Introduction
Acute monocytic leukemia (AML) is a serious life
threatening clinical disorder and its prognosis is usually poor due
to its resistance to the majority of chemotherapy treatments.
Therefore, it is important to seek novel agents to overcome this
disorder (1).
In normal cells, glucose is firstly metabolized
through oxidative phosphorylation in mitochondria when oxygen is
available. However, if oxygen is deficient, pyruvate is converted
to lactate in the cytoplasm and then lactate is metabolized through
glycolysis for further metabolism to produce ATP. In cancer cells,
even with sufficient oxygen supply, cells prefer to undertake a
glycolytic process to generate ATP and lactate production. This
cancer metabolic phenotype is characterized by high glucose uptake,
increased aerobic glycolysis, decreased mitochondrial activity and
is known as the Warburg effect (2,3).
Previously, targeting glucose metabolism has been used as an
anticancer strategy (4), for
example, aminopterin is targeted to induce remission of leukemia by
blocking the activity of the enzyme dihydrofolate reductase, an
NADPH-dependent enzyme, which has a central role in nucleic acid
synthesis, which is activated by the high redox state induced by
aerobic glycolysis and/or glutamine breakdown. Methotrexate, is
another example of a related aminopterin compound that is a
clinically used anticancer drug (5–7). The
use of citrate has been reported in the treatment for a number of
cancers, however, the underlying mechanism remains unknown
(4,8–10).
The transcription factor hypoxia-inducible factor 1α
(HIF-1α) has been implicated in regulating genes which are
responsible for the metabolic differences between cancer and normal
cells (11,12). HIF-1α is closely associated with
multiple oncogenes, including ras, FOXO3a, bcl-2, VEGFA and PKM2
(11,13,14).
Exploiting HIF-1α to switch to glycolysis in the process of glucose
metabolism to produce ATP for cancer growth is a primary function
of the oncogenes. However, it remains unclear how this aerobic
glycolysis is regulated to sustain the growth of tumor cells
(11,15,16).
New data suggest that HIF-1α exerts its role by increasing
glycolysis rather than by suppressing mitochondrial activity
(17,18). In addition, studies on tumor
glycolysis have identified pyruvate kinase-M2 (19) as an intriguing novel interacting
partner for HIF-1α (14,20–22),
revealing a potential mechanism for the Warburg effect and this
characteristic may be exploited in targeting cancer (23–25).
The present study investigated the effects of
citrate on the monocytic leukemia cell line U937 in vitro
and a number of genes involved in glycolysis and cell death,
including bcl-2, caspases-9 and -3, and HIF-1α have been assessed
to explore the mechanisms underlying the anticancer activity of
citrate.
Materials and methods
Cell line and culture
The human established monocytic leukemia cell line,
U937, was commercially obtained from the Institute of Biochemistry
and Cell Biology, SIBS, Chinese Academy of Science (Shanghai,
China). The human lymphoblast cell line HYM2CIR, a de novo
cell line derived from a 56 year-old patient was a gift from
Yongchuan Wang (Department of Surgery, Shanghai First People’s
Hospital, Shanghai, China). The de novo cell line was
established following written consent from the patient and this
study was approved by the Ethics Committee of Shanghai First
People’s Hospital, Shanghai Jiaotong University, Shanghai, China
(approval number: 2012K071). The cell lines were cultured in
RMPI-1640 medium, 10% fetal calf serum and 1% (v/v)
penicillin/streptomycin (all Gibco-BRL, Carlsbad, CA, USA). DMEM
(no glucose) medium was purchased from Gibco-BRL. Cells were
maintained in a 5% CO2 humidified atmosphere at 37ºC and
powered tribasic sodium citrate was obtained from Sigma-Aldrich
(St. Louis, MO, USA).
Lactate measurement
Following termination of incubation, medium was
separated by centrifugation at 200 × g for 5 min. Lactate was
measured on an ABL700/800 series analyzer (Radiometer, Copenhagen,
Denmark) with a lower detection limit of 0.1 mM/l.
Cell viability measured by MTT
assays
Cell viability was measured using the CCK-8 assay at
48 h following citrate treatment. Briefly, U937 or HYM2CIR cells
(3×103), were seeded in each well in 96-well plates and
cultured overnight. The culture medium was removed and cells were
incubated with fresh medium containing citrate at concentrations
ranging between 0.125 and 4 mg/ml. Following 48 h, plates were
incubated with 10 μl CCK-8 for 4 h and measured with a Bio-Rad 680
microtiter plate reader (Bio-Rad, Hercules, CA, USA) at 450 nm. The
viability of cells treated with citrate was compared with untreated
cells. All experiments were performed a minimum of three times and
triplicate wells were used in each experiment.
HIF-1α silencing
Small interfering RNA (siRNA) was performed by
transfection using HIF-1α directed or control (Luciferase GL2;
Thermo Scientific, Waltham, MA, USA) double-stranded RNA
oligonucleotides. HIF-1α was synthesized by Eurofins MWG Operon
(Ebersberg, Germany). The target sequences are labeled ‘siRNA
Target Sequences’ and the sequence is as follows: HIF-1α forward
5′-CCACAGGACAGTACAGGATG-3′ and reverse
5′-TCAAGTCGTGCTGAATAATACC-3′. Cells (1.5×105) were
seeded in 12.5-cm2 flasks 24 h prior to treatment with
siRNA. At the time of transfection, the proliferation state of the
cell was in the exponential (40–50%) phase. Different
concentrations and incubation times of siRNA were analyzed in pilot
experiments. siRNA (75 nM) was transfected into U937 cells using
Interferin (Polyplus-transfection Inc., NY, USA) following the
manufacturer’s instructions. Cells were pre-treated with siRNA for
4 h at 37ºC under normoxic conditions and treated following
incubation.
Hoechst 33258 staining
U937 cells (1×105 cell/ml) were seeded in
24-well plates and treated with concentrations of citrate between
0.77 and 1.55 mM/l for 12–24 h. The apoptotic morphology of U937
cells was evaluated by Hoechst 33258 (KeyGen Biotech Co., Ltd.,
Nanjing, Jiangsu, China) staining according the kit’s
instructions.
Western blot analysis
Cells were centrifuged following treatment and
rinsed with ice-cold PBS, lysed in RIPA buffer (150 mM NaCl, 50 mM
Tris HCl pH 8, 1% Triton™ X-100, 4 mM PMSF, 2 mM aprotinin, 5 mM
EDTA, 10 mM NaF, 10 mM NaPPi and 1 mM Na3VO4) for 30 min on ice.
Lysates were clarified by centrifugation at 14,000 rpm for 15 min
at 4ºC and protein concentrations were determined using the BCA
assay (Bio-Rad). Equal amounts of total cellular protein (40 μg)
were resolved in a Bis-tris-HCL buffered (pH 6.4) 10–12%
polyacrylamide gel (Bio-Rad) for 90 min at 120 V and
electrophoretically transferred onto a PVDF membrane (Bio-Rad) for
75 min at 300 MA. The membrane was blocked for 1 h at room
temperature in T-TBS (132 mM NaCl, 20 mM Tris-HCl pH 7.6 and 0.05%
Tween-20) supplemented with 5% non-fat dry milk. The membrane was
incubated for 1 h at room temperature in T-TBS-milk with the
following primary antibodies: anti-Bcl-2, anti-caspases-9 and -3
(all 1:1,000, Cell Signaling Technology, Inc., Danvers, MA, USA),
anti HIF-1α (1:500, Bioword Technology, Visalia, CA, USA),
anti-glut-1 and anti-β-actin (1:500 and 1:3,000;
Sigma-Aldrich).
Statistical analysis
Statistical analysis was performed using the SPSS
software version 13.0 (SPSS, Inc., Chicago, IL, USA). Student’s
t-test was also used. P<0.05 was considered to indicate a
statistically significant difference.
Results
Citrate inhibits cellular proliferation
and induces apoptosis of U937
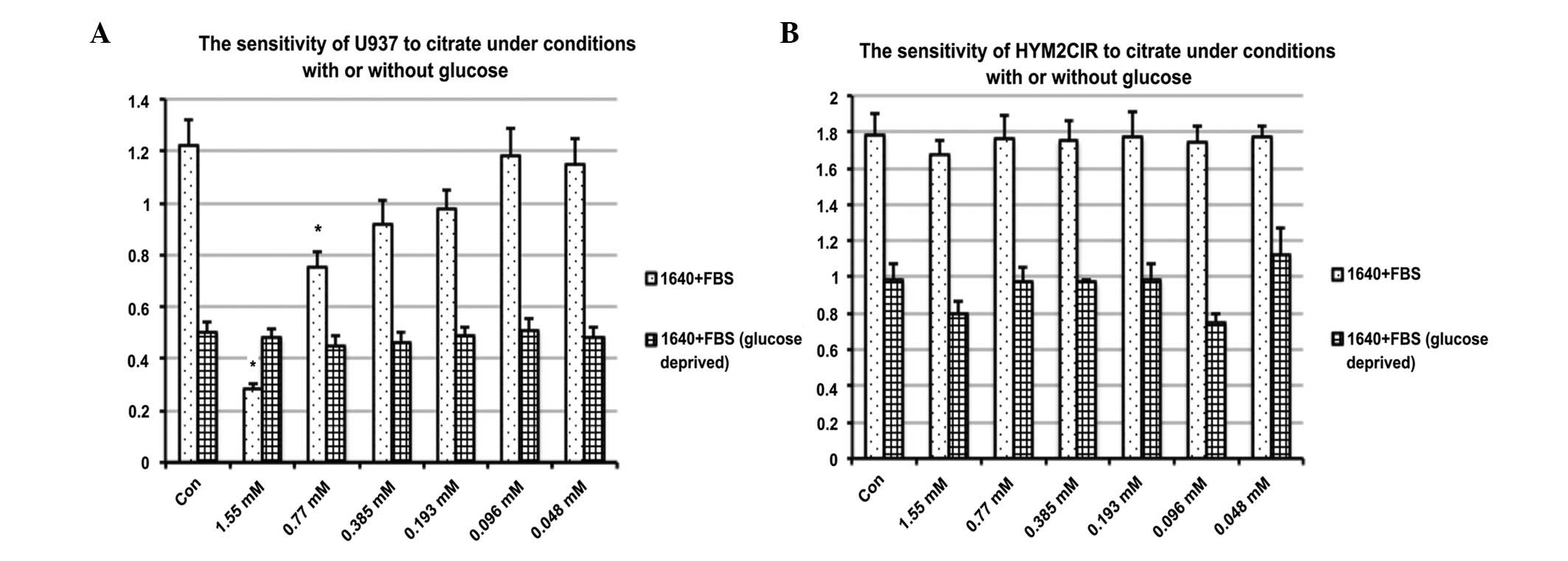
Cell viability was assessed using a CCK-8 assay.
Briefly, cells were treated with citrate at concentrations ranging
between 0.77 and 1.55 mM/l for 48 h with or without glucose in the
medium. The proliferation of cells was significantly inhibited in
U937 compared with control (P<0.05; Fig. 1). The IC50 values of
citrate for U937 were 0.86 mM/l. However, it did not significantly
affect the viability of HYM2CIR. This suggested that the
proliferation inhibition of U937 by citrate is associated with
glucose metabolism with little effect on normal cells.
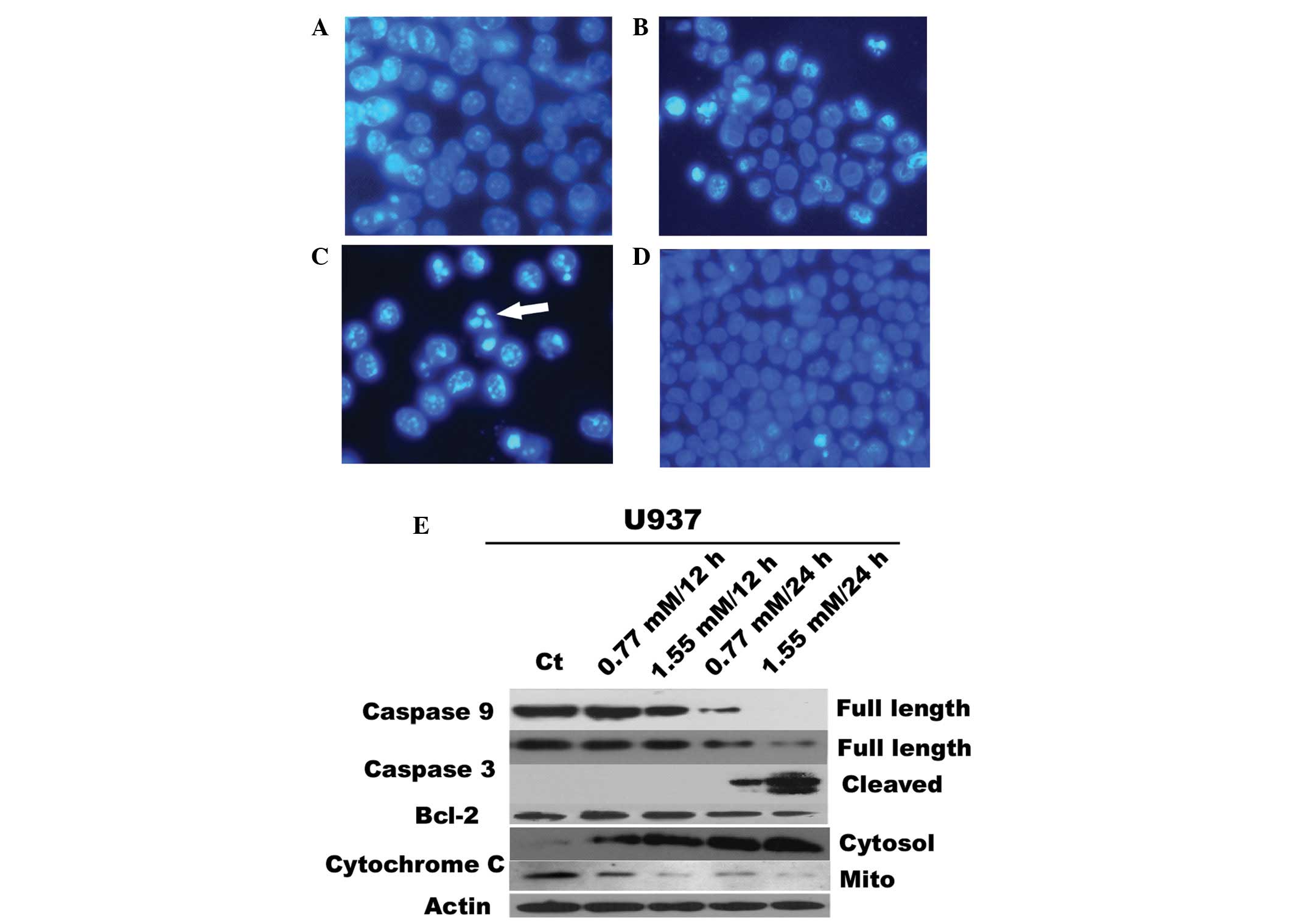
To probe the mechanisms by which citrate inhibits
cell proliferation, the morphological changes in citrate-treated
cells were examined. Apoptosis was evaluated by the appearance of
condensed and fragmented nuclei by Hoechst 33258 staining. Compared
with controls, more nuclei of U937 cells showing condensed and
apoptotic bodies were observed, due to apoptosis (Fig. 2B and C) in a dose- and
time-dependent manner.
To determine the apoptotic mechanisms, western
blotting was performed to assess the expression of Bcl-2 and
activation of caspases-3 and -9. The results showed that citrate
significantly inhibits Bcl-2 protein expression in U937 in a dose-
and time-dependent manner accompanied by the cleavage activation of
caspase-3 or -9 (Fig. 2E).
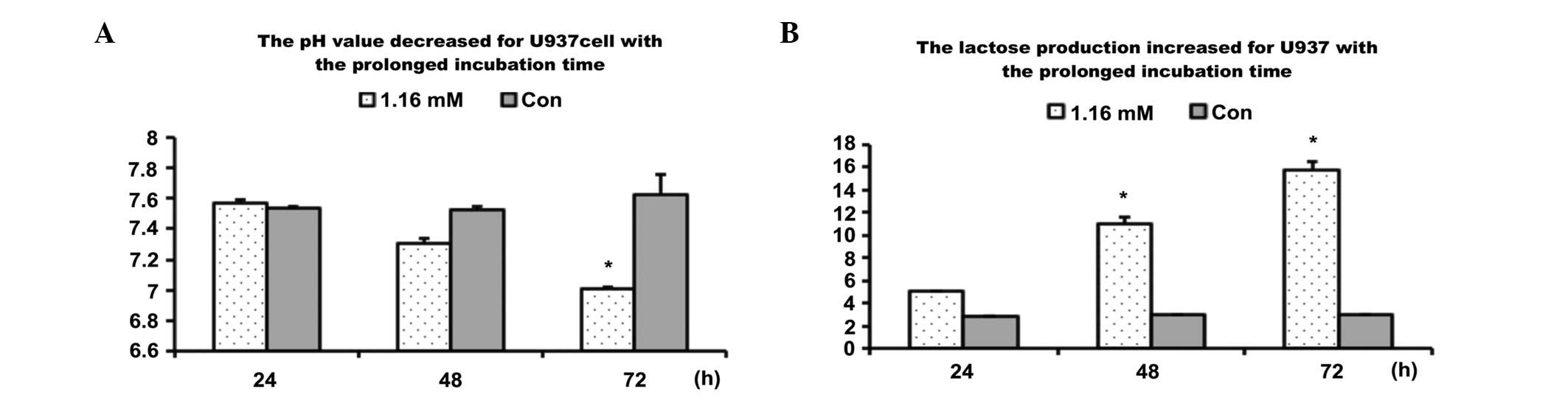
Lactate production of U937
Cells were incubated with citrate at a dose of 1.16
mM/l for 24, 36 or 72 h. Lactate production was compared between
treatment and control groups. The results showed that citrate
markedly reduced lactate production and increased the pH value
(Fig. 3), suggesting that U937
cell death treatment by citrate is induced by intervening in the
glycolysis process.
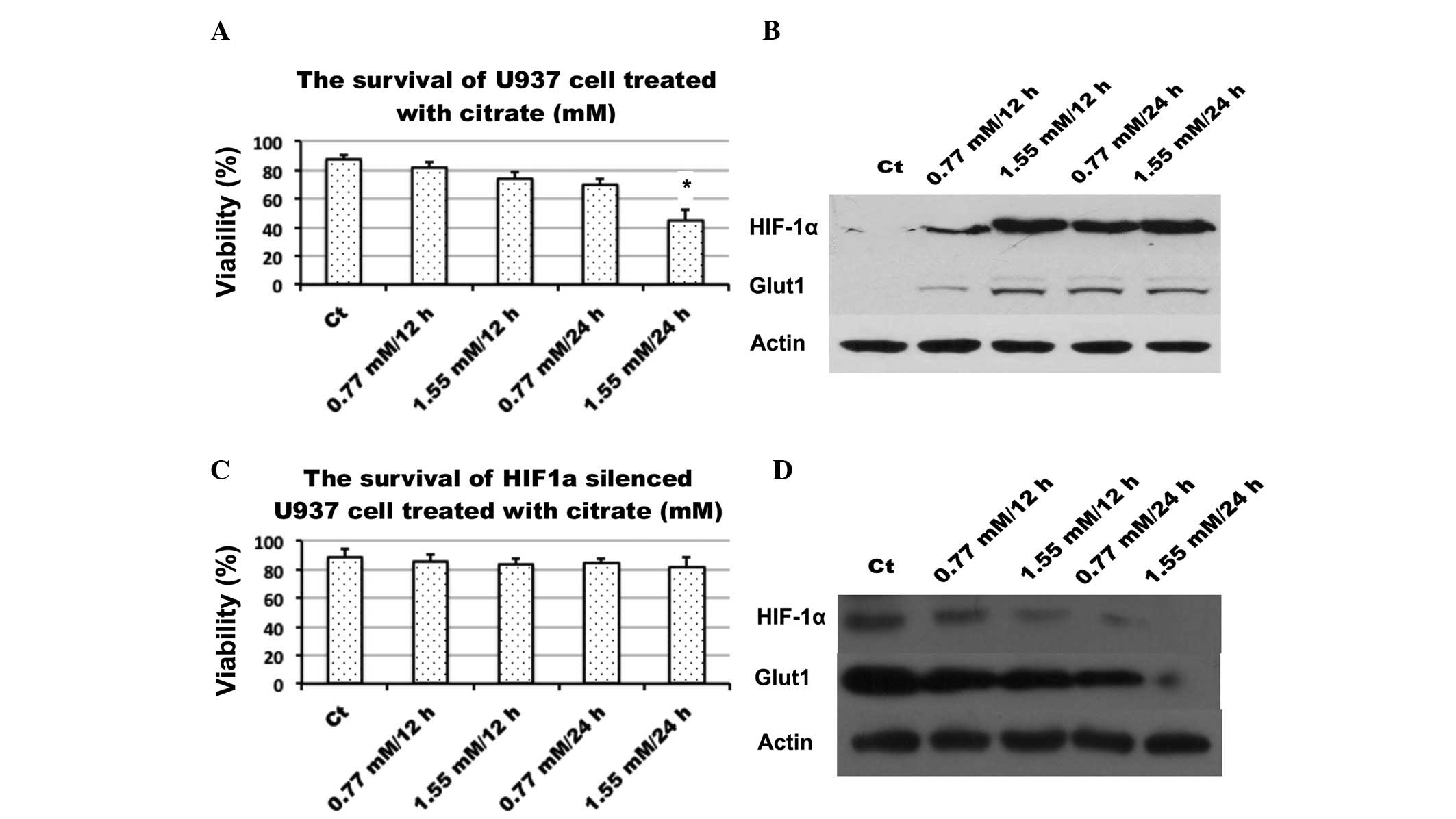
Citrate induces the expression of HIF-1α
and GLUT-1
To determine the role which citrate plays in
glycolysis inhibition, its effect on the expression of HIF-1α, an
important signaling molecule which is closely associated with
glucose metabolism, was examined. Following treatment with citrate
for 24 h, western blot analysis revealed that citrate up-regulated
the expression of HIF-1α and another important protein, glucose
transporter-1, which is a target of HIF-1α, the primary transporter
involved in glucose influx in U937 cells. By contrast, U937 cell
viability is also assayed following HIF-1α silencing (Fig. 4).
Discussion
Otto Warburg initially observed that, under aerobic
conditions, normal cells metabolize glucose to pyruvate through
glycolysis in the cytosol and then the pyruvate enters the
mitochondria for oxidative phosphorylation to produce ATP, while,
under anaerobic conditions, glycolysis is the primary mechanism to
obtain energy. By contrast, in cancer cells, even under conditions
of abundant oxygen, cancer cells consume glucose for their energy
production through glycolysis, this is known as the Warburg effect
(26,27). The Warburg effect is regarded as
one of the most significant hallmarks in cancer cells and targeting
glucose metabolism as an anticancer strategy has been attracting
attention over the past decade (26,28,29).
HIF-1α has been shown to be important in the altered metabolism of
cancer cells as it causes increased glycolysis and decreased
mitochondrial function in tumors by regulating a number of genes
involved in these processes (11,30,31).
HIF-1α plays its role by dimerizing with HIF-1β to activate a
number of enzymes involved in glycolysis and suppresses genes
involved in mitochondrial biogenesis (30,32).
For example, previous studies revealed that fibroblasts harboring
activated HIF-1α showed a marked reduction in Cav-1 levels and a
shift towards aerobic glycolysis and hence, a loss of mitochondrial
activity and an increase in lactate production (18,33).
It has been reported that fibroblasts expressing activated HIF-1α
have an increased tumor mass by ~2-fold and tumor volume by ~3-fold
and HIF-1α induces an increase in the lymph node metastasis of
cancer cells (34), suggesting a
complex role played by HIF-1α. Previous studies have observed that
HIF-1α overexpression is an indicator of poor prognosis in T1 and
T2 squamous cell carcinoma of the oral floor (11,12).
In the present study, the induction of HIF-1α was observed to be
associated with the expression of Glut1 and affects cell viability.
The difference between the current observation and the reports are
hypothesized to be attributed to cell type specificity. However,
the present results suggest that HIF-1α may be a promising target
in the treatment of monocytic leukemia.
Citrate is an intermediate in the tricarboxylic acid
cycle and glycolysis in glucose metabolism (2,23),
it is capable of inducing apoptosis for malignant pleural
mesothelioma cells by depletion of ATP as well as leading to the
diminution of the expression of the anti-apoptotic proteins and
inhibition of hexokinase due to the inhibition of
phosphofructokinase (10,35). Thus, it is logical that citrate
induces apoptosis by intervening in the glucose metabolic process.
It is well established that all cells survive of ATP supply and the
fast growing cells require more energy supply to meet the biomass
requirement of growth and proliferation, thus, it is possible that
cancer cells are more sensitive to lack of energy, specifically
lack of glucose. It is understandable that malignant cells are
prone to death once energy supply is insufficient (8,36).
In the current experiments, the AML U937, treated, with citrate was
observed to exhibit an elevation in lactate production and an
increase in pH value and thus, citrate exhibited a potent apoptotic
inducing activity towards AML U937 cells while sparing the normal
control cell. This suggests that citrate may kill tumor cells while
having little effect on healthy cells, possibly by regulating
HIF-1α and other associated signaling. The suppression of the
monocytic cell line U937 by citrate is hypothesized to be closely
associated with HIF-1α signaling, which may explain, at least
partially, the relatively specific targeting effects of citrate on
HIF-1α signaling in monocytic leukemia.
The intrinsic pathway of apoptosis is a major
pathway and it is triggered by signals of intrinsic cell damage or
multiple stresses. These signals lead to the activation of the
pro-death Bcl2 family proteins, including Bax and Bak, resulting in
oligomerization of these proteins and the formation of Bak or Bax
based pores in the outer mitochondrial membrane, followed by
cytochrome c release from the mitochondria into the cytosol
through these pores. Once translocated into the cytosol, cytochrome
c assembles with Apaf-1 and caspase-9 to form an apoptosome,
which leads to the activation of caspase-9. Caspase-9 subsequently
activates the executioner caspases-3 and -7, ultimately resulting
in apoptosis (9,37). From the observations that citrate
is a calcium chelate used clinically as an anticoagulant, a number
of experiments have been performed to determine whether citrate is
significant at a concentration that kills tumor cells, but spares
the normal cell. Notably, by supplementing calcium in vitro
and in vivo, studies demonstrated that citrate induces tumor
cell death by activating apical apoptotic molecules of the type one
apoptotic pathway, including caspases-8 and -3, and investigators
attributed the activation of apoptotic effector proteins, including
bax and cytochrome c to the its kosmotropic properties
(4,9,10).
Multiple genes in the apoptotic process were investigated. The
results showed that the expression of Bcl-2 is down regulated and
that caspases-9 and -3 were activated. According to the
observations, an intrinsic apoptotic pathway is hypothesized to be
important during the process of AML cell apoptosis induced by
citrate.
Acknowledgements
The authors would like to thank Mrs. Tang (The
Center of Experimental Animal at Shanghai First People’s Hospital)
for her technical assistance and Tao Kaizhong (Scientific Service
of Shanghai First People’s Hospital) for his instructive suggestion
in this study. Xiaowei Xu performed the whole study; Peijie Huang,
Xinjian Wan and Youwen Qin participated in the animal model
experiment; Baiwen Li, Lili Zhou, Huixia Liu and Haitao Bai
participated in the pathological and statistical sections; Yanrong
Gao was responsible for blood cell assay and MTT test and Chun Wang
and Xiangjun Meng were responsible for the design of the study and
the interpretation of the results, including the manuscript
preparation. This study was supported by grants from Shanghai
Pujiang Talent Program Fund [no. 21PJ1408500] and NSFC (no.
81072007).
References
|
1
|
Gupta K, Chakrabarti A, Rana S, Ramdeo R,
Roth BL, Agarwal ML, Tse W, Agarwal MK and Wald DN: Securinine, a
myeloid differentiation agent with therapeutic potential for AML.
PloS One. 6:e212032011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Cairns RA, Harris IS and Mak TW:
Regulation of cancer cell metabolism. Nat Rev Cancer. 11:85–95.
2011. View
Article : Google Scholar : PubMed/NCBI
|
|
3
|
Garber K: Energy deregulation: licensing
tumors to grow. Science. 312:1158–1159. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lu Y, Zhang X, Zhang H, Lan J, Huang G,
Varin E, Lincet H, Poulain L and Icard P: Citrate induces apoptotic
cell death: a promising way to treat gastric carcinoma? Anticancer
Res. 31:797–805. 2011.PubMed/NCBI
|
|
5
|
Agarwal NK, Mueller GA, Mueller C, Streich
JH, Asif AR and Dihazi H: Expression proteomics of acute
promyelocytic leukaemia cells treated with methotrexate. Biochim
Biophys Acta. 1804:918–928. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Morshed SR, Tokunaga T, Otsuki S, Takayama
F, Satoh T, Hashimoto K, Yasui T, Okamura M, Shimada J, Kashimata M
and Sakagami H: Effect of antitumor agents on cytotoxicity
induction by sodium fluoride. Anticancer Res. 23:4729–4736.
2003.PubMed/NCBI
|
|
7
|
Verburg M, Renes IB, Einerhand AW, Büller
HA and Dekker J: Isolation-stress increases small intestinal
sensitivity to chemotherapy in rats. Gastroenterology. 124:660–671.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Bayley JP and Devilee P: Warburg tumours
and the mechanisms of mitochondrial tumour suppressor genes.
Barking up the right tree? Curr Opin Genet Dev. 20:324–329. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kruspig B, Nilchian A, Orrenius S,
Zhivotovsky B and Gogvadze V: Citrate kills tumor cells through
activation of apical caspases. Cell Mol Life Sci. 69:4229–4237.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhang X, Varin E, Allouche S, Lu Y,
Poulain L and Icard P: Effect of citrate on malignant pleural
mesothelioma cells: a synergistic effect with cisplatin. Anticancer
Res. 29:1249–1254. 2009.PubMed/NCBI
|
|
11
|
Cairns RA, Papandreou I, Sutphin PD and
Denko NC: Metabolic targeting of hypoxia and HIF1 in solid tumors
can enhance cytotoxic chemotherapy. Proc Natl Acad Sci USA.
104:9445–9450. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Fillies T, Werkmeister R, van Diest PJ,
Brandt B, Joos U and Buerger H: HIF1-alpha overexpression indicates
a good prognosis in early stage squamous cell carcinomas of the
oral floor. BMC Cancer. 5:842005. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Denko NC: Hypoxia, HIF1 and glucose
metabolism in the solid tumour. Nat Rev Cancer. 8:705–713. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Doe MR, Ascano JM, Kaur M and Cole MD: Myc
posttranscriptionally induces HIF1 protein and target gene
expression in normal and cancer cells. Cancer Res. 72:949–957.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Bohuslavová R, Kolář F, Kuthanová L,
Neckář J, Tichopád A and Pavlinkova G: Gene expression profiling of
sex differences in HIF1-dependent adaptive cardiac responses to
chronic hypoxia. J Appl Physiol. 109:1195–1202. 2010.PubMed/NCBI
|
|
16
|
Sitkovsky M and Lukashev D: Regulation of
immune cells by local-tissue oxygen tension: HIF1 alpha and
adenosine receptors. Nat Rev Immunol. 5:712–721. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kumar A, Kant S and Singh SM: Novel
molecular mechanisms of antitumor action of dichloroacetate against
T cell lymphoma: Implication of altered glucose metabolism, pH
homeostasis and cell survival regulation. Chem Biol Interact.
199:29–37. 2012. View Article : Google Scholar
|
|
18
|
Priebe A, Tan L, Wahl H, Kueck A, He G,
Kwok R, Opipari A and Liu JR: Glucose deprivation activates AMPK
and induces cell death through modulation of Akt in ovarian cancer
cells. Gynecol Oncol. 122:389–395. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Sklar P, Ripke S, Scott LJ, Andreassen OA,
Cichon S, Craddock N, Edenberg HJ, Nurnberger JI Jr, Rietschel M,
Blackwood D, et al: Large-scale genome-wide association analysis of
bipolar disorder identifies a new susceptibility locus near ODZ4.
Nat Genet. 43:977–983. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
20
|
Simon F, Bockhorn M, Praha C, Baba HA,
Broelsch CE, Frilling A and Weber F: Deregulation of HIF1-alpha and
hypoxia-regulated pathways in hepatocellular carcinoma and
corresponding non-malignant liver tissue - influence of a modulated
host stroma on the prognosis of HCC. Langenbecks Arch Surg.
395:395–405. 2010. View Article : Google Scholar
|
|
21
|
Yang W, Xia Y, Ji H, Zheng Y, Liang J,
Huang W, Gao X, Aldape K and Lu Z: Nuclear PKM2 regulates β-catenin
transactivation upon EGFR activation. Nature. 480:118–122.
2011.
|
|
22
|
Luo W and Semenza GL: Pyruvate kinase M2
regulates glucose metabolism by functioning as a coactivator for
hypoxia-inducible factor 1 in cancer cells. Oncotarget. 2:551–556.
2011.PubMed/NCBI
|
|
23
|
Dang CV, Hamaker M, Sun P, Le A and Gao P:
Therapeutic targeting of cancer cell metabolism. J Mol Med (Berl).
89:205–212. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Mazurek S: Pyruvate kinase type M2: a key
regulator of the metabolic budget system in tumor cells. Int J
Biochem Cell Biol. 43:969–980. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Pan JG and Mak TW: Metabolic targeting as
an anticancer strategy: dawn of a new era? Sci STKE.
2007:pe142007.PubMed/NCBI
|
|
26
|
Hsu PP and Sabatini DM: Cancer cell
metabolism: Warburg and beyond. Cell. 134:703–707. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Vander Heiden MG, Cantley LC and Thompson
CB: Understanding the Warburg effect: the metabolic requirements of
cell proliferation. Science. 324:1029–1033. 2009.PubMed/NCBI
|
|
28
|
Koppenol WH, Bounds PL and Dang CV: Otto
Warburg’s contributions to current concepts of cancer metabolism.
Nat Rev Cancer. 11:325–337. 2011.
|
|
29
|
Maxwell RE: Calibration of Warburg
Manometers. Science. 110:403–404. 1949. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Tennant DA: PK-M2 Makes Cells Sweeter on
HIF1. Cell. 145:647–649. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Wittwer JA, Robbins D, Wang F, Codarin S,
Shen X, Kevil CG, Huang TT, Van Remmen H, Richardson A and Zhao Y:
Enhancing mitochondrial respiration suppresses tumor promoter
TPA-induced PKM2 expression and cell transformation in skin
epidermal JB6 cells. Cancer Prev Res (Phila). 4:1476–1484. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Christofk HR, Vander Heiden MG, Harris MH,
Ramanathan A, Gerszten RE, Wei R, Fleming MD, Schreiber SL and
Cantley LC: The M2 splice isoform of pyruvate kinase is important
for cancer metabolism and tumour growth. Nature. 452:230–233. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Koukourakis MI, Giatromanolaki A, Chong W,
Simopoulos C, Polychronidis A, Sivridis E and Harris AL: Amifostine
induces anaerobic metabolism and hypoxia-inducible factor 1 alpha.
Cancer Chemother Pharmacol. 53:8–14. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Chiavarina B, Whitaker-Menezes D, Migneco
G, Martinez-Outschoorn UE, Pavlides S, Howell A, Tanowitz HB,
Casimiro MC, Wang C, Pestell RG, et al: HIF1-alpha functions as a
tumor promoter in cancer associated fibroblasts, and as a tumor
suppressor in breast cancer cells: Autophagy drives
compartment-specific oncogenesis. Cell Cycle. 9:3534–3551. 2010.
View Article : Google Scholar
|
|
35
|
Zhang X, Varin E, Briand M, Allouche S,
Heutte N, Schwartz L, Poulain L and Icard P: Novel therapy for
malignant pleural mesothelioma based on anti-energetic effect: an
experimental study using 3-Bromopyruvate on nude mice. Anticancer
Res. 29:1443–1448. 2009.PubMed/NCBI
|
|
36
|
Bayet-Robert M, Lim S, Barthomeuf C and
Morvan D: Biochemical disorders induced by cytotoxic marine natural
products in breast cancer cells as revealed by proton NMR
spectroscopy-based metabolomics. Biochem Pharmacol. 80:1170–1179.
2010. View Article : Google Scholar
|
|
37
|
Boros LG, Lee WN and Go VL: A metabolic
hypothesis of cell growth and death in pancreatic cancer. Pancreas.
24:26–33. 2002. View Article : Google Scholar : PubMed/NCBI
|