62. 1994
|
|
6
|
Bors W, Michel C and Stettmaier K:
Antioxidant effects of flavonoids. Biofactors. 6:399–402. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Halliwell B: Drug antioxidant effects. A
basis for drug selection? Drugs. 42:569–605. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ahmed AS, Nakamura N, Meselhy MR, Makhboul
MA, El-Emary N and Hattori M: Phenolic constituents from
Grevillea robusta. Phytochemistry. 53:149–154. 2000.
View Article : Google Scholar
|
|
9
|
He QQ, Liu MS, Jin DJ and Kong LY:
Phenolic glycosides from leaves of Hopiciopsis lobata. J
Asian Natur Prod Res. 8:373–377. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Adisa RA, Abass Khan A, Oladosu I, Ajaz A,
Choudhary MI, Olorunsogo OO and Ur Rahman A: Purification and
characterization of phenolic compounds from the leaves of
Cnestis ferruginea (De Candolle): Investigation of
antioxidant property. Res J Phytochem. 5:177–189. 2011. View Article : Google Scholar
|
|
11
|
Boakye-Yiadom K and Konning GH: Incidence
of antibacterial activity in the Connaraceae. Planta Med.
28:397–400. 1975. View Article : Google Scholar
|
|
12
|
Akiu S, Suzuki Y, Fujinuma Y, Asahara T
and Fukada M: Inhibitory effect of Arbutin on melanogenesis:
Biochemical study in cultured B16 cells and effect on the
UV-induced pigmentation in human skin. Proc Jpn Soc Invest
Dermatol. 12:138–139. 1988.(In Japanese).
|
|
13
|
World Health Organization. Cause-specific
mortality and morbidity. http://www.who.int/whosis/whostat/EN_WHS09_Table2.pdf.
Accessed July 10, 2013
|
|
14
|
Ferlay J, Shin HR, Bray F, Forman D,
Mathers C and Parkin DM: Estimates of worldwide burden of cancer in
2008: GLOBOCAN 2008. Int J Cancer. 127:2893–2917. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Grever MCB: Cancer drug discovery and
development. Cancer: Principles and Practice of Oncology. De Vita
VT Jr, Hellman S and Rosenberg SA: Lippincott Raven; Philadelphia,
PA: pp. 328–339. 2001
|
|
16
|
Peters GJ, Backus HH, Freemantle S, Van
Triest B, Codacci-Pisanelli PG, et al: Induction of thymidylate
synthase as a 5-fluorouracil resistance mechanism. Biochim Biophys
Acta. 1587:194–205. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Desagher S and Martinou JC: Mitochondria
as the central control point of apoptosis. Trends Cell Biol.
10:369–377. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Bernardi P: Mitochondrial transport of
cations: channels, exchangers and permeability transition. Physiol
Rev. 79:1127–1155. 1999.PubMed/NCBI
|
|
19
|
Crompton M: The mitochondrial permeability
transition pore and its role in cell death. Biochem J. 341:233–249.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Halestrap AP, McStay GP and Clarke SJ: The
permeability transition pore complex: another view. Biochimie.
84:153–166. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Rasola A, Sciacovelli M, Pantic B and
Bernardi P: Signal transduction to the permeability transition
pore. FEBS Lett. 584:1989–1996. 2010. View Article : Google Scholar
|
|
22
|
Fadeel B, Orrenius S and Zhivotovsky B:
Apoptosis in human disease: a new skin for the old ceremony?
Biochem Biophys Res Commun. 266:699–717. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Endrini S, Jaksa S, Marsiati H, Othman F
and Rahmat A: Effects of cola nut (Cola nitida) on the
apoptotic cell of human breast carcinoma cell lines. J Medicinal
Plant Res. 5:2393–2397. 2011.
|
|
24
|
Martin KR: Targeting apoptosis with
dietary bioactive agents. Exper Biol Med (Maywood). 231:117–129.
2006.PubMed/NCBI
|
|
25
|
Berridge MV, Herst PM and Lawen A:
Targeting mitochondrial permeability in cancer drug development.
Mol Nutr Food Res. 53:76–86. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Salako TA, Adisa RA, Alao OO, Adeniran OO,
Atanu FO and Olorunsogo OO: Effects of methanolic and chloroform
extracts of leaves of Alstonia boonei on rat liver
mitochondrial membrane permeability transition pore. Afr J Med Med
Sci. 39(Suppl 1): 109–116. 2010.PubMed/NCBI
|
|
27
|
Johnson D and Lardy H: Isolation of liver
or kidney mitochondria. Methods Enzymol. 10:94–96. 1967. View Article : Google Scholar
|
|
28
|
Lowry OH, Rosenbrough NJ, Farr AL and
Randall RJ: Protein measurement with Folin phenol reagent. J Biol
Chem. 193:265–275. 1951.PubMed/NCBI
|
|
29
|
Varshney R and Kale RK: Effect of
calmodulin antagonists on radiation induced-lipid peroxidation in
microsomes. Internatl J Radiat Biol. 58:733–743. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Farombi EO, Ogundipe OO, Samuel Uhunwangho
E, Adeyanju MA and Olarenwaju Moody J: Antioxidant properties of
extracts from Alchornea laxiflora (Benth) Pax and Hoffman.
Phytother Res. 17:713–716. 2003. View
Article : Google Scholar : PubMed/NCBI
|
|
31
|
Lapidus RG and Sokolove PM: Inhibition by
spermine of the inner membrane permeability transition of isolated
rat heart mitochondria. FEBS Lett. 313:314–318. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Shawi AA, Rasul A, Khan M, Iqbal F and
Tonghui M: Eupatilin: A flavonoid compound isolated from the
artemisia plant induces apoptosis and G2/M phase cell cycle arrest
in human melanoma A375 cells. Afr J Pharm Pharmacol. 5:582–588.
2011. View Article : Google Scholar
|
|
33
|
Nugraheni M, Santoso U, Suparmo H and
Wuryastuti H: Potential of Coleus tuberosus as an
antioxidant and cancer chemoprevention agent. Int Food Res J.
18:1471–1480. 2011.
|
|
34
|
Choi S, Jang HJ, Choi JY, Kim MS, Lee YR,
Kim HS, et al: Antioxidant and anticancer activity of fractions of
the ethanol extracts of Naematoloma sublateritium. J Med
Plant Res. 6:1344–1352. 2012.
|
|
35
|
Stadtman ER: Metal ion-catalyzed oxidation
of proteins: biochemical mechanism and biological consequences.
Free Radical Biol Med. 9:315–325. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Lapidus RG and Sokolove PM: Spermine
inhibition of the permeability transition of isolated rat liver
mitochondria: an investigation of mechanism. Arch Biochem Biohphys.
306:246–253. 1993. View Article : Google Scholar : PubMed/NCBI
|