Introduction
Tight junctions are intercellular structures,
located at the top of junctional complexes, which are critical in
maintaining the fence, barrier and signal transduction functions in
epithelial and endothelial cells (1–3).
Tight junctions are composed of three types of integral membrane
proteins: occludins, claudins and junction adhesion molecules and
also contain peripheral plasmosins, including ZO-1, -2 and -3
(4). Claudins, which contain at
least 27 members, have been shown to be the most important backbone
protein of tight junctions (5).
Claudin-6 is a protein in the claudin family with a molecular
weight of ~23 kDa and whose gene is located at chromosome
16p13.3.
There is increasing evidence indicating that tight
junctions are involved in cell migration, proliferation,
differentiation and apoptosis. Disruption or loss of tight junction
structure and function has been reported in tumors of human
epithelial origin and it has been shown that these changes are
associated with variations in tumor growth, invasion and metastasis
(6–8). Thus, the loss or downregulated
expression of claudin and other tight junction proteins is
hypothesized to be important steps for tumor development and
metastasis (9).
Previously, claudin-6 was identified as a potential
breast cancer suppressor gene, which may contribute to a breast
cancer resistant phenotype observed in Copenhagen rats (10). In addition, overexpression of
claudin-6 may abate the malignant phenotype of the human breast
cancer cell line MCF-7 (11),
indicating that claudin-6 may play a role in suppressing the
development of breast cancer.
In the current study, RNA interference (RNAi) was
used to knock down the expression of claudin-6 in the human breast
epithelium cell line HBL-100. By examining the junctional function,
proliferation and migration, the aim was to determine whether the
loss of claudin-6 may have any effects on HBL-100 cells. The
results clearly showed that the downregulation of claudin-6 in
HBL-100 cells leads to a higher growth rate and migratory ability,
accompanied with an increased MMP-2 expression and activity, which
may be mediated via the p38 MAPK pathway. These observations
support the hypothesis that a decreased expression of claudin-6
contributes to the malignant progression of breast cancer.
Materials and methods
Cell line
Human breast epithelial cell line HBL-100 was
obtained from the American Type Culture Collection and maintained
in DMEM medium (Gibco-BRL, Carlsbad, CA, USA) supplemented with 10%
fetal bovine serum (Gibco) at 37ºC in 5% CO2.
Short hairpin RNA (shRNA)
transfection
Four shRNAs were designed, based on the claudin-6
mRNA sequence NM_021195 and were constructed into the vector
pGCsilencer™U6/Neo/GFP (GeneChem, Shanghai, China). The shRNAs were
transfected into cells using SuperFect® transfection
reagent (Qiagen, Germantown, MD, USA). Transfected cells were
treated with 400 μg/ml G418 (Sigma-Aldrich, St. Louis, MO, USA) for
10 days. Populations of knockdown cells were pooled following 10
days G418 selection. A negative control cell line was generated by
transfecting cells with the vector constructed by targeting a
sequence that did not yield any appreciable knockdown of the
protein production. shRNA sequences were as follows: i)
caGTCAAGCTATGGAACTAATTTCAAGAG AATTAGTTCCATCATAGCTTGACtg; ii)
aaCTGAGCC AAGGTGTTGACTTTCAAGAGAAGTCAACACCTTGGC TCAGtt; iii)
gcCAGATGCAGTGCAAGGTGTTTCAAGAG AACACCTTGCAACTGCATCTGgc and iv) caGTG
CAAGGTGTACGACTCATTCAAGAGATGAGTCGTACAC CTTGCACtg.
Reverse transcription-polymerase chain
reaction (RT-PCR)
Total RNA was extracted using TRIzol reagent
(Invitrogen Life Technologies, Carlsbad, CA, USA) and
reverse-transcribed with M-MLV reverse transcriptase (Takara Bio
Inc., Shiga, Japan). PCR was performed to analyze claudin-6 mRNA
expression (Applied Biosystems, Carlsbad, CA, USA). Forward and
reverse primers of claudin-6 used were: 5′-CAC TGCCACTTCTGGATGG-3′
and 5′-CAGTGCAGCTCCTT CAACCT-3′.
Measurement of transepithelial electrical
resistance (TER)
Monolayers of cells were grown in filters (Millicell
culture plate inserts; Millipore, Billerica, MA, USA). The TER was
measured when the cells had grown to full confluency, using a
voltmeter (Millicell-ERS). TER (Ω × cm2) = (total
resistance − blank resistance) (Ω) × area (cm2). Three
replicates each of three independent experiments were
performed.
Cell proliferation analysis
To measure cell proliferation, cells were seeded at
a low density (2×104/well) in 24-well plates and
cultured as described earlier. The number of cells was counted
every 24 h in the following 6 days. A growth curve was constructed
according to the data. Three replicates each of three independent
experiments were performed.
Wound-healing assay
Cells were grown to confluency and wounded by
dragging a 10-μl pipette tip through the monolayer. The cells were
washed to remove cellular debris and allowed to migrate for 48 h.
Images were captured by microscopy (Olympus, Tokyo, Japan) at 0, 24
and 48 h time-points following wounding. The widths of the wounded
areas were measured at 0 h (W0) and 48 h
(W48) time-points. The relative migration distance (% of
recovery) was calculated as (W0 −
W48)/W0 × 100%. Five replicates each of three
independent experiments were performed.
Western blot analysis
Cells were harvested and lysed in ice-cold lysis
buffer containing 50 mmol/l Tris (pH 7.4), 150 mmol/l NaCl, 1
mmol/l NaHCO3, 1% Triton X-100, 20 mmol/l EDTA, 1 mmol/l
NaF, 1 μg/ml leupeptine and 10 mmol/l phenylmethyl
sulfonylfluoride. The protein concentration was determined by BCA
protein assay kit (Pierce Biotechnology, Inc., Rockford, IL, USA).
Twenty micrograms of each sample were applied to 10%
SDS-polyacrylamide gel. Proteins were transferred onto a
nitrocellulose membrane (Millipore) and incubated with each primary
antibody, diluted at 1:1,000 (Santa Cruz Biotechnology, Inc., Santa
Cruz, CA, USA) overnight at 4ºC, followed by incubation for 1 h at
room temperature with a horseradish peroxidase-conjugated secondary
antibody diluted 1:1,000. The blots were then stained using an ECL
western blotting system (GE, Fairfield, CT, USA).
Gelatin zymography
The gelatinolytic activity of MMP-2 in the
conditioned medium was determined using zymography. Cells were
cultured in serum-free DMEM for 24 h and the supernatants were
collected following centrifugation at 500 × g for 10 min. Equal
amounts of total protein were loaded onto a zymogram gel composed
of 10% polyacrylamide with 1 mg/ml gelatin. Samples were
electrophoresed at a constant voltage of 130 V for 2 h. The gel was
washed for 40 min in 2.5% Triton X-100 to renature the proteins and
incubated for 40 h in 50 mmol/l Tris-HCl (pH 7.6) containing 5
mmol/l CaCl2 at 37ºC. Following staining with 1%
Coomassie blue and destaining, the proteolytic activity was
detected as clear bands on blue background gel. Three independent
experiments were performed.
Statistical analysis
Statistical analysis was performed using the SPSS
software package (version 13.0.0; SPSS, Inc., Chicago, IL, USA).
The Student’s unpaired t-test was performed to determine
statistically significant differences. P<0.05 was considered to
indicate a statistically significant difference.
Results
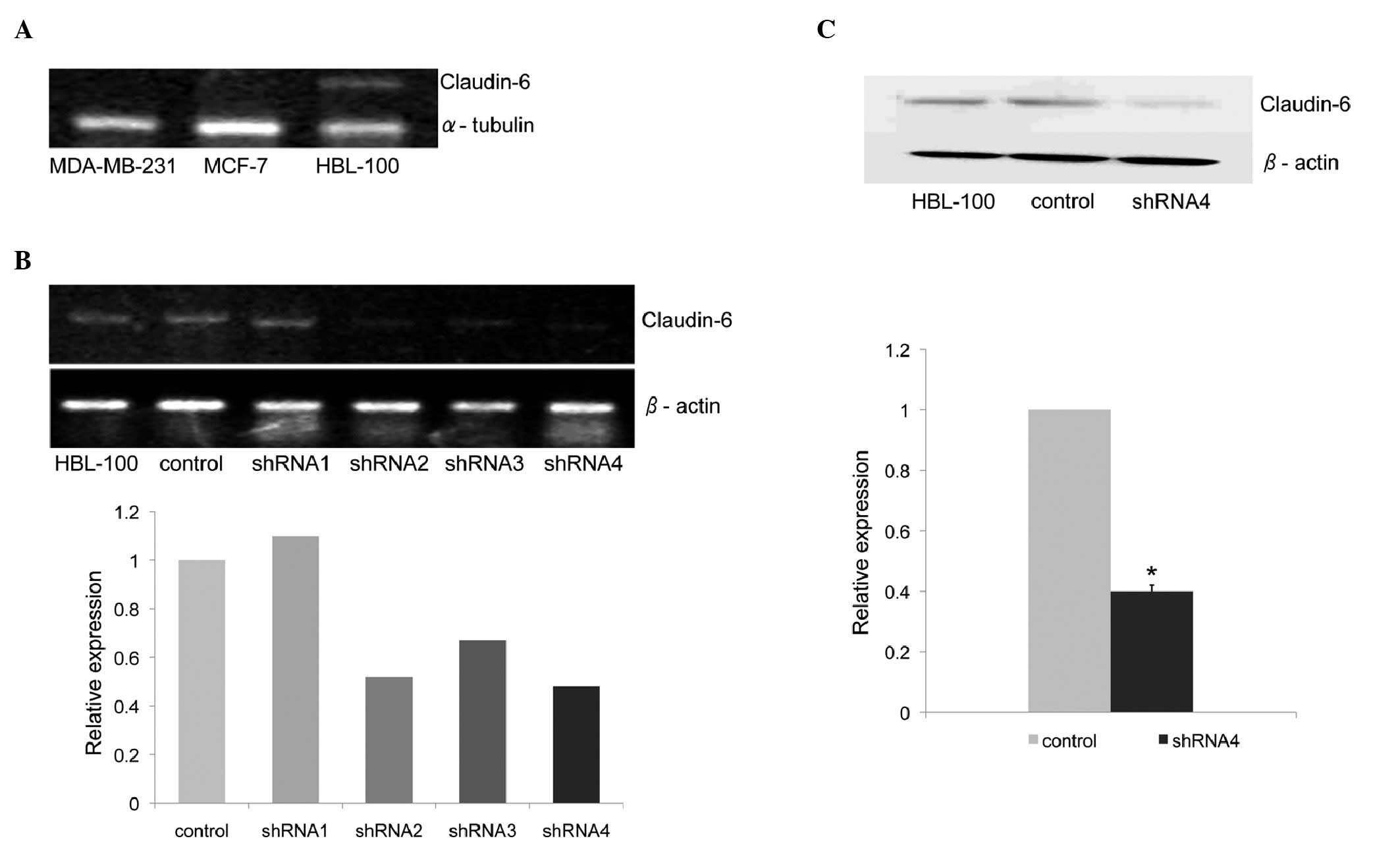
Claudin-6 expression in the HBL-100 cell
line is knocked down by shRNA
Claudin-6 expression was examined by RT-PCR in the
breast cancer cell lines MDA-MB-231 and MCF-7 and in the normal
human breast epithelial cell line HBL-100. Claudin-6 mRNA
expression was detected only in HBL-100 but not in the other two
breast cancer cell lines (Fig.
1A).
To investigate whether claudin-6 plays any role in
suppressing the development of breast cancer, shRNA was used to
knock down claudin-6 expression in the HBL-100 cell line. The
expression of claudin-6 mRNA was reduced by 50% in
shRNA-4-expressing cells, which was the most significantly
decreased of the four shRNA sequences. Claudin-6 expression
remained unchanged in the cells expressing control shRNA (Fig. 1B). The decreased expression of
claudin-6 protein in shRNA-4-expressing cells was confirmed by
western blot analysis using anti-claudin-6 antibody (Fig. 1C).
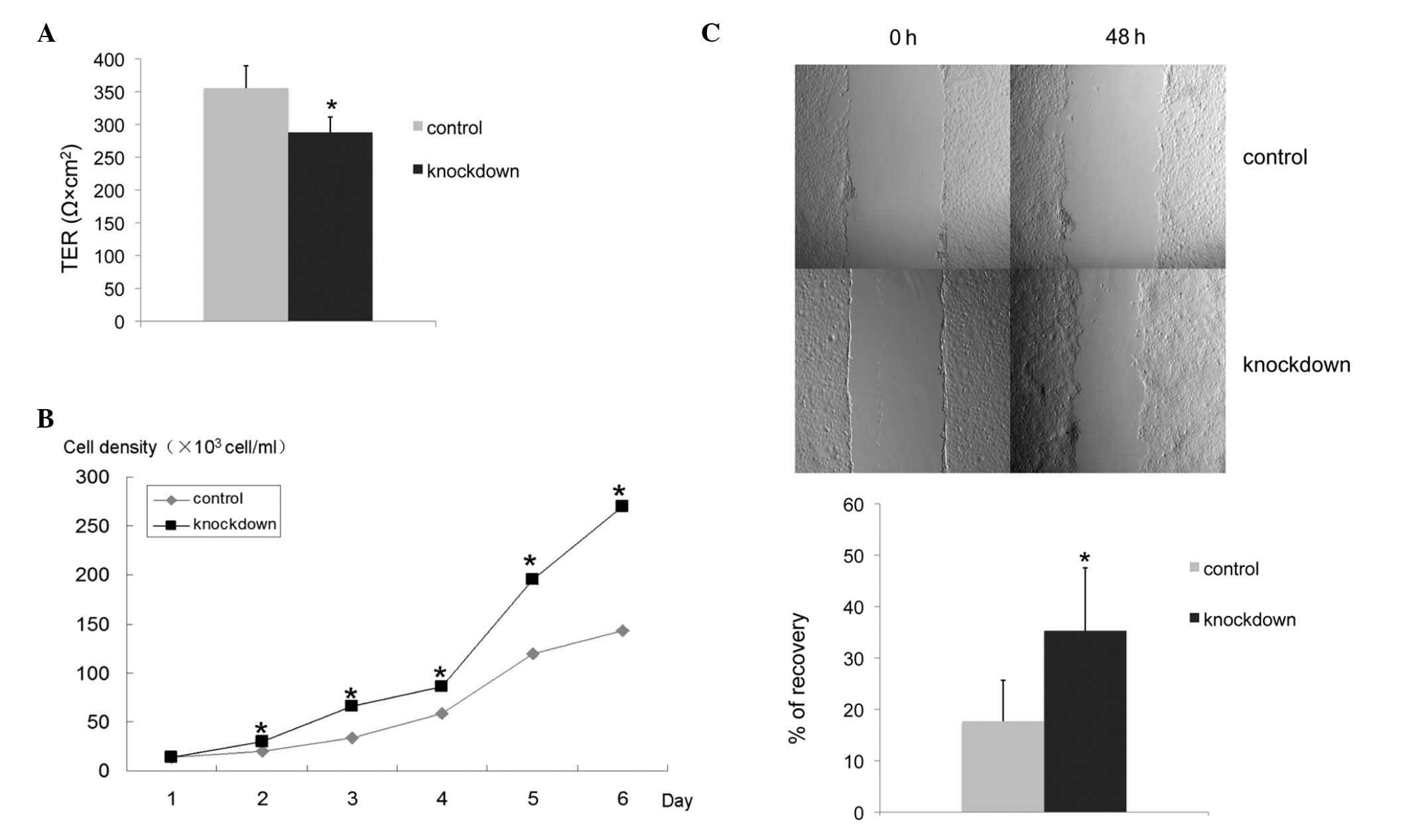
TER is decreased in claudin-6 knockdown
cells
Claudin expression is known to be important for
tight junction formation. TER across confluent epithelial cell
layers is a measurement of tight junction function. When cells
reached confluency, we measured TER to evaluate whether knockdown
of claudin-6 in HBL-100 cells may result in any changes in the
function of tight junctions. Compared with control cells, the TER
of claudin-6 knockdown cells was decreased and the differences were
found to be statistically significant (P<0.001; Fig. 2A). The results indicate that the
function of tight junction is changed by knockdown of claudin-6 in
HBL-100 cells.
Claudin-6 knockdown cells have a higher
proliferation rate and migratory ability
To determine the effect of claudin-6 knockdown on
the proliferation of HBL-100 cells, growth curves were constructed
over a period of 6 days by manual cell counting. The proliferation
rate of claudin-6 knockdown cells was significantly higher than
that in the control cells (P<0.001; Fig. 2B). The cell migratory ability was
compared between the claudin-6 knockdown cells and control cells,
using a wound healing assay. Following 24 and 48 h wound generation
in the monolayers of cells, the wound of the control cells was
wider than that of the claudin-6 knockdown cells. Claudin-6
interference resulted in a 100% increase in the migration rate
(P<0.001; Fig. 2C).
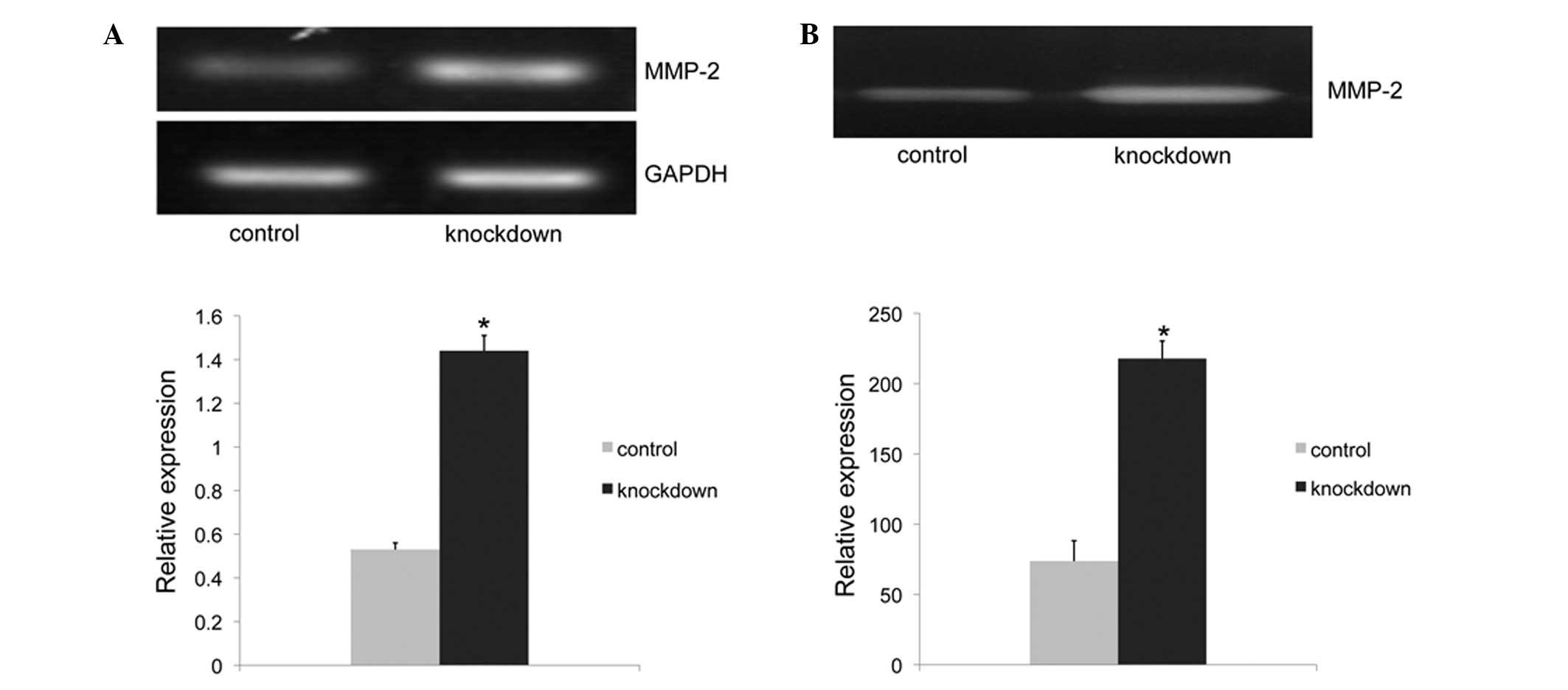
Claudin-6 knockdown cells have higher
expression and activity of MMP-2
As expression of MMP-2 is associated with cell
migration, changes in MMP-2 expression and activity were examined
by RT-PCR and gelatin zymography assay to investigate associations
with the migratory phenotype. The MMP-2 mRNA expression of the
claudin-6 knockdown cells was 2.6-fold higher than that of the
control cells (P<0.001; Fig.
3A). While the zymography assay result revealed that the MMP-2
activity of claudin-6 knockdown cells was 3-fold higher than that
of the control cells (P<0.001; Fig.
3B). The increase in MMP-2 activity caused by claudin-6
knockdown was associated with the results of cell migration,
indicating that claudin-6 knockdown may increase the cell migration
ability through activation of MMP-2.
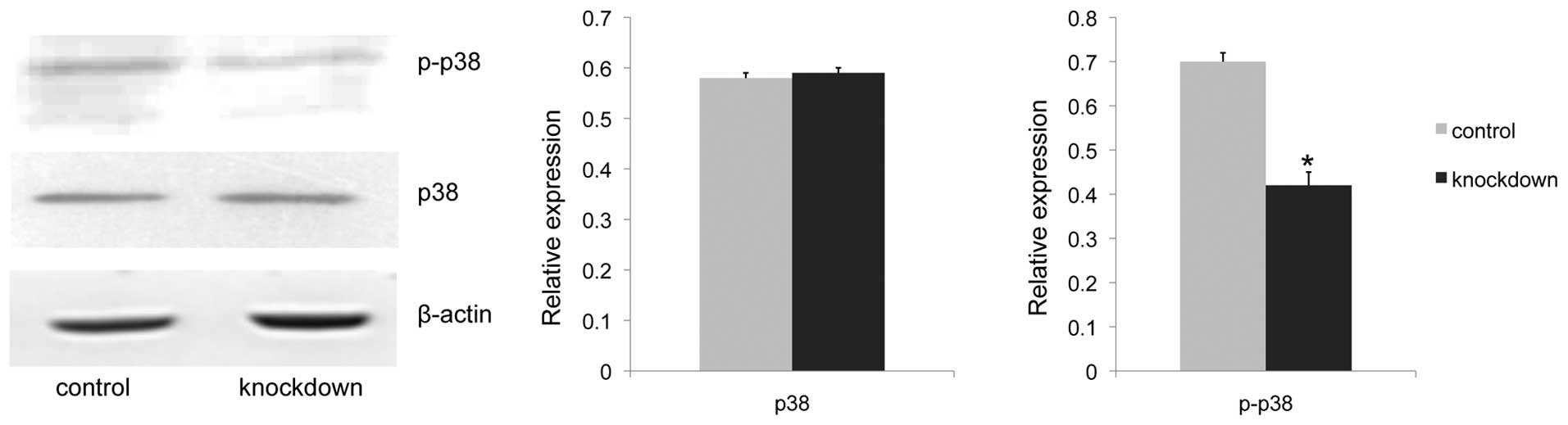
Claudin-6 knockdown cells have lower
phosphorylated p38 MAPK expression
It has been reported that the p38 MAPK signaling
pathway was involved in cell proliferation and MMP activation in
other cell lines (12–14). To test whether claudin-6 knockdown
had any effect on the p38 MAPK pathway, the level of p38 MAPK and
phosphorylated p38 MAPK protein expression was investigated by
western blot analysis. Results showed a 41% decrease in
phosphorylated p38 MAPK expression of claudin-6 knockdown cells
(P<0.001), while the p38 MAPK expression remained unchanged
(Fig. 4). The present results
confirmed the correlation between claudin-6 expression and p38 MAPK
pathway activation.
Discussion
One of the first steps in cancer metastasis is loss
of cell-to-cell adhesion (15,16).
Claudins are known to be important for tight junction formation and
the changes in their expression or localization often leads to the
disruption or deregulation of the tight junction structure and
function, which play a critical role in cancer progression,
invasion and metastasis. TER across the confluent epithelial cell
layer is a measurement of tight junction function. Overexpression
of various claudins, including claudin-1 and -4, leads to increased
TER (17,18), while the downregulation of various
types of claudins may lead to decreased TER and altered tight
junction structures, including claudin-3, -4 (19), -5 (20) and -7 (21). Overexpression of claudin-6 was
observed to lead to an increased TER in the MCF-7 breast cancer
cell line (11). Consistent with
this previous observation, the current study found that the TER of
claudin-6 knockdown cells was lower than the control cells,
indicating that the organization or function of tight junction is
disrupted by the downregulation of claudin-6 in the HBL-100 cell
line.
In the current study, significantly higher rates of
proliferation in claudin-6 knockdown cells, compared with control
cells were observed. In addition, the downregulated expression of
claudin-6 was found to lead to an increased migratory ability in
HBL-100 cells with a higher mRNA expression and activity of MMP-2,
suggesting that the cells gained a relatively, more malignant
phenotype following claudin-6 knockdown mediated via the activation
of MMP-2. Furthermore, a decreased expression of phosphorylated p38
MAPK in claudin-6 knockdown cells was also found in the current
study, indicating that p38 MAPK may be one of the signaling
pathways involved in altering the phenotype of the cells following
claudin-6 knockdown. To the best of our knowledge, this is the
first report in which silencing of the claudin-6 gene enhanced cell
proliferation and migration, accompanied with MMP-2 activation via
the p38 MAPK pathway in HBL-100 cells.
Cell proliferation and migration are essential for
cancer progression, invasion and metastasis. Evidence has shown the
effect of claudin on cell proliferation; findings of a previous
study showed that the overexpression of claudin-6 resulted in a
lower proliferation rate of MCF-7 cells (11) and it has been reported by Li et
al(22) that the
overexpression of miR-7 and miR-218 was accompanied by reactivation
of claudin-6 gene, leading to the inhibition of the cell cycle and
clone formation of breast cancer cells, which markedly supports the
results of the current hypothesis. Sun et al(23) have also reported that the
downregulated expression of claudin-3 reduces cell proliferation.
The mechanism by which claudins affect proliferation is complex and
involves a number of signaling pathways, including p38 MAPK.
It has been reported that the p38 MAPK signaling
pathway negatively regulates cell proliferation in other cell
lines. The mechanism by which p38 MAPK regulates cell proliferation
remains unclear, however there are a number of studies suggesting
the involvement of the JNK/c-Jun pathway (24,25),
EGFR (26) and p53 (27). p38 also is involved in p21
upregulation, subsequently resulting in cell growth inhibition
(28). Increased proliferation of
hepatocytes has been observed during liver regeneration in mice
lacking p38 α in the liver (12).
As well as the changed proliferation ability, higher
migration ability in claudin-6 knockdown cells was observed. Cell
migration requires MMPs, serine proteases and a number of other
proteases. Numerous types of claudin have been shown to change
cancer metastasis by the regulation of MMP activity. For example,
overexpression of claudin-4 increased MMP-2 and -9 activity in
Caco-2 cells (29) and knockdown
of claudin-2 increased cell migration and MMP-9 activity in MDCK
cells (30). These studies suggest
the potential involvement of claudins in cancer metastasis via MMP
activation. Consistent with the current observations, Osanai et
al(31) demonstrated that
knockdown of claudin-6 resulted in increased MMP activity in MCF-7
cells. Although claudins have been shown to activate MMPs (8,32),
the mechanism of the enhancement of MMP-2 activity by claudin-6
knockdown is not yet understood. In the current study, a decreased
expression of phosphorylated p38 MAPK following claudin-6 knockdown
was observed. p38 MAPK has been shown to be involved in MMP-2 and
-9 regulation. Green et al have reported that inhibition of
the p38 MAPK pathway resulted in a 228% increase in MMP-2 secretion
(13) and Kim et al
demonstrated that TGF-β induced the upregulation of MMP-2 and -9
through p38 MAPK signaling in the human breast epithelial cell line
MCF10A (14), providing increasing
evidence of the correlation between MMP-2 activation and the p38
MAPK pathway. Since there is little understanding of the mechanism
by which p38 MAPK pathway is involved in MMP-2 activity, the
potential transcription factors involved in it are now under
investigation. However, further studies are required to address the
exact mechanism by which claudin-6 function is regulated through
the p38 MAPK signaling pathway to alter the phenotype of cells.
In conclusion, the current study has demonstrated
that downregulation of claudin-6 expression leads to a relatively
malignant phenotype mediated through p38 MAPK signaling pathway in
the human breast epithelium cell line HBL-100, further supporting
the hypothesis that claudin-6 is likely to be a suppressor in a
number of types of cancer. Decreased expression of claudin-6 may
contribute to the malignant progression of breast cancer.
Acknowledgements
This study was supported by grants from the National
Natural Science Foundation of China (no. 81172499) and Science and
Technology Development Plan of the Office of Science and Technology
Project in Jilin Province (no. 201115113). The authors would like
to thank William Orr, Department of Pathology, University of
Manitoba, Canada, for providing language help with this
manuscript.
References
|
1
|
Elkouby-Naor L and Ben-Yosef T: Functions
of claudin tight junction proteins and their complex interactions
in various physiological systems. Int Rev Cell Mol Biol. 279:1–32.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Tsukita S, Furuse M and Itoh M:
Multifunctional strands in tight junctions. Nat Rev Mol Cell Biol.
2:285–293. 2001. View
Article : Google Scholar : PubMed/NCBI
|
|
3
|
Matter K, Aijaz S, Tsapara A and Balda MS:
Mammalian tight junctions in the regulation of epithelial
differentiation and proliferation. Curr Opin Cell Biol. 17:453–458.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
González-Mariscal L, Betanzos A, Nava P
and Jaramillo BE: Tight junction proteins. Prog Biophys Mol Biol.
81:1–44. 2003.
|
|
5
|
Takahashi A, Kondoh M, Kodaka M and Yagi
K: Peptides as tight junction modulators. Curr Pharm Des.
17:2699–2703. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lee KW, Lee NK, Kim JH, et al: Twist1
causes the transcriptional repression of claudin-4 with prognostic
significance in esophageal cancer. Biochem Biophys Res Commun.
423:454–460. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Tokés AM, Kulka J, Paku S, et al:
Claudin-1, -3, and -4 proteins and mRNA expression in benign and
malignant breast lesions: a research study. Breast Cancer Res.
7:R296–R305. 2005.PubMed/NCBI
|
|
8
|
Miyamori H, Takino T, Kobayashi Y, Tokai
H, Itoh Y, Seiki M and Sato H: Claudin promotes activation of
pro-matrix metalloproteinase-2 mediated by membrane-type matrix
metalloproteinases. J Biol Chem. 276:28204–28211. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Turksen K and Troy TC: Junctions gone bad:
claudins and loss of the barrier in cancer. Biochim Biophys Acta.
1816:73–79. 2011.PubMed/NCBI
|
|
10
|
Quan C and Lu SJ: Identification of genes
preferentially expressed in mammary epithelial cells of Copenhagen
rat using subtractive hybridization and microarrays.
Carcinogenesis. 24:1593–1599. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wu Q, Liu Y, Ren Y, Xu X, Yu L, Li Y and
Quan C: Tight junction protein, claudin-6, downregulates the
malignant phenotype of breast carcinoma. Eur J Cancer Prev.
19:186–194. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Hui L, Bakiri L, Stepniak E and Wagner EF:
p38alpha: a suppressor of cell proliferation and tumorigenesis.
Cell Cycle. 6:2429–2433. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Green JA, Dholakia S, Friedland JS, et al:
Mycobacterium tuberculosis-infected human monocytes down-regulate
microglial MMP-2 secretion in CNS tuberculosis via TNFα, NFκB, p38
and caspase 8 dependent pathways. J Neuroinflammation.
8:462011.PubMed/NCBI
|
|
14
|
Kim ES, Kim MS and Moon A:
TGF-beta-induced upregulation of MMP-2 and MMP-9 depends on p38
MAPK, but not ERK signaling in MCF10A human breast epithelial
cells. Int J Oncol. 25:1375–1382. 2004.PubMed/NCBI
|
|
15
|
Hazan RB, Qiao R, Keren R, Badano I and
Suyama K: Cadherin switch in tumor progression. Ann NY Acad Sci.
1014:155–163. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Jordan NV, Johnson GL and Abell AN:
Tracking the intermediate stages of epithelial-mesenchymal
transition in epithelial stem cells and cancer. Cell Cycle.
10:2865–2873. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Bhat AA, Sharma A, Pope J, Krishnan M,
Washington MK, Singh AB and Dhawan P: Caudal homeobox protein cdx-2
cooperates with Wnt pathway to regulate claudin-1 expression in
colon cancer cells. PLoS One. 7:e371742012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Neesse A, Griesmann H, Gress TM and Michl
P: Claudin-4 as therapeutic target in cancer. Arch Biochem Biophys.
524:64–70. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Lambert D, Padfield PJ, McLaughlin J,
Cannell S and O’Neill CA: Ochratoxin A displaces claudins from
detergent resistant membrane microdomains. Biochem Biophys Res
Commun. 358:632–636. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Haorah J, Heilman D, Persidsky Y, et al:
Ethanol-induced activation of myosin light chain kinase leads to
dysfunction of tight junctions and blood-brain barrier compromise.
Alcohol Clin Exp Res. 29:999–1009. 2005. View Article : Google Scholar
|
|
21
|
Oshima T, Miwa H and Joh T: Aspirin
induces gastric epithelial barrier dysfunction by activating p38
MAPK via claudin-7. Am J Physiol Cell Physiol. 295:C800–C806. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Li Q, Zhu F and Chen P: miR-7 and miR-218
epigenetically control tumor suppressor genes RASSF1A and Claudin-6
by targeting HoxB3 in breast cancer. Biochem Biophys Res Commun.
424:28–33. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Sun C, Yi T, Song X, et al: Efficient
inhibition of ovarian cancer by short hairpin RNA targeting
claudin-3. Oncol Rep. 26:193–200. 2011.PubMed/NCBI
|
|
24
|
Hui L, Bakiri L, Mairhorfer A, et al:
p38alpha suppresses normal and cancer cell proliferation by
antagonizing the JNK-c-Jun pathway. Nat Genet. 39:741–749. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Perdiguero E, Ruiz-Bonilla V, Gresh L, et
al: Genetic analysis of p38 MAP kinases in myogenesis: fundamental
role of p38alpha in abrogating myoblast proliferation. EMBO J.
26:1245–1256. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ventura JJ, Tenbaum S, Perdiguero E, Huth
M, Guerra C, Barbacid M, Pasparakis M and Nebreda AR: p38alpha MAP
kinase is essential in lung stem and progenitor cell proliferation
and differentiation. Nat Genet. 39:750–758. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Bulavin DV and Fornace AJ Jr: p38 MAP
kinase’s emerging role as a tumor suppressor. Adv Cancer Res.
92:95–118. 2004.
|
|
28
|
Lee B, Kim CH and Moon SK: Honokiol causes
the p21WAF1-mediated G(1)-phase arrest of the cell cycle through
inducing p38 mitogen activated protein kinase in vascular smooth
muscle cells. FEBS Lett. 580:5177–5184. 2006. View Article : Google Scholar
|
|
29
|
Takehara M, Nishimura T, Mima S, Hoshino T
and Mizushima T: Effect of claudin expression on paracellular
permeability, migration and invasion of colonic cancer cells. Biol
Pharm Bull. 32:825–831. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Ikari A, Takiguchi A, Atomi K, Sato T and
Sugatani J: Decrease in claudin-2 expression enhances cell
migration in renal epithelial Madin-Darby canine kidney cells. J
Cell Physiol. 226:1471–1478. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Osanai M, Murata M, Chiba H, Kojima T and
Sawada N: Epigenetic silencing of claudin-6 promotes
anchorage-independent growth of breast carcinoma cells. Cancer Sci.
98:1557–1562. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Agarwal R, D’Souza T and Morin PJ:
Claudin-3 and claudin-4 expression in ovarian epithelial cells
enhances invasion and is associated with increased matrix
metalloproteinase-2 activity. Cancer Res. 65:7378–7385. 2005.
View Article : Google Scholar : PubMed/NCBI
|