Introduction
Despite the significant advances in
immunosuppression, acute rejection remains a crucial barrier to
long-term survival following kidney transplantation (1,2). An
early diagnosis of acute rejection is critical for graft survival.
Renal biopsy is currently the primary method to monitor the dynamic
changes of graft rejection; however, this technique is invasive and
graft damage is detected at a late stage. Although there have been
efforts to identify non-invasive biomarkers for the early diagnosis
of acute graft rejection (3–5),
current acute rejection diagnostic methods are not specific or
sensitive enough. Certain chemokines and chemokine receptor
pathways have been shown to be critical in acute allograft
rejection (6–8). The interferon-γ
(IFNγ)-CXCR3-chemokine-dependent inflammatory loop is crucial in
recruiting T lymphocytes during acute rejection following renal
transplantation (9–11). Monokine induced by IFNγ (MIG,
CXCL9), IFN-induced protein 10 (IP-10, CXCL10) and IFN-induced
T-cell chemoattractant (I-TAC, CXCL11) are CXCR3-specific ligands
induced by IFNγ in a wide variety of cell types. These ligands
direct migration and stimulate the adhesion of activated Th1 cells
and cytoxic T lymphocytes (CTLs) via the IFNγ-CXCR3-chemokine loop
(12–14). Multiple chemokines act as
proinflammatory cytokines and produce signals for the dynamic
trafficking and recruitment of leukocytes, which leads to an
inflammatory response (12,15).
We hypothesized that determining MIG, IP-10 and
I-TAC levels using Luminex assays may offer a non-invasive means to
diagnose T cell-mediated acute rejection in renal allograft
recipients. The Luminex method is a high-throughput tool, which
detects numerous chemokines and cytokines simultaneously using only
25 μl of serum. It is a more efficient and practical method
compared with ELISA. In the present study, we collected the serum
of patients who either had biopsy-confirmed T cell-mediated acute
renal allograft rejections during the first month after
transplantation or were diagnosed as stable kidney transplant
recipients. Using the Luminex method, the levels of MIG, IP-10 and
I-TAC in the serum of patients were detected. It was concluded that
the concentrations of MIG, IP-10 and I-TAC in the serum during an
acute rejection episode were significantly higher compared with
those of stable patients. The joint detection of MIG, IP-10 and
I-TAC in the serum using Luminex analysis may constitute a
non-invasive and efficient method for the early prediction of T
cell-mediated acute rejection following kidney transplantation.
Materials and methods
Study population
The study protocol was approved by the Ethics
Committee of the Chinese PLA 309th Hospital (Beijing, China) and
informed consent was obtained from each patient. Seventy patients
undergoing kidney transplantation were included in this
retrospective study. All the patients had no other co-morbidities.
Thirty-two patients had biopsy-confirmed T cell-mediated acute
renal allograft rejection during the first month after
transplantation (Fig. 1). The
indications of renal needle biopsy were serum creatinine increase,
hypourocrinia and hardened texture of the renal graft and
ultrasonography revealed the increase of renal vascular resistance
after transplantation. Thirty-eight patients with stable function
underwent protocol biopsies one month post-transplantation and were
diagnosed as stable kidney transplant recipients. All the patients
were administered triple therapy based on a combination of
calcineurin inhibitors (CNIs), mycophenolate mofetil (MMF) and
steroids [8–10 mg/kg/day methylprednisolone (MP) for 3 days and
prednisone gradually reduced to 10 mg/day] for the maintenance of
immunosuppression. When acute rejection was diagnosed, the patients
were typically treated with high-dose corticosteroids for 3 days.
Patient demographic information and the parameters of kidney
transplantation are shown in Table
I.
 | Table IPatient demographic information and
parameters of kidney transplantation (mean ± SD). |
Table I
Patient demographic information and
parameters of kidney transplantation (mean ± SD).
| Parameters | Acute rejection
patients | Stable function
patients |
|---|
| Patient no. | 32 | 38a |
| Male gender, n
(%) | 24 (75) | 18 (47)a |
| Age (years) | 39.0±11.0 | 40.5±12.1a |
| HLA mismatch | 1.7±0.8 | 1.5±0.8a |
| PRA (%) | 2.6±0.7 | 2.5±0.8a |
| Warm ischemia times
(min) | 5.2±2.0 | 4.4±2.4a |
| Cold ischemia times
(min) | 472.4±276.1 | 447.8±285.1a |
| Serum creatinine
(μM) | 219.39±74.15 | 74.29±15.72b |
| Immunosuppression
protocol, n (%) |
| Corticosteroids +MMF
+ CsA | 16 (50) | 18 (47)a |
| Corticosteroids +
MMF + FK506 | 15 (47) | 20 (53)a |
| Corticosteroids +
AZA + CsA | 1 (3) | 0a |
Renal needle biopsy of the renal
graft
Patients were in the supine position and were kept
in this position for <8 h. Color Doppler ultrasound was used as
a guide to ensure blood vessels and the renal pelvis were avoided
and the inferior pole of the kidney was obliquely punctured with a
BARD biopsy needle (Bard Biopsy Systems, Tempe, AZ, USA). The depth
of needle penetration was ~2.2 cm and two punctures were carried
out at separate sites. Following the needle biopsy, the puncture
site and local area were covered and appropriately compressed with
a pressure dressing. Hemostasis and anti-infection treatment were
administered. The blood pressure, heart rate, urine output and
urine color of patients were closely monitored.
Pathological examinations
Graft biopsy specimens were immediately immersed in
formaldehyde solution, followed by careful identification of the
specimen to determine whether it was renal tissue, perirenal fat or
a different tissue type. An additional renal needle biopsy at a
different puncture site was performed when necessary, in order to
improve the success rate of the biopsy. Pathological examinations
of the biopsy specimens were performed immediately, including
paraffin embedding, sectioning and hematoxylin and eosin (H&E),
Periodic acid-Schiff (PAS) and Masson staining. Pathological
changes were observed using a light microscope. In specimens with
suspected rejection, C4d immunohistochemical staining was also
performed.
Immunohistochemical analysis
All the renal allograft specimen sections were
deparaffinized and rehydrated through a series of xylene and graded
alcohols. Endogenous peroxidase was blocked in 3%
H2O2 for 5 min. Antigen retrieval was carried
out by placing the slides in a Black & Decker vegetable steamer
in Maixin-Bio retrieval solution (pH 6.1; Maixin Biotechnology
Company, Shanghai, China). The primary polyclonal rabbit anti-human
CXCR3 antibody (cat. no. PA1-32503; Thermo Fisher Scientific, Inc.,
Rockford IL, USA) was applied at a dilution of 1:100 at 4°C
overnight. The IP-10 staining procedure was performed using the
Elivision™ Plus kit (cat. no. kit-9901; Maixin Biotechnology
Company). Following primary antibody incubation, a polymer enhancer
from the kit was added at room temperature for 20 min. Polymerized
HRP-Anti Ms/Rb IgG from the kit was also applied at room
temperature for 30 min. A detection kit (DAB-0031; Maixin
Biotechnology Company) was used with DAB as a chromogen. The slides
were counterstained with hematoxylin (CTS-1099; Maixin
Biotechnology Company), dehydrated through graded alcohols, cleared
in xylene and coverslipped with CytoSeal.
Histopathological evaluation and
immunohistochemical quantification
Samples from each organ were studied using
hematoxylin and eosin staining of the paraffin-embedded sections.
Histopathological evaluation was performed by two pathologists who
specialized in rejection diagnosis according to the Banff ‘05
classification (16,17). The number of infiltrating cells was
measured in at least 20 randomly selected high-power fields (hpf;
×400) by two independent observers. The final count was calculated
as the mean of the two measures. The inter-observer variability was
not >15% at any point.
Preparation of serum samples and
chemokine bead arrays
Serum samples were collected from the peripheral
blood (10 ml) and drawn into additive-free Vacutainer tubes
(Insepack, Beijing, China) following the acute rejection diagnosis
in the rejection group and one month following transplantation in
the stable patients. Serum was separated by centrifugation,
aliquoted into Axygen cryovial tubes and stored at −80°C. The
Luminex assays were performed on single-thawed serum samples.
The serum concentrations of MIG, IP-10 and I-TAC in
25 μl were assayed simultaneously using a human cytokine/chemokine
bead kit (Milliplex, Billerica, MA, USA) and a Luminex-200 array
assay reader (Luminex Corporation, Austin, TX, USA) according to
the manufacturer’s instructions. Serum samples were randomly
assigned to the plates to avoid assay bias and inter- and
intra-assay reproducibility was confirmed. The data were analyzed
with a five-parameter curve fit using the Milliplex®
Analyst software (Milliplex). The concentration of each chemokine
was detected as the mean fluorescence intensity (MFI) and was
subsequently converted to pg/ml of chemokine using a simultaneously
generated standard curve (18).
Statistical analysis
A Mann-Whitney U test was used to compare the MIG,
IP-10 and I-TAC levels between the acute rejection and stable
groups. P<0.05 was considered to indicate a statistically
significant difference.
A logistic model of multiple chemokine synergy was
established by logistic regression. Based on the logistic model,
the receiver-operating characteristic (ROC) curves were generated
to determine the highest diagnostic accuracy in distinguishing
patients with acute renal allograft rejection from control groups.
The area under the curve (AUC) was calculated. An AUC of 1.0 would
indicate a perfect test, whereas a test that is no better than
expected by chance would have an AUC of 0.5 (19).
The expression of CXCR3 in different groups was
assessed using Student’s t-test. P<0.05 was considered to
indicate a statistically significant difference. Statistical
analysis was carried out using SPSS 13.0 software (SPSS Inc.,
Chicago, IL, USA).
Results
Serum chemokine levels
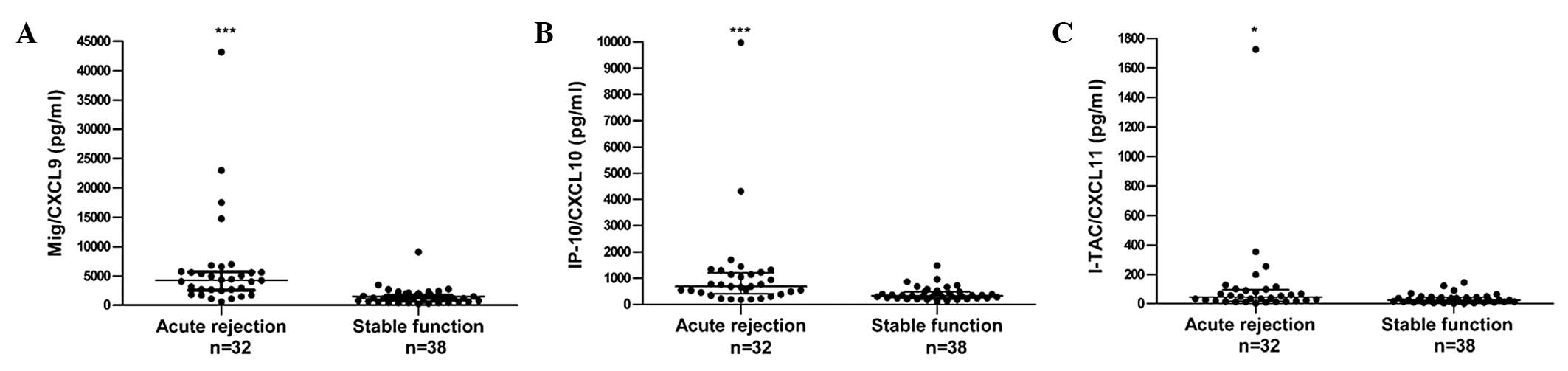
The median concentrations of MIG, IP-10 and I-TAC
were 4,271, 686.7 and 44.32 pg/ml, respectively. The Mann-Whitney U
test indicated that the serum concentrations of MIG, IP-10 and
I-TAC measured during an episode of T cell-mediated acute rejection
were significantly increased compared with those of the stable
patients (MIG, P<0.0001; IP-10, P=0.0002; I-TAC, P=0.0103;
Table II and Fig. 2).
 | Table IILevels of chemokines (median, pg/ml)
in the serum of patients following transplantation. |
Table II
Levels of chemokines (median, pg/ml)
in the serum of patients following transplantation.
| Type of
chemokine | Patients | P-value |
|---|
|
|---|
| Acute rejection | Stable function |
|---|
| MIG | 4,271 | 1,148 | <0.0001 |
| IP-10 | 686.7 | 332.2 | 0.0002 |
| I-TAC | 44.32 | 22.92 | 0.0103 |
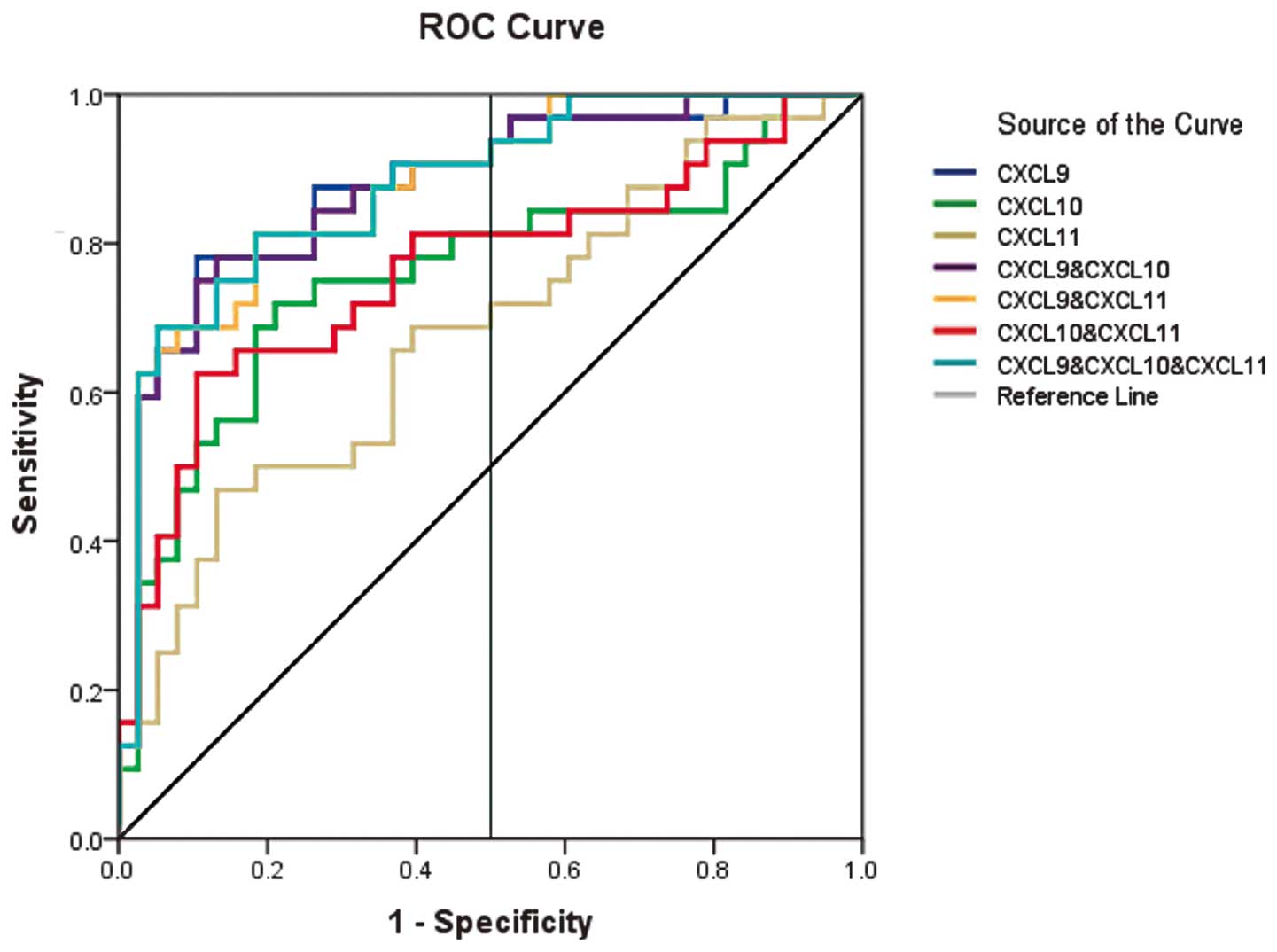
ROC curve analysis of chemokine
levels
The ROC curves show the true-positive (sensitivity)
and false-positive fractions (1 - specificity) for detecting each
chemokine alone and the synergy of multiple chemokines (Fig. 3). The AUC in the different assays
is shown in Table III. Thus,
joint detection of MIG, IP-10 and I-TAC is the best method to
predict acute rejection following kidney transplantation.
 | Table IIIThe calculated area under the curve
(AUC) for chemokine levels. |
Table III
The calculated area under the curve
(AUC) for chemokine levels.
| Type of
chemokine | AUC | SD | P-value | 95% CI |
|---|
| MIG | 0.877 | 0.043 | <0.0001 | 0.794–0.961 |
| IP-10 | 0.760 | 0.061 | <0.0001 | 0.641–0.879 |
| I-TAC | 0.679 | 0.064 | 0.010 | 0.553–0.806 |
| MIG + IP-10 | 0.876 | 0.042 | <0.0001 | 0.793–0.959 |
| MIG + I-TAC | 0.875 | 0.042 | <0.0001 | 0.793–0.957 |
| IP-10 + I-TAC | 0.765 | 0.060 | <0.0001 | 0.648–0.882 |
| MIG + IP-10 +
I-TAC | 0.878 | 0.041 | <0.0001 | 0.797–0.959 |
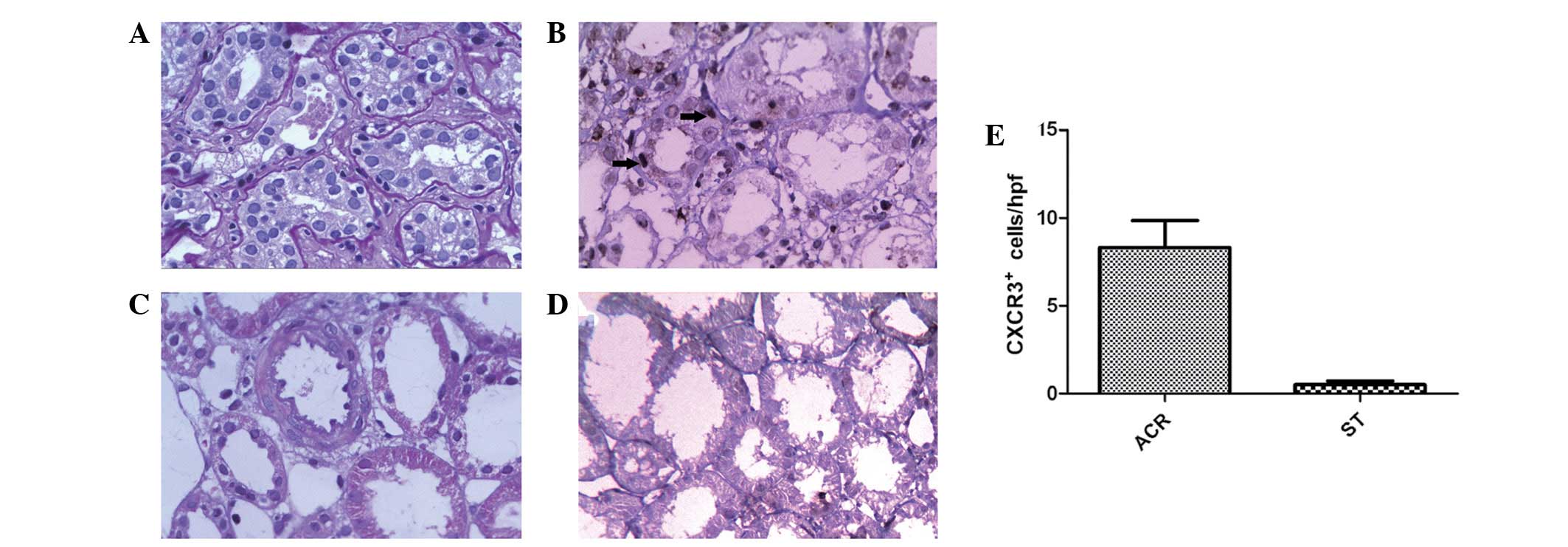
CXCR3 protein expression following
transplantation in renal biopsy patients with acute rejection and
stable renal allograft function
CXCR3+ cells were barely detectable in
biopsies with stable graft function. A significant increase in the
number of CXCR3+ cells in graft biopsies with ACR was
observed (P=0.015; Fig. 1).
Discussion
CD4+ T cells and effector CD8+
T cells play a key role in acute rejection episodes (20). During the course of acute
rejection, CD4+ and CD8+ T cells
differentiate into Th1 cells and CTLs, respectively, due to the
effect of specific cytokines (15). The induction of Th1 and CTLs is
closely linked to the upregulation of the chemokine receptor CXCR3.
CXCR3 binds three chemokines, MIG (CXCL9), IP-10 (CXCL10) and I-TAC
(CXCL11), to induce activated T-cell migration in vitro and
in vivo. As suggested by their original names, all three of
the CXCR3 ligands are induced by IFNγ, which is secreted by
CXCR3+ effector cells. The increased secretion of CXCR3
ligands promotes the additional recruitment of CXCR3+
effector cells. Subsequently, these effectors locally secrete IFNγ,
which further amplifies the infiltration of effector cells. This
inflammatory loop allows CXCR3 and its ligands to coordinate T-cell
responses in the inflamed periphery (21,22).
Previously published studies have demonstrated that competing for
binding with IP-10, I-TAC and MIG or inhibiting CXCR3 disrupts
CXCR3+ Th1 cell trafficking and may be a novel
anti-inflammatory strategy (23,24).
The expression of MIG, IP-10 and I-TAC has been
revealed to play a role, not only in various autoimmune and
infectious diseases that are associated with an increased
expression of IFNγ (Th1-type diseases) (21,25–27),
but also in hypoxia-induced inflammation associated with solid
organ transplantation, including heart and lung transplants
(6,8,28). A
number of chemokines and their receptors in human renal
transplantation have shown an increased expression in allograft
acute rejection, including IP-10, MIG and CXCR3 (29,30).
In the present study, we hypothesized that CXCR3 and
its ligands, MIG, IP-10 and I-TAC, may be reliable biomarkers for T
cell-mediated acute rejection. The detection of MIG, IP-10 and
I-TAC in the serum is easier and more efficient compared with the
determination of CXCR3 on the cell surface. Therefore, it is
suggested that the detection of MIG, IP-10 and I-TAC in the serum
of recipients may constitute a method for diagnosing acute
rejection episodes after kidney transplantation. This study has
shown that the serum levels of MIG, IP-10 and I-TAC in T
cell-mediated acute rejection patients were significantly higher
compared with those in stable patients. Immunohistochemical
analysis was also performed to confirm that the increased CXCR3
ligands trigger an additional recruitment of CXCR3+
effector cells in allografts.
Although the concept of an
IFNγ-CXCR3-chemokine-dependent inflammatory loop has been firmly
established, the differential induction of these CXCR3 ligands may
be due to different cellular sources. In the cerebral malaria
model, Campanella et al(21) demonstrated that endothelial cells
predominantly expressed MIG and that neurons predominantly
expressed IP-10. This finding may explain the non-overlapping roles
of the two CXCR3 ligands in the pathogenesis of cerebral malaria
(21). As compared with the
remaining two CXCR3 ligands, MIG is more dependent on and strongly
induced by IFNγ (22). The present
study also suggests that MIG is a better indicator of acute
rejection compared with the other two chemokines. According to the
study mentioned above, initial innate challenges activate the
endothelial cells to secrete MIG, which recruits Th1 cells and CTLs
to the target tissue in T cell-mediated acute rejection after
kidney transplantation. Th1 cells and CTLs produce large amounts of
IFNγ, which further induces resident tissue cells to produce more
MIG and additional CXCR3 ligands. Based on the ROC curve, the joint
detection of MIG, IP-10 and I-TAC is the best method to more
specifically and effectively predict T cell-mediated acute
rejection.
Increased chemokine release amplifies inflammation,
leading to further recruitment of CXCR3-expressing Th1 T cells and
CTLs. Acute rejection becomes more serious via the
IFNγ-CXCR3-chemokine-dependent inflammatory loop.
Thus, blocking the IFNγ-CXCR3-chemokine-dependent
inflammatory loop may be an effective immunosuppressive therapy and
has the potential to be applied as a therapeutic method for
allograft rejection, and should be investigated further..
In the present study, all the patients were
separately administered three different therapy protocols for the
maintenance of immunosuppression. However, previous studies have
shown that cyclosporine A affects the recruitment of chemokines
(31,32). In the present study, chemokine
levels in the serum of patients who were treated with three
different therapy regimens were not significantly different
(Table I).
The one-year follow-up of all the patients indicated
that three patients were diagnosed with acute rejection after renal
transplantation. Following steroid pulse therapy, 2 cases became
chronic allograft rejections and 1 had graft loss. Several of the
remaining patients had complications, however, their graft function
was stable. No significant correlation was identified between CXCR3
ligands and graft survival or rejection one year after
transplantation.
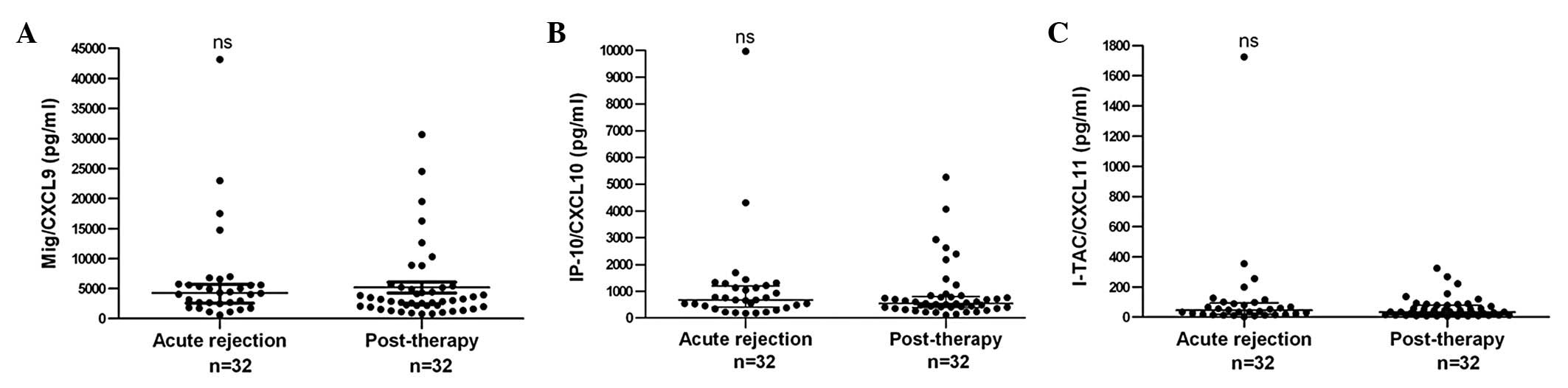
To detect the alterations in chemokine levels
following anti-rejection therapy, the serum of patients who were
administered routine anti-rejection therapy (4–6 mg/kg MP) for 3–5
days was also collected. It was demonstrated that serum creatinine
was returned to normal levels. There was no significant difference
in the CXCR3 ligand level prior to and following anti-rejection
therapy (Fig. 4). This may
indicate that the recovery of chemokine levels require more time
compared with the recovery of kidney function. Future studies
investigating this further should be conducted.
During the last decade, despite abundant
improvements in the investigation of the molecular basis of
allograft rejections, renal biopsy remains the primary method to
monitor the dynamic changes of graft rejection. However, the
detection and screening of chemokines using immunohistochemistry in
allograft biopsies is inefficient and invasive. Luminex analysis,
as a high-throughput and non-invasive tool for the measurement of
different cell products, is increasingly facilitated.
In the present study, Luminex analysis was applied
to detect the chemokine levels in serum and to demonstrate that
higher concentrations of MIG, IP-10 and I-TAC in the serum of
recipients after kidney transplantation constitutes a risk factor
for T cell-mediated acute rejection episodes. The joint detection
of MIG, IP-10 and I-TAC in the serum using Luminex analysis may be
a non-invasive and efficient method for the early-stage prediction
of T cell-mediated acute rejection.
Acknowledgements
This study was supported by the Key Projects in the
National Science and Technology Pillar Program in the Eleventh
Five-year Plan Period (2008BAI60B04) and the National Natural
Science Foundation of China (30801124 and 81170692).
References
|
1
|
Matas AJ: Acute rejection is a major risk
factor for chronic rejection. Transplant Proc. 30:1766–1768. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Gwinner W: Renal transplant rejection
markers. World J Urol. 25:445–455. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Mihovilović K, Kardum-Skelin I, Ljubanović
D, Sabljar-Matovinović M, Vidas Z and Knotek M: Urine
immunocytology as a noninvasive diagnostic tool for acute kidney
rejection: a single center experience. Coll Antropol. 34:63–67.
2010.PubMed/NCBI
|
|
4
|
Hollander Z, Lin D, Chen V, et al: Whole
blood biomarkers of acute cardiac allograft rejection:
double-crossing the biopsy. Transplantation. 90:1388–1393. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Viklicky O, Hribova P, Volk HD, et al:
Molecular phenotypes of acute rejection predict kidney graft
prognosis. J Am Soc Nephrol. 21:173–180. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Fahmy NM, Yamani MH, Starling RC, et al:
Chemokine and chemokine receptor gene expression indicates acute
rejection of human cardiac transplants. Transplantation. 75:72–78.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zhang Z, Kaptanoglu L, Tang Y, et al:
IP-10-induced recruitment of CXCR3 host T cells is required for
small bowel allograft rejection. Gastroenterology. 126:809–818.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zerwes HG, Li J, Kovarik J, et al: The
chemokine receptor Cxcr3 is not essential for acute cardiac
allograft rejection in mice and rats. Am J Transplant. 8:1604–1613.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Anders HJ, Vielhauer V and Schlöndorff D:
Chemokines and chemokine receptors are involved in the resolution
or progression of renal disease. Kidney Int. 63:401–415. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Panzer U, Reinking RR, Steinmetz OM, et
al: CXCR3 and CCR5 positive T-cell recruitment in acute human renal
allograft rejection. Transplantation. 78:1341–1350. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kanmaz T, Feng P, Torrealba J, et al:
Surveillance of acute rejection in baboon renal transplantation by
elevation of interferon-gamma inducible protein-10 and monokine
induced by interferon-gamma in urine. Transplantation.
78:1002–1007. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wen X, Kudo T, Payne L, Wang X, Rodgers L
and Suzuki Y: Predominant interferon-γ-mediated expression of
CXCL9, CXCL10, and CCL5 proteins in the brain during chronic
infection with Toxoplasma gondii in BALB/c mice resistant to
development of toxoplasmic encephalitis. J Interferon Cytokine Res.
30:653–660. 2010.
|
|
13
|
McInnis KA, Britain A, Lausch RN and Oakes
JE: Synthesis of alpha-chemokines IP-10, I-TAC, and MIG are
differentially regulated in human corneal keratocytes. Invest
Ophthalmol Vis Sci. 46:1668–1674. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Campanella GS, Medoff BD, Manice LA,
Colvin RA and Luster AD: Development of a novel chemokine-mediated
in vivo T cell recruitment assay. J Immunol Methods. 331:127–139.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Bromley SK, Mempel TR and Luster AD:
Orchestrating the orchestrators: chemokines in control of T cell
traffic. Nat Immunol. 9:970–980. 2008. View
Article : Google Scholar : PubMed/NCBI
|
|
16
|
Solez K, Colvin RB, Racusen LC, et al:
Banff ‘05 Meeting Report: differential diagnosis of chronic
allograft injury and elimination of chronic allograft nephropathy
(‘CAN’). Am J Transplant. 7:518–526. 2007.
|
|
17
|
Han Y, Guo H, Xu YJ, et al: Pathological
findings and clinical analyses on delayed renal graft function. SR
Essays. 6:4744–4748. 2011.
|
|
18
|
Xu X, Huang H, Cai M, et al: Serum
hematopoietic growth factors as diagnostic and prognostic markers
of acute renal allograft rejection: a potential role for serum stem
cell factor. Cytokine. 56:779–785. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Chen W, Pan X and Ni Z: Evaluation of 4
markers in the combining screening test for coronary heart disease.
Mod Prev Med. 33:723–724. 2006.
|
|
20
|
Guo WH, Tian L, Chan KL, Dallman M and Tam
PK: Role of CD4+ and CD8+ T cells in early
and late acute rejection of small bowel allograft. J Pediatr Surg.
36:352–356. 2001.
|
|
21
|
Campanella GS, Tager AM, El Khoury JK, et
al: Chemokine receptor CXCR3 and its ligands CXCL9 and CXCL10 are
required for the development of murine cerebral malaria. Proc Natl
Acad Sci USA. 105:4814–4819. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Groom JR and Luster AD: CXCR3 in T cell
function. Exp Cell Res. 317:620–631. 2011. View Article : Google Scholar
|
|
23
|
Ranjbaran H, Wang Y, Manes TD, et al:
Heparin displaces interferon-gamma-inducible chemokines (IP-10,
I-TAC, and Mig) sequestered in the vasculature and inhibits the
transendothelial migration and arterial recruitment of T cells.
Circulation. 114:1293–1300. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Seung E, Cho JL, Sparwasser T, Medoff BD
and Luster AD: Inhibiting CXCR3-dependent CD8+ T cell
trafficking enhances tolerance induction in a mouse model of lung
rejection. J Immunol. 186:6830–6838. 2011.PubMed/NCBI
|
|
25
|
Christen U, McGavern DB, Luster AD, von
Herrath MG and Oldstone MB: Among CXCR3 chemokines, IFN-gamma-
inducible protein of 10 kDa (CXC chemokine ligand (CXCL) 10) but
not monokine induced by IFN-gamma (CXCL9) imprints a pattern for
the subsequent development of autoimmune disease. J Immunol.
171:6838–6845. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Menke J, Zeller GC, Kikawada E, et al:
CXCL9, but not CXCL10, promotes CXCR3-dependent immune-mediated
kidney disease. J Am Soc Nephrol. 19:1177–1189. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Thapa M, Welner RS, Pelayo R and Carr DJ:
CXCL9 and CXCL10 expression are critical for control of genital
herpes simplex virus type 2 infection through mobilization of
HSV-specific CTL and NK cells to the nervous system. J Immunol.
180:1098–1106. 2008. View Article : Google Scholar
|
|
28
|
Agostini C, Calabrese F, Rea F, et al:
Cxcr3 and its ligand CXCL10 are expressed by inflammatory cells
infiltrating lung allografts and mediate chemotaxis of T cells at
sites of rejection. Am J Pathol. 158:1703–1711. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Romagnani PL, Lazzeri E, Lasagni L, et al:
High expression of chemokines interferon-γ-inducible protein of 10
kDa (IP-10), monokine induced by interferon-γ-(Mig) and of their
receptor (CXCR3) in acute renal rejection. Am J Transplant.
1:S3432001.
|
|
30
|
Inston NG and Cockwell P: The evolving
role of chemokines and their receptors in acute allograft
rejection. Nephrol Dial Transplant. 17:1374–1379. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Naidu BV, Krishnadasan B, Byrne K, et al:
Regulation of chemokine expression by cyclosporine A in alveolar
macrophages exposed to hypoxia and reoxygenation. Ann Thorac Surg.
74:899–905. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Li J, Xia J, Zhang K and Xu L: Suppression
of acute and chronic cardiac allograft rejection in mice by
inhibition of chemokine receptor 5 in combination with cyclosporine
A. J Surg Res. 157:81–90. 2009. View Article : Google Scholar : PubMed/NCBI
|