Introduction
Nanomaterials have been widely studied over the past
decades, due to their unique chemical and physical properties. At a
nanoscale, the properties of materials depend significantly on
their particle size and morphology. Silver nanoparticles (AgNPs)
are one of the most common commercialized nanomaterials used in key
biological and medical studies for applications such as
antimicrobial agents, drug and gene delivery vehicles and
biosensors. (1,2)
AgNPs have been demonstrated to effectively inhibit
the replication of hepatitis B virus (HBV) (3). Injection of 10 and 50 nm diameter
AgNPs into patients with HBV reduced the quantity of HBV by 40%
within 10 min and by ~90% after 1 h (3). In addition, these nanoparticles have
been used to target cancer cells with the controlled uptake into
specific cellular compartments. Specifically, nanoparticles may be
delivered to specific tissues and subcellular compartments or to
malignant cells in circulation via a combination of antibody-based
targeting of ligands, changing of surface charge and material
composition (4). These studies
provided the basis for the prevention and cure of HBV infection and
also provided a reference for chronic liver disease, such as
hepatic fibrosis.
In the clinic, conventional anti-fibrotic treatments
and chemotherapy are of limited success in chronic liver injury,
predominantly due to non-specific drug effects and the development
of drug tolerance. Thus, AgNPs may provide a more efficacious and
safer therapeutic approach. The possibility of this approach is
important for hepatic stellate cells (HSCs), which are considered
to be targets for the therapy of hepatic fibrosis and liver
cirrhosis. Thus, investigation of the cytotoxicity of AgNPs in HSCs
may be useful in determining their potential use in clinical
applications.
In this study, the cytotoxicity of
polyvinylpyrrolidone (PVP)-coated AgNPs in primary HSCs derived
from fresh rat livers was determined. The biological responses of
HSCs, such as the morphological changes in subcellular structure,
proliferation, apoptosis, cell movement and cytokine secretion,
were determined following the treatment of cells with AgNPs.
Different particle sizes and concentrations of AgNPs were also used
to study their effects on the cytotoxicity of AgNPs.
Materials and methods
Preparation and characterization of
AgNPs
PVP-coated AgNPs with diameters of 10 and 30–50 nm
were received in solution from Dr Jie Liu (Duke University, Durham,
NC, USA). The size, morphology and dispersion of the nanoparticles
were characterized using a Tecnai™ G2 Twin Transmission Electron
Microscope (FEI, Hillsboro, OR, USA) and dynamic light scattering
(Compact Goniometer System 3; ALV-GmbH, Langen, Germany). The ζ
potential was determined using a Zetasizer Nano ZS (Malvern
Instruments, Malrem, UK). X-ray diffraction experiments were
performed on powder samples and were analyzed using an X’Pert PRO
MRD HR diffractometer with a Cu Kα radiation (1.5405 Å) at 5 kV and
40 mA (PANalytical, Almelo, Netherlands). X-ray photoelectron
spectroscopy (Kratos Analytical Inc., Chestnut Ridge, Spring
Valley, NY, USA) was used to determine the composition of the
surface coating.
Isolation and culture of rat HSCs
HSCs were isolated from the livers of six normal
Buffalo rats aquired from the Chinese Academy of Sciences
(Shanghai, China) using the improved Friedman method (5). The study was approved by the Ethics
Committee of Fudan University, Shanghai, China. Following
isolation, the cells were cultured in Dulbecco’s modified Eagle’s
medium, supplemented with 10% fetal bovine serum, in a humidified
incubator at 37°C with 5% CO2. The morphology and
features of HSCs were observed with light and fluorescence
microscopes. Cell purity was determined by immunofluorescence
staining for desmin and smooth muscle actin (SMA) using anti-α-SMA
antibodies from Sigma-Aldrich (St. Louis, MO, USA). Cells were
counterstained with fluorescein isothiocyanate (FITC)-conjugated
goat anti-mouse IgG (Becton-Dickinson, New York, NY, USA) and
nuclei were stained with DAPI. The purity of HSCs (%) was
calculated as the number of desmin-positive (or α-SMA-positive)
cells divided by the total number of cells multiplied by 100%. HSC
activation was determined by α-SMA immunofluorescence staining.
Transmission electron microscopy
observation
Transmission electron microscopy (TEM; CM12;
Philips, Amsterdam, Netherlands) was used to examine the morphology
of HSCs. HSCs were seeded in 100-mm tissue culture dishes, cultured
for 2 days and treated with different concentrations of AgNPs for
24 h.
Assessment of in vitro cytotoxicity
Cell proliferation was evaluated with a Cell
Counting Kit-8 (CCK-8; Becton Dickinson). Approximately
5×103 cells were plated in each well of a 96-well plate.
The cells were divided into seven groups. Group 1 served as a blank
control; groups 2, 3, and 4 were treated with 30–50 nm AgNPs at 20,
100 and 250 μg/ml, respectively; and groups 5, 6 and 7 were treated
with 10 nm AgNPs at 20, 100 and 250 μg/ml, respectively. Following
incubation for 96 h, 10 μl WST-8 was added to the wells and the
absorbance at 450 nm was determined using a microplate reader
(Thermo Fisher Scientific, Inc., Waltham, MA, USA). The
aforementioned samples were analyzed for 24 h.
Apoptosis detection
The FITC-Annexin V and propidium iodide (PI) double
staining method was used to detect apoptosis induced by AgNPs.
Freshly isolated HSCs were cultured in 96-well plates for 2 days
and were divided into seven groups as described previously for the
cytotoxicity studies. Following 24 h of nanoparticle treatment, the
cells were washed with cold phosphate-buffered saline (4°C) and
stained using the FITC-Annexin V Apoptosis Detection kit (ExCell
Biology, Inc., Shanghai, China). The stained cells were analyzed by
flow cytometry by a trained laboratory technician according to the
experimental protocol.
Analysis of AgNP acute toxicity by a
lactase dehydrogenase (LDH) activity assay
Rat LDH kits (Sigma-Aldrich) were used to conduct
the LDH leakage assay. Two days following seeding in 24-well
plates, the cells were divided into two groups. One group was
treated with AgNPs of different diameters and different
concentrations, and the other group was treated as a control
without nanoparticle treatment. The two groups were incubated at
37°C with 5% CO2 for 4 h according to the manufacturer’s
instructions. The cell culture medium (50 μl) was collected from
each well and analyzed using a spectrometer (UV-Vis-NIR
spectrophotometer; Agilent, Santa Clara, CA, USA).
Cell IQ
The cell biological behaviors, including the total
cell number, number of dead cells and cell movement, were measured
using a real-time cell-monitoring system with a cell-culturing
platform (Cell-IQ; Chip-Man Technologies, Tampere, Finland). HSCs
were cultured in the Cell-IQ system in 24-well plates
(1×104 cells/well) for 72 h. Cells were divided into
five groups with two wells per group. One group served as a blank
control and the remaining four groups were treated with large and
small AgNPs at 20 and 100 μg/ml, respectively.
Cytokine detection
The HSCs were adjusted to a concentration of
5×105 cells/ml and cultured in 24-well plates. The 24
wells were divided into three groups; one group served as the blank
control and the other two groups were treated with AgNPs (with
diameters of either 10 or 30–50 nm) at a concentration of 20 μg/ml.
The conditioned medium was collected after 2 days and the quantity
of hepatocyte growth factor (HGF), interleukin (IL)-6, transforming
growth factor (TGF)-β1, tumor necrosis factor (TNF)-α, matrix
metallopeptidase (MMP)-2 and MMP-9 in the serum-free
HSC-conditioned medium was quantified using an enzyme-linked
immunosorbent assay (ELISA) kit (ExCell Biology, Inc.) according to
the manufacturer’s instructions.
Statistical analysis
All experiments were repeated at least three times,
and the data are presented as the mean ± standard deviation.
Student’s t-test was performed to determine the statistical
significance of the difference between untreated cells (blank
control) and cells treated with AgNPs. P<0.05 was considered to
indicate a statistically significant difference.
Results
Characterization of AgNPs
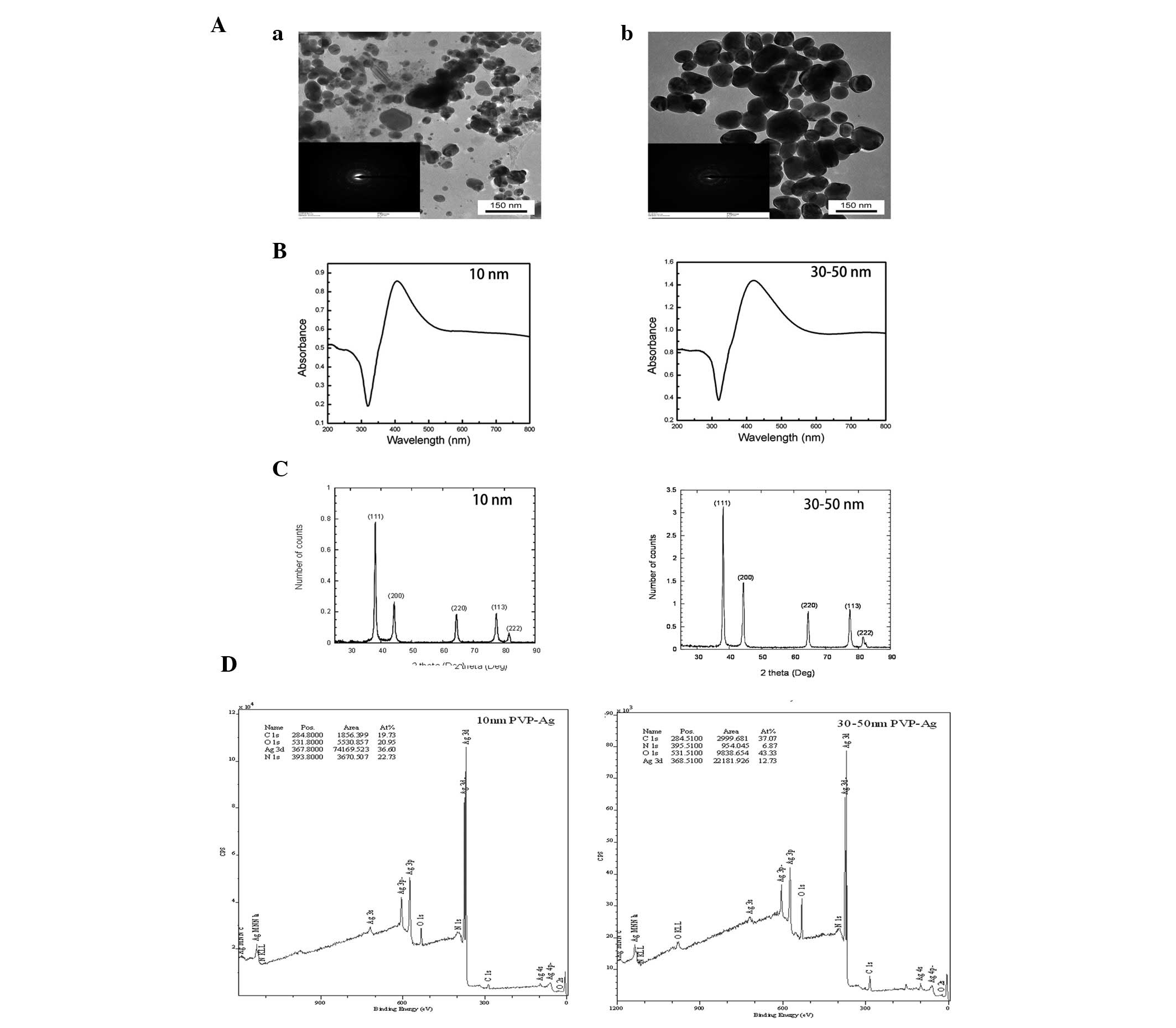
TEM images of the AgNPs used in this study are shown
in Fig. 1. The nanoparticles were
all roughly spherical in shape, although a few aggregates were also
observed. The diameters of these two types of AgNPs were 30±10 and
80±40 nm as determined by TEM. The nanoparticles consisted of C, O,
N and Ag, were metallic silver with a face-centered cubic lattice
and were polydispersed. The broad absorption at wavelengths >500
nm as shown in the ultraviolet-visible absorption spectra also
indicated the possible existence of aggregates (Fig. 1). The results of the other
characterization experiments are summarized in Table I.
 | Table ICharacterization of silver
nanoparticles. |
Table I
Characterization of silver
nanoparticles.
| Silver
nanoparticle |
|---|
|
|
|---|
| Property | Small | Large |
|---|
| Nominal diameter
(nm) | 10 | 30–50 |
| TEM diameter
(nm) | 30±10 | 80±40 |
| Hydrodynamic radius
using DLS (nm) | 28.6±0.61 (PDI:
0.395) | 58.74±1.49 (PDI:
0.491) |
| ζ Potential (mV) | −24.5±17.2 | −28.6±5.54 |
| Silver recovery using
ICP-MS (%) | 85.2 | 87.3 |
Identification of HSCs
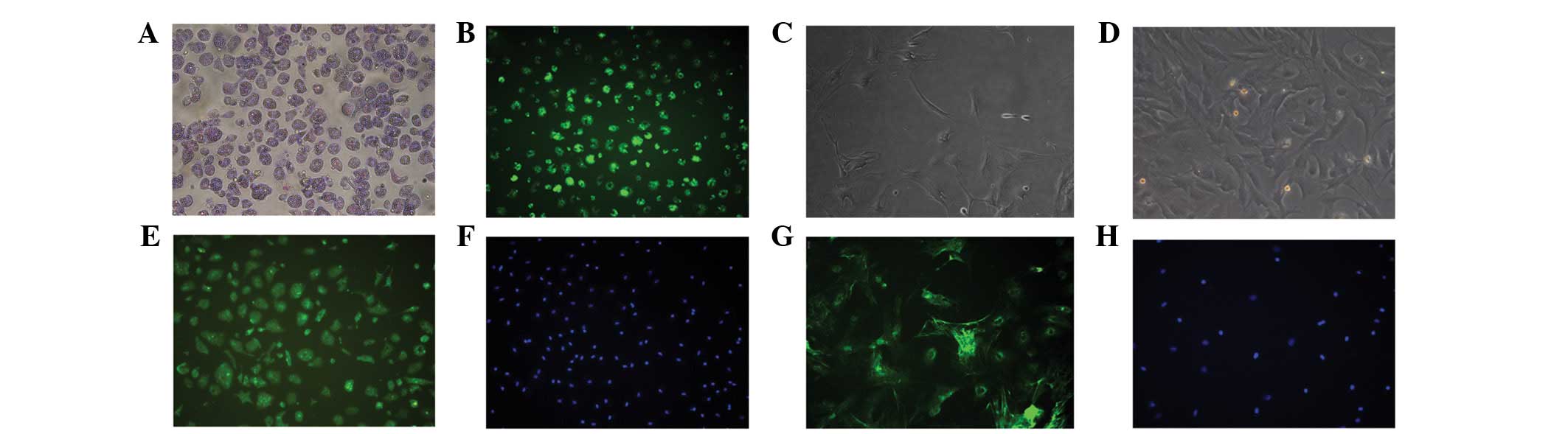
Approximately 2×107 HSCs were isolated
from each rat. The morphology of freshly isolated HSCs exhibited no
obvious features (Fig. 2A).
Abundant lipid droplets were observed with light microscopy and the
cyan vitamin A autofluorescence was excited at 328 nm, as observed
with fluorescence microscopy (Fig.
2B). However, activated HSCs demonstrated star-like morphology
(Fig. 2C) and developed into
fibroblast-like cells 7 days following isolation (Fig. 2D). More than 95% of the HSCs showed
positive desmin staining, indicating that the population consisted
of pure HSCs (Fig. 2E and F). In
addition, >95% of the HSCs were positive for α-SMA, also
suggesting a pure population (Fig. 2G
and H).
Ultrastructural characteristics of
AgNP-treated HSCs
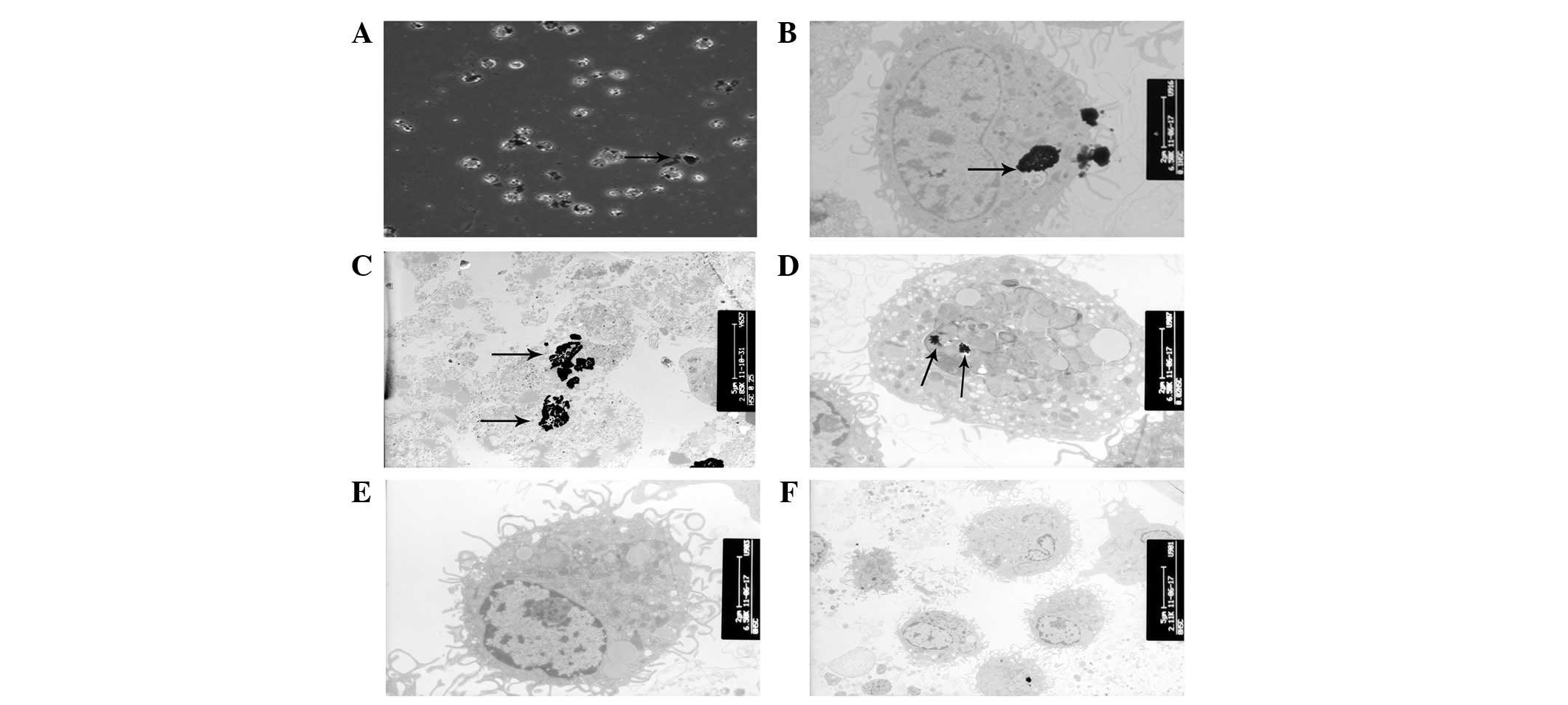
According to the TEM analysis, AgNPs were rapidly
internalized by HSCs, although the majority of the nanoparticles
were observed on the cell surface and between the cells (Fig. 3A). The TEM images of AgNP-treated
cells demonstrated the presence of electron-dense, aggregated
regions, which were thought to be AgNPs in the cytoplasm (Fig. 3B). Moreover, AgNP-treated HSCs
exhibited karyolysis and ruptured cell membranes (Fig. 3C). The presence of large vacuoles
and the swelling of the mitochondria indicated the destruction of
organelles in the treated cells (Fig.
3D).
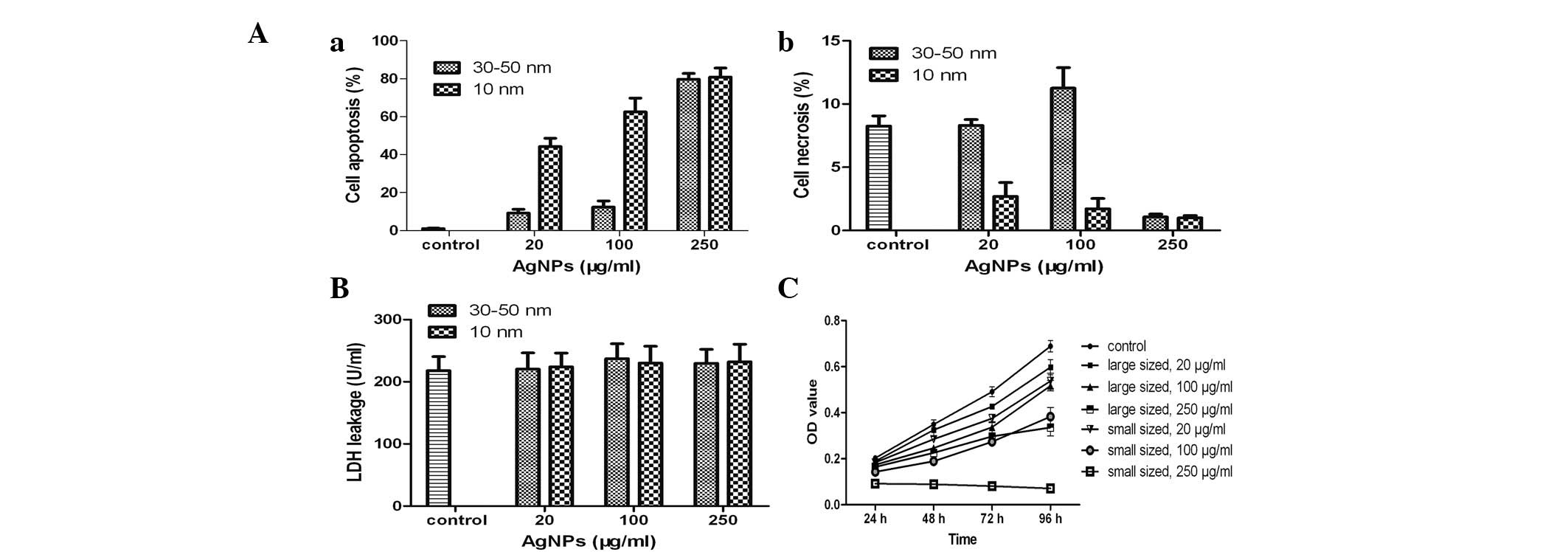
Apoptosis detection
To investigate the mechanism of cell death induced
by AgNPs, the treated cells were stained with FITC-Annexin V and
PI. The smaller AgNPs exhibited a greater ability to induce
apoptosis and necrosis in the HSCs than the larger AgNPs (Fig. 4A). Morever, treatment of HSCs with
higher concentrations of AgNPs induced greater rates of apoptosis
and necrosis than the lower concentrations of AgNPs. Cell death was
primarily due to a size-dependent increase in apoptosis induced by
the smaller AgNPs. In addition, the large and small AgNPs induced
greater apoptosis than necrosis in treated HSCs (Fig. 4Aa). Notably, AgNPs induced the
greatest apoptosis and the least necrosis at the highest
concentration tested (250 μg/ml).
Acute HSC cytotoxicity and AgNPs
The effect of AgNPs on the plasma membrane was not
statistically significant at any of the concentrations tested
(P>0.05; Fig. 4B). Thus, the
HSC death was not able to be correlated with the acute toxicity of
AgNPs. The aforementioned results also indicated the primary cause
of HSC death was not due to the acute toxicity of AgNPs.
In vitro cytotoxicity of AgNPs
The time-dependent cytotoxicity of AgNPs was
assessed using the CCK-8 assay (Fig.
4C). HSC growth was arrested in the presence of the two sizes
of nanoparticles at various concentrations. The influence of
particle size and concentration on cell proliferation was
determined. Treatment of cells with 100 μg/ml AgNPs resulted in a
decrease in cell proliferation after 96 h of exposure. These
effects were also dependent on the AgNP diameter size. In
particular, the smaller nanoparticles induced a greater decrease in
cell viability than larger ones. Furthermore, in cases of treatment
with identically sized nanoparticles, there was a greater
inhibitory effect with an increased concentration.
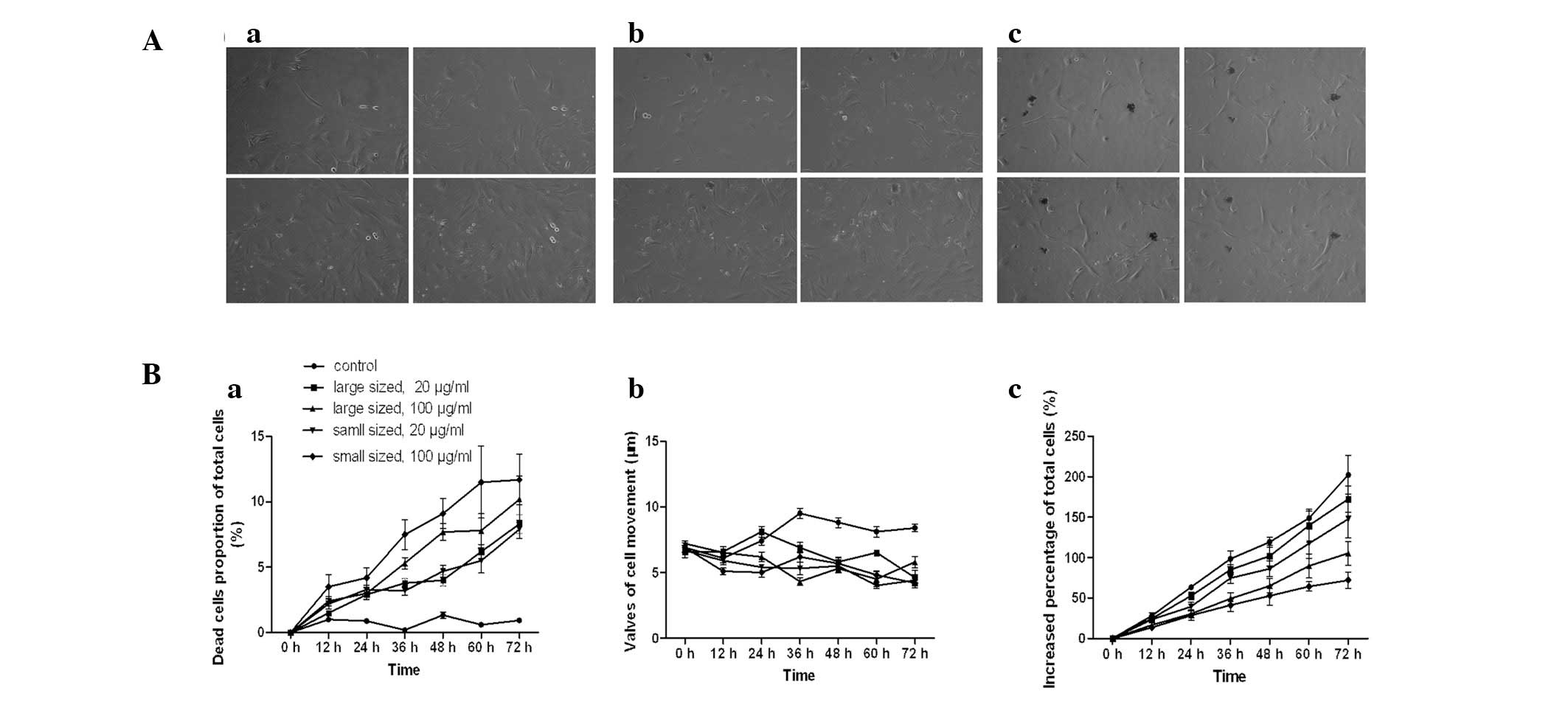
Inhibitory effects of AgNPs on cell
biological behaviors
Dynamic alterations of the total cell number, number
of dead cells and cell movement were measured using a Cell-IQ
culturing platform. The increased percentage of the total cell
number was significantly lower following incubation with AgNPs,
than in untreated cells (P<0.05). The inhibitory effects of
AgNPs were dependent on the size and dose (Fig. 5Bc). The number of dead cells as a
percentage of total cells in the control group was significantly
lower than that in the groups treated with AgNPs (P<0.05), and
the effect was dose- and size-dependent (Fig. 5Ba). Similarly, the movement of
AgNP-treated HSCs was inhibited (P<0.05). The diameter of the
AgNPs, however, was not significantly correlated with cell
migration at high concentrations (P>0.05).
Effect of AgNPs on various cytokines
Analysis of cytokine levels following treatment of
HSCs with AgNPs was performed using ELISA. Production of HGF, IL-6,
TGF-β1, and TNF-α was not significantly different between the
treated and untreated groups (Fig.
6). The cells treated with AgNPs produced less MMP-2 and MMP-9
than the control cells (P<0.05).
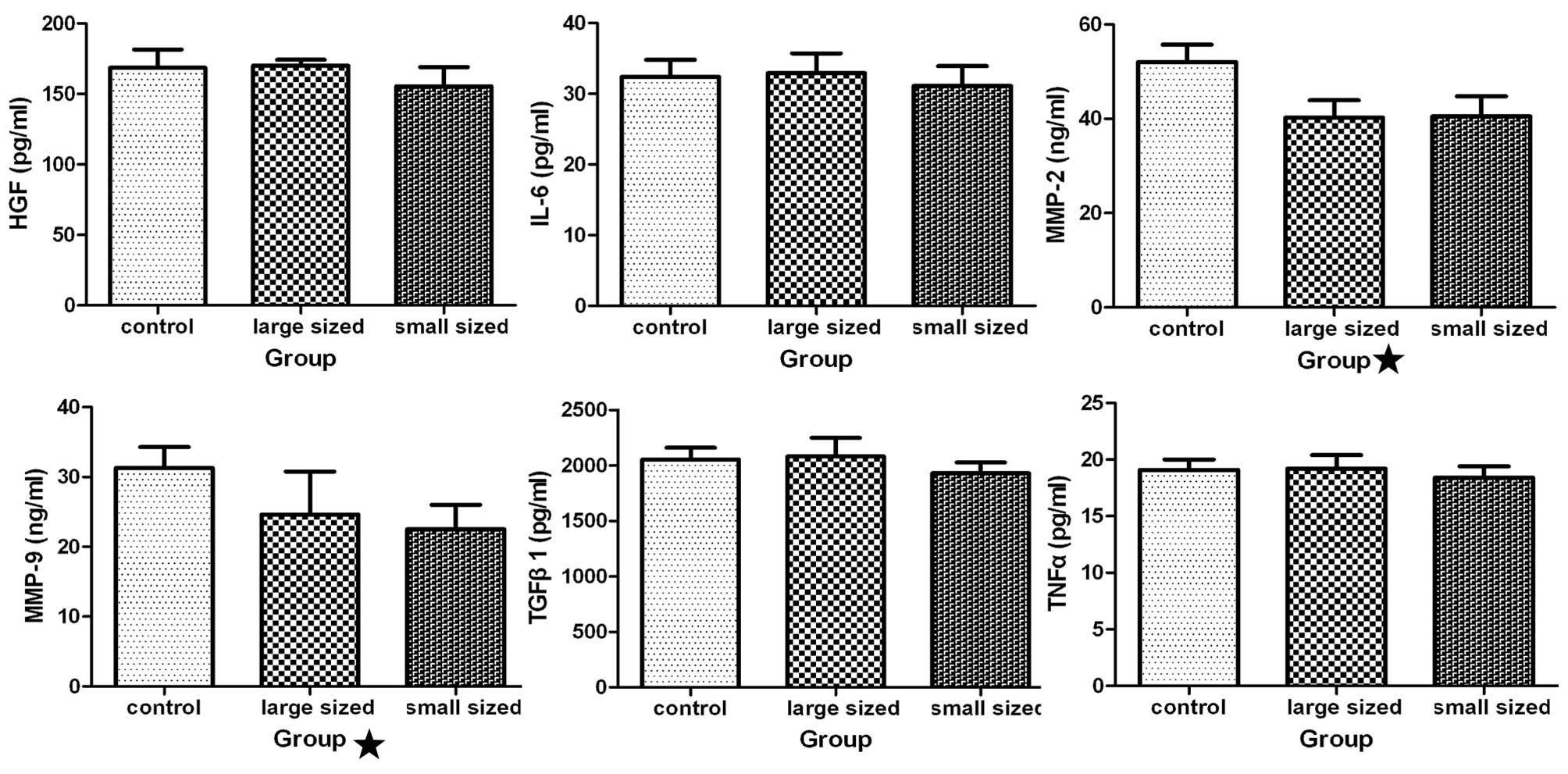 | Figure 6Influence of silver nanoparticles
(AgNPs) on cytokine production. Following incubation of hepatic
stellate cells (HSCs) with AgNPs of different sizes at 0.2 mg/ml
for 48 h, the levels of various cytokines [hepatocyte growth factor
(HGF), interleukin (IL)-6, transforming growth factor (TGF)-β1,
tumor necrosis factor (TNF)-α, matrix metallopeptidase (MMP-2) and
MMP-9] in the medium were measured using an enzyme-linked
immunosorbent assay kit. Statistical analysis showed that the
levels of MMP-2 and −9 were significantly different in cultures of
cells incubated with AgNPs as compared with the medium from the
untreated cells (P<0.05), while the other four groups were not
significantly different compared with the control group
(P>0.05). Control, HSCs treated without AgNPs; large sized, HSCs
treated with AgNPs, with a diameter of 30–50 nm; small sized, HSCs
treated with AgNPs, with a diameter of 10 nm. |
Discussion
Previously, AgNPs have been studied as a result of
their ability to inhibit HBV and their function in drug delivery
and targeting, which may lead to their application for treating
liver diseases (1,6,7).
With such considerable interest in the development of AgNPs for
medical applications, there is concern regarding their cytotoxicity
and its potential mechanism of action. Consequently, this study
investigated the cytotoxic effects of AgNPs on HSCs. The study
demonstrated three key results: i) the AgNPs exerted a strong
negative effect on HSCs, even at low concentration; ii) the
particle size of the AgNPs affected the cytotoxicity, as the
smaller nanoparticles exerted larger effects on cell bioactivity
than the larger nanoparticles; and iii) AgNPs altered the secretion
of cytokines by HSCs, which may further affect the microenvironment
of HSC activation.
Previous studies have demonstrated the impact of
nanoparticle size on cellular uptake and consequent cytotoxicity
(8,9). In the present study, the cytotoxic
effects of AgNPs, according to their size and concentration, were
determined by the investigation of AGNPs of two distinct sizes at
various concentrations. The results of the CCK-8 assay demonstrated
that the cytotoxic effects exerted by AgNPs on HSCs are size- and
dose-dependent. Smaller AgNPs induced greater HSC cytotoxicity than
the larger particles, and at higher concentrations, these
nanoparticles induced greater apoptosis. The death of HSCs was not
due to acute toxicity, according to our studies, which confirmed
the relatively safe use of HSCs in vivo. Morever, the
mechanism of cell death induced by AgNPs was analyzed and it was
demonstrated that the cell death of AgNP-treated HSCs was
predominantly due to apoptosis. The induced HSC apoptosis primarily
occurred in cells treated with the smaller nanoparticles and the
apoptotic behavior was dose dependent. To enter the cell nucleus,
nanoparticles must be small enough to pass through the nuclear pore
complex on the nuclear membrane. The size of AgNPs is therefore
significant in cellular uptake, and thus may impact their
bioactivity (10). As a result,
consideration of the particle size is required in the design of
nanoparticles for biomedical uses.
The changes in HSC morphology were studied using
phase contrast microscopy and TEM prior to and following incubation
with two sizes of AgNPs at various concentrations. These studies
indicated that AgNPs induced HSCs apoptosis or necrosis. The
ultrastructural characteristics of apoptosis are cell shrinkage,
karyopyknosis, karyorrhexis and karyolysis. Karyorrhexis is a type
of destructive fragmentation of the nucleus and is preceded by
pyknosis and followed by karyolysis (11). This fragmentation appeared
predominantly in HSCs treated with 250 μg/ml of smaller AgNPs
(Fig. 3C). Karyotheca
disintegration followed by karyolysis is the complete dissolution
of the chromatin matter of a dying cell. Cell membrane rupture and
the eventual fragmentation of the cell into apoptotic bodies that
are engulfed by neighboring cells or phagocytes was observed in
HSCs treated with AgNPs (12). The
cellular uptake of smaller nanoparticles may be easier than the
uptake of larger nanoparticles, although this hypothesis requires
additional confirmation by quantitative analysis. Necrosis,
however, occurs when the cell is sacrificed and is characterized by
cell swelling, cell organelle swelling and the formation of
microvesicles (Fig. 3D). This
cellular swelling may be accompanied by an increase in
intracellular pressure that leads to the breakage of the cell
membrane, which allows leakage of the cytoplasm into the
intercellular space (13).
The mitochondrial swelling observed following
incubation of HSCs with AgNPs indicated that the AgNPs in the
cytoplasm primarily reside in the mitochondria, affecting their
function and consequently exerting effects on cell metabolism.
Mitochondria are important signaling centers during apoptosis, and
the loss of mitochondrial integrity is induced and inhibited by
numerous regulators of apoptosis (14). For example, Bcl-2 prevents the
opening of the mitochondrial membrane pore, whereas Bax accelerates
this opening (15). AgNPs have
been demonstrated to activate the intrinsic apoptotic pathway,
which is characterized by the modulation of Bax and Bcl-2
expression, disruption of the mitochondrial membrane potential, and
cytochrome c release from the mitochondria (16). During the apoptotic process, the
mitochondrial membrane pores are opened and the mitochondrial
membrane potential is disrupted (17). Loss of mitochondrial membrane
potential is followed by cytochrome c release from the
mitochondria, resulting in the activation of caspase-9 and −3.
Activated HSCs are important in chronic liver
disease through the production of various cytokines. The ELISA
assay confirmed that AgNPs inhibited the production of MMP-2 and
−9, which are known to be crucial in chronic liver injury. During
hepatic inflammation and hepatocellular necrosis, MMP-2 and −9 are
predominantly produced by myofibroblasts, which transdifferentiate
from activated HSCs (18). In
chronic liver disease, increases in MMP-2 and −9 are associated
with fibrosis and the development of cirrhosis through altered
matrix production and degradation (19). In addition, the overexpression of
MMP-2 and −9 has been detected in hepatocellular carcinoma (HCC)
(20). Moreover, the
overexpression of MMP-2 and −9 in HCC tissues is correlated with
liver cirrhosis, capsular invasion, the presence of intrahepatic
metastasis, vascular invasion and higher tumor-node-metastasis
stage (20). Therefore, the
inhibitory effects of AgNPs on certain cytokines may destroy the
microenvironment in hepatic fibrosis and cirrhosis; however, the
detailed mechanisms require further investigation.
In conclusion, the cytotoxicity of AgNPs was
investigated by various biochemical approaches and was particle
size- and dose-dependent. Morphology alterations may be an
indication of metabolic and structural disturbances of HSCs caused
by AgNPs. Thus, it was concluded that these alterations were
size-dependent, as smaller particles induced greater cellular
damage at the same concentrations. The results of the LDH assay
confirmed that the necrosis or apoptosis of HSCs was not due to the
acute toxicity of AgNPs but due to the effects of the nanoparticles
on the physiological activities of the cells. These findings may
illustrate the relative safety of AgNP application to the human
body. AgNPs also affected the HSC cytokine secretion, as
demonstrated by the inhibitory effects of AgNPs on the production
of MMP-2 and −9. These results suggested that AgNPs may be used in
the treatment of hepatic fibrosis, including HCC; however, the
molecular mechanisms of nanoparticle cytotoxicity remain unclear.
Therefore, further studies are required to elucidate particle-cell
interactions and the metabolic and immunological responses
activated in HSCs in the presence of AgNPs.
Acknowledgements
This study was conducted at Duke University and was
supported by the National Science Foundation (NSF) and the
Environmental Protection Agency (EPA) under NSF Cooperative
Agreement EF-0830093, Center for the Environmental Implications of
NanoTechnology. Any opinions, findings, conclusions or
recommendations expressed in this material are those of the
author(s) and do not necessarily reflect the views of the NSF or
the EPA. This study has not been subjected to EPA review and no
official endorsement should be inferred.
References
|
1
|
AshaRani PV, Low Kah Mun G, Hande MP and
Valiyaveettil S: Cytotoxicity and genotoxicity of silver
nanoparticles in human cells. ACS Nano. 3:279–290. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Xu X, Yang Q, Bai J, Lu T, Li Y and Jing
X: Fabrication of biodegradable electrospun
poly(L-lactide-co-glycolide) fibers with antimicrobial nanosilver
particles. J Nanosci Nanotechno. 8:5066–5070. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Lu L, Sun RW, Chen R, Hui CK, Ho CM, Luk
JM, Lau GK and Che CM: Silver nanoparticles inhibit hepatitis B
virus replication. Antivir Ther. 13:253–262. 2008.PubMed/NCBI
|
|
4
|
Schroeder S, Heller DA, Winslow MM, et al:
Treating metastatic cancer with nanotechnology. Nat Rev Cancer.
12:39–50. 2011. View
Article : Google Scholar
|
|
5
|
Weiskirchen R and Gressner AM: Isolation
and culture of hepatic stellate cells. Methods Mol Med. 117:99–113.
2005.PubMed/NCBI
|
|
6
|
Lara HH, Ayala-Nuñez NV, Ixtepan-Turrent L
and Rodriguez-Padilla C: Mode of antiviral action of silver
nanoparticles against HIV-1. J Nanobiotechnology. 8:1–10. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Gopinath P, Gogoi SK, Chattopadhyay A and
Ghosh SS: Implications of silver nanoparticle induced cell
apoptosis for in vitro gene therapy. Nanotechnology. 19:104–113.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Yuan Y, Liu C, Qian J, Wang J and Zhang Y:
Size-mediated cytotoxicity and apoptosis of hydroxyapatite
nanoparticles in human hepatoma HepG2 cells. Biomaterials.
31:730–740. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Abdelhalim MA and Jarrar BM: Gold
nanoparticles induced cloudy swelling to hydropic degeneration,
cytoplasmic hyaline vacuolation, polymorphism, binucleation,
karyopyknosis, karyolysis, karyorrhexis and necrosis in the liver.
Lipids Health Dis. 10:1662011. View Article : Google Scholar
|
|
10
|
Li Y, Tian X, Lu Z, Yang C, Yang G, Zhou
X, Yao H, Zhu Z, Xi Z and Yang X: Mechanism for
alpha-MnO2 nanowire-induced cytotoxicity in Hela cells.
J Nanosci Nanotechnol. 10:397–404. 2010.PubMed/NCBI
|
|
11
|
Zamzami N and Kroemer G: Condensed matter
in cell death. Nature. 401:127–128. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Urne AG and Vaux DL: Molecular and
clinical aspects of apoptosis. Pharmacol Ther. 72:37–50. 1996.
View Article : Google Scholar
|
|
13
|
Schrand AM, Rahman MF, Hussain SM,
Schlager JJ, Smith DA and Syed AF: Metal-based nanoparticles and
their toxicity assessment. Wiley Interdiscip Rev Nanomed
Nanobiotechnol. 2:544–568. 2010. View
Article : Google Scholar : PubMed/NCBI
|
|
14
|
Green DR and Reed JC: Mitochondria and
apoptosis. Science. 281:1309–1312. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zamzami N, Marchetti P, Castedo M, Zanin
C, Vayssière JL, Petit PX and Kroemer G: Reduction in mitochondrial
potential constitutes an early irreversible step of programmed
lymphocyte death in vivo. J Exp Med. 181:1661–1672. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Piao MJ, Kang KA, Lee IK, Kim HS, Kim S,
Choi JY, Choi J and Hyun JW: Silver nanoparticles induce oxidative
cell damage in human liver cells through inhibition of reduced
glutathione and induction of mitochondria-involved apoptosis.
Toxicol Lett. 201:92–100. 2011. View Article : Google Scholar
|
|
17
|
Kroemer G, Zamzami N and Susin SA:
Mitochondrial control of apoptosis. Immunol Today. 18:44–51. 1997.
View Article : Google Scholar
|
|
18
|
Chung TW, Kim JR, Suh JI, Lee YC, Chang
YC, Chung TH and Kim CH: Correlation between plasma levels of
matrix metalloproteinase (MMP)-9/MMP-2 ratio and alpha-fetoproteins
in chronic hepatitis carrying hepatitis B virus. J Gastroen
Hepatol. 19:565–571. 2004. View Article : Google Scholar
|
|
19
|
Lichtinghagen R, Huegel O, Seifert T, et
al: Expression of matrix metalloproteinase-2 and −9 and their
inhibitors in peripheral blood cells of patients with chronic
hepatitis C. Clin Chem. 46:183–192. 2000.
|
|
20
|
Chen JS, Wang Q, Fu XH, Huang XH, Chen XL,
Cao LQ, Chen LZ, Tan HX, Li W, Bi J and Zhang LJ: Involvement of
PI3K/PTEN/AKT/mTOR pathway in invasion and metastasis in
hepatocellular carcinoma: Association with MMP-9. Hepatol Res.
39:177–186. 2009. View Article : Google Scholar : PubMed/NCBI
|