Introduction
Pulmonary embolism (PE), together with deep venous
thrombosis (DVT), is termed venous thromboembolism (VTE). Acute
venous thrombosis is a type of red thrombus. A large number of
cells gather in red thrombus irregularly and the majority are
neutrophils. Smeeth et al observed that VTE was associated
with infection, and the risks of DVT and PE were significantly
raised in the first two weeks of diagnosis (1). The current study observed that
compromised immunity is associated with the occurrence of VTE
(2). In 2009, it was reported
(3) that the mRNA expression of
natural killer (NK) cells and T lymphocytes in PE patients were
significantly downregulated. It has recently been reported that the
immunity of CD3+ and CD8+ T cells in patients
with acute PE were reduced (1) and
the immunity of CD3+ and CD8+ T cells in
patients with chronic thromboembolic pulmonary hypertension were
also reduced (2). These
observations indicate that integrins are the core proteins in VTE
and β2 integrins, which are distributed in the leukocyte and are
involved in the occurrence of VTE.
Integrins are type I transmembrane glycoproteins. In
humans, there are 24 integrins formed by specific non-covalent
associations of 18 α and 8 β subunits (4). A type of integrin may be distributed
in a number of cells and a number of integrins are expressed in
signal cells.
Numerous integrins are specially expressed on
specific types of cells. For example, β2 integrins are only
expressed on the cytomembrane of leukocytes, thus, β2 integrins are
also known as leukocyte integrins.
A recent study suggested that the associated mRNA
expression of integrins, which were distributed in leukocytes and
platelets, were upregulated significantly (5). It is unclear how the
integrin-mediated signal transduction pathway and signaling
proteins function. To investigate the differential expression of
associated mRNAs, which were found in the signal transduction
pathway of β2 integrins in PE patients, the whole human genome
oligo microarray was employed to systematically investigate the
expression differences of associated mRNAs.
Materials and methods
Patient information
A total of 20 patients were enrolled in the PE group
and were admitted to Tongji Hospital (Shanghai, China) during 2007,
including 11 males and 9 females, with an average age of 70±14
years (44–89 years old). All patients were diagnosed with PE on the
basis of a minimum of two of the following criteria: i) selective
pulmonary arteriography showing a filling defect or blockage; ii)
pulmonary ventilation perfusion scanning exhibiting single or
multiple blood flow perfusion defects with normal or abnormal
ventilation and mismatched ratio of ventilation/perfusion and iii)
other clinical characteristics, including a typical manifestation
of PE. Arterial blood gas analysis, D-dimer test, ultrasound
cardiogram and chest computerized tomography were used to support
the diagnosis and exclude other cardiac and pulmonary disorders. A
further 20 patients (11 males, 9 females; 44–91 years of age with a
mean age of 72±14) with ischemic heart disease admitted during the
same period, without PE, DVT and other congenital bleeding and
thrombosis diseases, with comparative clinical presentation were
enrolled in the control group. The study was approved by the Ethics
Committee of Tongji University (Shanghai, China) and informed
consent was obtained from all patients in accordance with the
Declaration of Helsinki.
Total RNA isolation
A total of 5 ml of peripheral blood samples
anti-coagulated with EDTA were drawn from patients suspected of
having PE and from those without PE, immediately following
admission to the hospital. Leukocytes were obtained by density
gradient centrifugation with Ficoll solution and the remaining red
blood cells were destroyed by erythrocyte lysis buffer (Qiagen,
Hilden, Germany). Total mononuclear cell RNA was extracted with
TRIzol (Invitrogen Life Technologies, Carlsbad, CA, USA) and
purified with RNeasy column (Qiagen), according to the
manufacturer’s instructions. The isolated total RNA was tested and
quantified using a Nanodrop ND-1000 spectrophotometer (Thermo
Fisher Scientific, Waltham, MA, USA).
Gene expression clip
Agilent G4112A Whole Human Genome Oligo Microarrays
were purchased from Agilent Technologies Inc. (Santa Clara, CA,
USA). The genes or transcripts included 314 negative control spots,
1,924 positive control spots and 359 blank spots. The functions of
>70% of the genes in the microarray have been previously
identified. All patients were subjected to clip analysis.
Target preparation and microarray
hybridization
The RNA samples of patients with confirmed diagnosis
of PE and controls were labeled using the indirect labeling method.
Briefly, 1 μg of total RNA was reverse transcribed. Second strand
cDNA was produced and purified, followed by in vitro
transcription (IVT) with T7 RNA Polymerase. During IVT, the
modified nucleotide, 5-(3-aminoallyl)-UTP (aaUTP) was incorporated
into the cDNA. Subsequently, the fluorescent Cy3 was chemically
coupled with the aaUTP, which contains a reactive primary amino
group on the C5 position of uracil. The dye incorporation rate was
assessed with a Nanodrop ND-1000 spectrophotometer and was found to
be between 1.2 and 1.4 pmol/μl. Hybridization was performed using
the Agilent Oligonucleotide Microarray in situ Hybridization
Plus kit (p/n 5,184-3,568), according to the manufacturer’s
instructions. Briefly, 750 ng of Cy3-labeled sample cDNA was
subjected to fragmentation (30 min at 60°C) and hybridization on
44K Human Whole-Genome 60-mer oligo-chips (G4112F, Agilent
Technologies) was performed in a rotary oven (10 rpm, 60°C, 17 h).
Slides were disassembled and washed in solutions I and II,
according to the manufacturer’s instructions.
Reverse transcription-polymerase chain
reaction (RT-PCR)
Three differential genes were selected and their
expression was confirmed by RT-PCR. Among the genes with
differential expression, seven genes were randomly selected and the
house keeping gene [glyceraldehyde 3-phosphate dehydrogenase
(GAPDH)] was subjected to RT-PCR. The relative expression levels
were indicated as the expression of the target genes normalized to
the expression of GAPDH (2−ΔΔCt). A melting curve and
the 2−ΔΔCt method were used to compare the differences
in the expression between the control and PE group. The results
from RT-PCR were consistent with the microarray analysis.
Statistical analysis
Measurement data are expressed as the mean ± SD. The
Agilent Feature Extraction software was used to collect the
original data from the microarray, followed by an analysis with a
robust multichip average. The gene intensity data between the PE
and control group were compared with a random variance
model-corrected Student’s t-test by SPSS 14.0 software packet
(SPSS, Inc., Chicago, IL, USA). Differentially expressed genes were
identified from whole genomes. P<0.05 was considered to indicate
a statistically significant difference.
Results
A total of 80 associated mRNAs were detected
(Tables I–VIII, Figs. 1–8). 14-3-3ζ occurred in bilateral signal
transduction pathways so was detected twice; once in inside-out
activation, once in outside-in activation.
 | Table ImRNA expression of genes related to
the subunits of β2 integrins. |
Table I
mRNA expression of genes related to
the subunits of β2 integrins.
| Subunit | Gene | PE | Control | P-value | P-value |
|---|
| β2 | ITGB2 | 17.9±0.40 | 17.88±0.29 | - | 0.834460 |
| αL | ITGAL | 14.00±0.64 | 13.51±0.67 | <0.05 | 0.024773 |
| αM | ITGAM | 16.89±0.60 | 16.34±0.31 | <0.01 | 0.000830 |
| αX | ITGAX | 13.91±0.60 | 12.84±0.43 | <0.01 | 0.001798 |
| αD | ITGAD | 9.118±0.82 | 9.38±1.13 | - | 0.446025 |
 | Table VIIIβ2 mRNA expression of proteins which
mediate cytoskeletal remodelling in outside-in activation. |
Table VIII
β2 mRNA expression of proteins which
mediate cytoskeletal remodelling in outside-in activation.
| Name | Gene | PE | Control | P-value | P-value |
|---|
| 14-3-3ζ | YWHAZ | 15.67±0.41 | 15.19±0.41 | <0.01 | 0.0008 |
| Rac | RAC1 | 14.37±0.41 | 13.72±0.30 | <0.01 | 0.00104 |
| cdc42 | CDC42 | 13.80±0.41 | 13.69±0.24 | - | 0.56060 |
| RhoA | RHOA | 15.22±0.37 | 14.86±0.22 | <0.01 | 0.00579 |
| ROCK1/2 | ROCK1 | 13.11±0.53 | 12.64±0.32 | <0.01 | 0.00739 |
| ROCK2 | 10.73±0.55 | 10.27±0.47 | <0.05 | 0.01867 |
| mDia | DIAPH1 | 14.76±0.35 | 14.31±0.35 | <0.01 | 0.00107 |
| DIAPH2 | 12.07±0.47 | 11.78±0.50 | <0.05 | 0.03168 |
| DIAPH3 | 5.382±0.94 | 5.332±0.98 | - | 0.29674 |
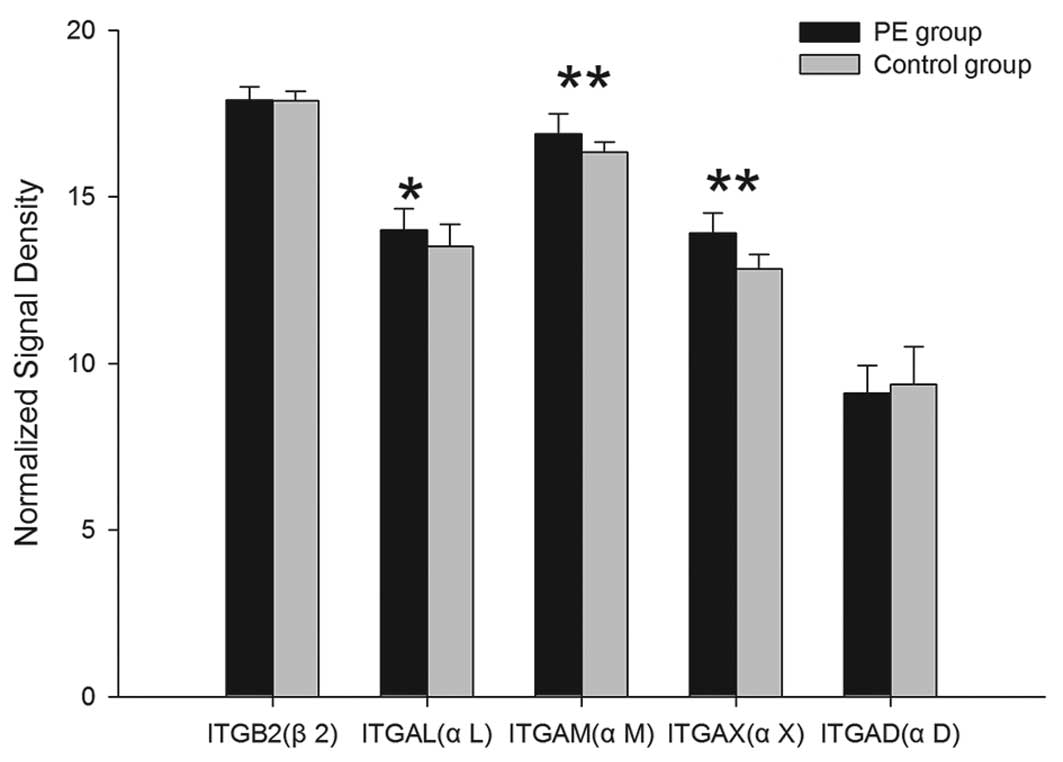
mRNA expression of α/β integrins
Among the 5 subunit-associated mRNAs, the mRNA
expression of ITGAL, ITGAM, ITGAX and ITGB2, which encode for the
subunits of αL, αM, αX, β2, were upregulated in the PE group and
the differences, with the exception of ITGB2, exhibited statistical
significance (P<0.05). Compared with the controls, the mRNA
expression of ITGAD in the PE group was downregulated, but there
was no significant difference (P>0.05) (Table I; Fig.
1).
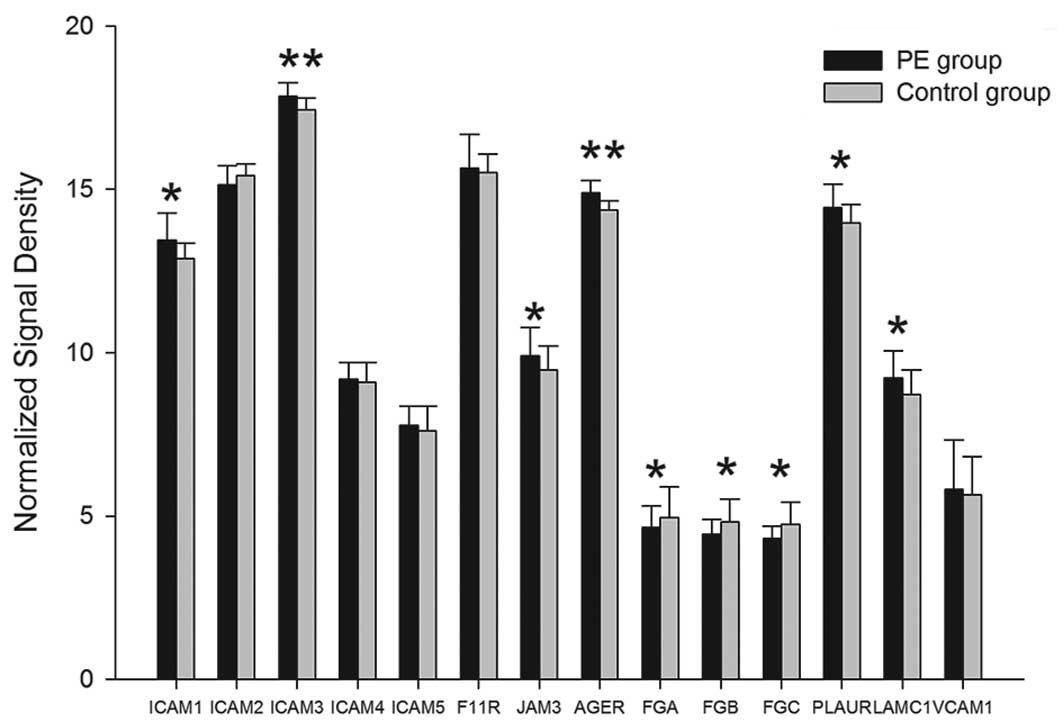
mRNA expression of 12 ligands of β2
integrins
Among the 14 associated mRNAs of 12 ligands, the
gene expression of 10 ligands, ICAM-1, ICAM-3, ICAM-4, ICAM-5,
JAM-1, JAM-3, RAGE, urokinase-type plasminogen activator receptor
(uPAR), laminin 8 and VCAM-1, were upregulated in the PE group and
the differences of ICAM-1, ICAM-3, JAM-3, AGER, PLAUR and LAMC1
exhibited statistical significance (P<0.05). Compared with the
controls, the mRNA expression of ICAM-2 and fibrinogen were
downregulated. The differences of FGA, FGB and FGG mRNA exhibited
statistical significance (P<0.05); however, there was no
significant difference for ICAM-2 (P>0.05) (Table II, Fig. 2).
 | Table IImRNA expression of genes related to
the ligand of β2 integrins. |
Table II
mRNA expression of genes related to
the ligand of β2 integrins.
| Ligand | Gene | PE | Control | P-value | P-value |
|---|
| ICAM-1 | ICAM1 | 13.45±0.82 | 12.88±0.48 | <0.05 | 0.01456 |
| ICAM-2 | ICAM2 | 15.13±0.60 | 15.42±0.36 | - | 0.07313 |
| ICAM-3 | ICAM3 | 17.84±0.43 | 17.43±0.37 | <0.01 | 0.00253 |
| ICAM-4 | ICAM4 | 9.18±0.51 | 9.09±0.61 | - | 0.61033 |
| ICAM-5 | ICAM5 | 7.78±0.59 | 7.60±0.77 | - | 0.30345 |
| JAM-1 | F11R | 15.64±1.04 | 15.5±0.56 | - | 0.45364 |
| JAM-3 | JAM3 | 9.9±0.88 | 9.47±0.74 | <0.05 | 0.03685 |
| RAGE | AGER | 14.90±0.37 | 14.37±0.28 | <0.01 | 1.1E-05 |
| Fibrinogen | FGA | 4.65±0.66 | 4.96±0.93 | <0.05 | 0.01806 |
| FGB | 4.44±0.45 | 4.82±0.70 | <0.05 | 0.04980 |
| FGC | 4.31±0.38 | 4.75±0.67 | <0.05 | 0.01541 |
| uPAR | PLAUR | 14.45±0.71 | 13.96±0.57 | <0.05 | 0.01965 |
| Laminin 8 | LAMC1 | 9.23±0.82 | 8.72±0.75 | <0.05 | 0.04718 |
| VCAM-1 | VCAM1 | 5.83±1.49 | 5.64±1.17 | - | 0.65764 |
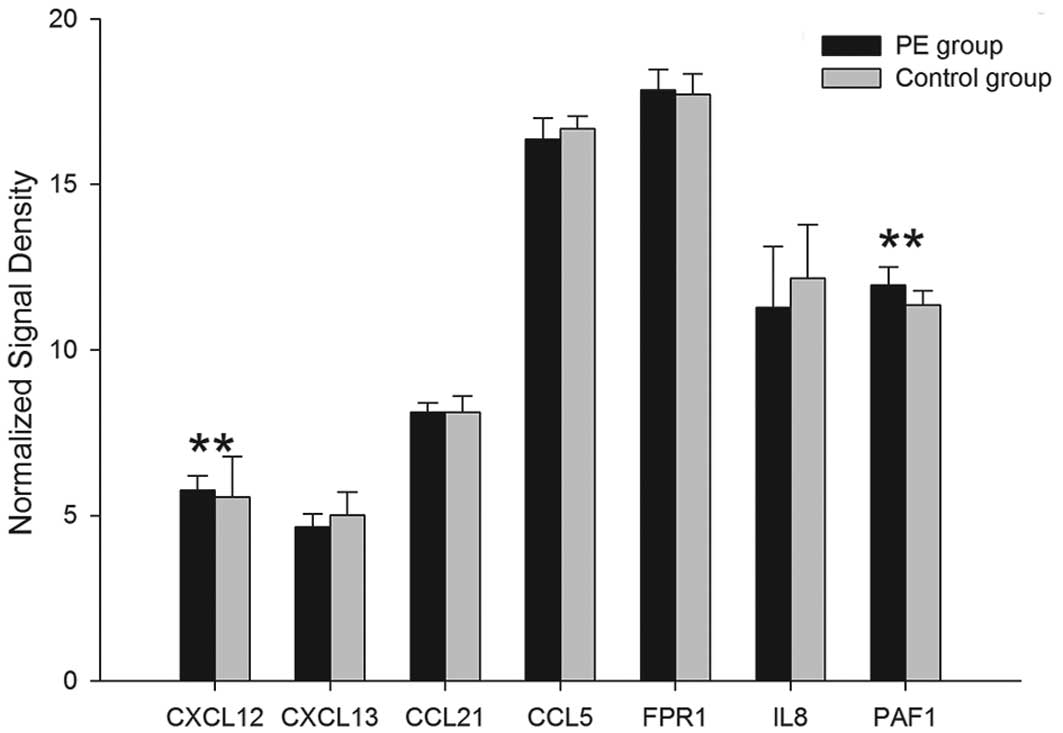
mRNA expression of 7 chemokines for β2
integrins
Among the 7 associated mRNAs of 7 chemokines for β2
integrins, the gene expression of 6 chemokines, SDF-1α, SLC,
RANTES, fMLP, IL-8 and PAF, were upregulated in the PE group and
the differences of CXCL12 and PAF1 mRNA exhibited statistical
significance (P<0.01). Compared with the controls, the mRNA
expression of CXCL13 in the PE group was downregulated; however,
this was not observed to be statistically significant (P>0.05)
(Table III; Fig. 3).
 | Table IIImRNA expression of genes related to
the chemokines. |
Table III
mRNA expression of genes related to
the chemokines.
| Chemokine | Gene | PE | Control | P-value | P-value |
|---|
| SDF-1α | CXCL12 | 5.76±0.43 | 5.56±1.22 | <0.01 | 0.00459 |
| BLC | CXCL13 | 4.65±0.40 | 5.01±0.69 | - | 0.05251 |
| SLC | CCL21 | 8.12±0.27 | 8.11±0.50 | - | 0.93293 |
| RANTES | CCL5 | 16.37±0.63 | 16.69±0.36 | - | 0.06193 |
| fMLP | FPR1 | 17.85±0.63 | 17.71±0.62 | - | 0.48139 |
| IL-8 | IL8 | 11.27±1.86 | 12.17±1.62 | - | 0.11033 |
| PAF | PAF1 | 11.96±0.54 | 11.35±0.43 | <0.01 | 0.00028 |
mRNA expression of 33 signal transduction
proteins
A total of 55 associated mRNAs of 33 signal
transduction proteins which related to the β2 integrins were
detected:
i) Inside-out activation of the β2
integrins
Among the 23 associated mRNAs of 17 inside-out
signal proteins which related to β2 integrins, the mRNA expression
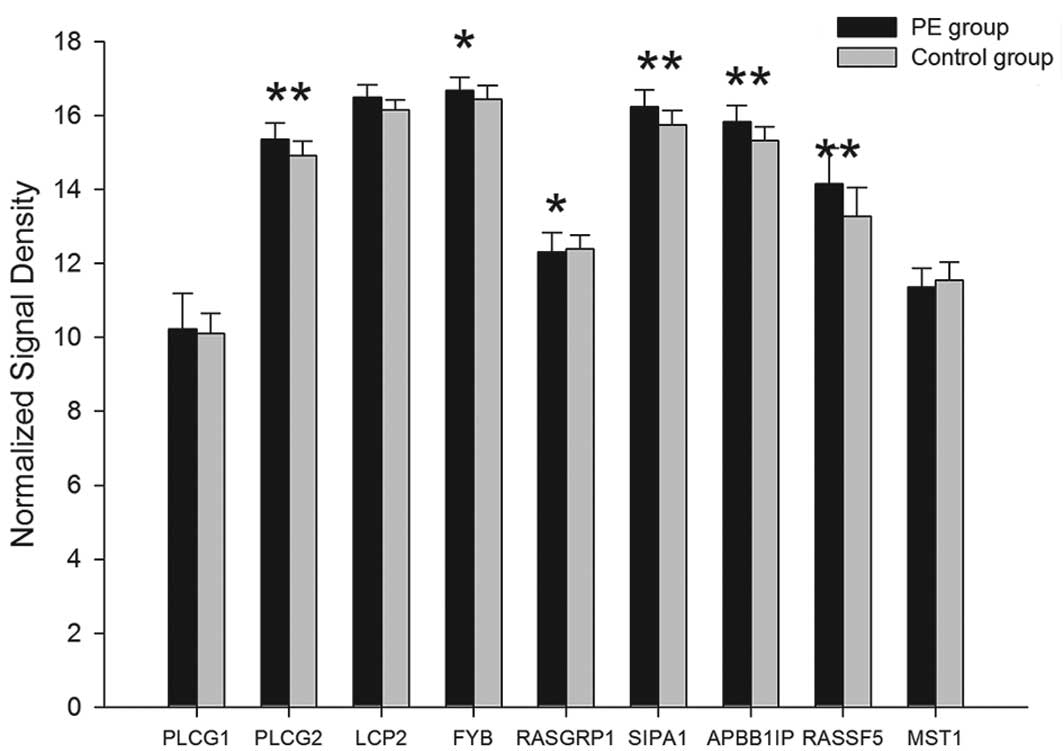
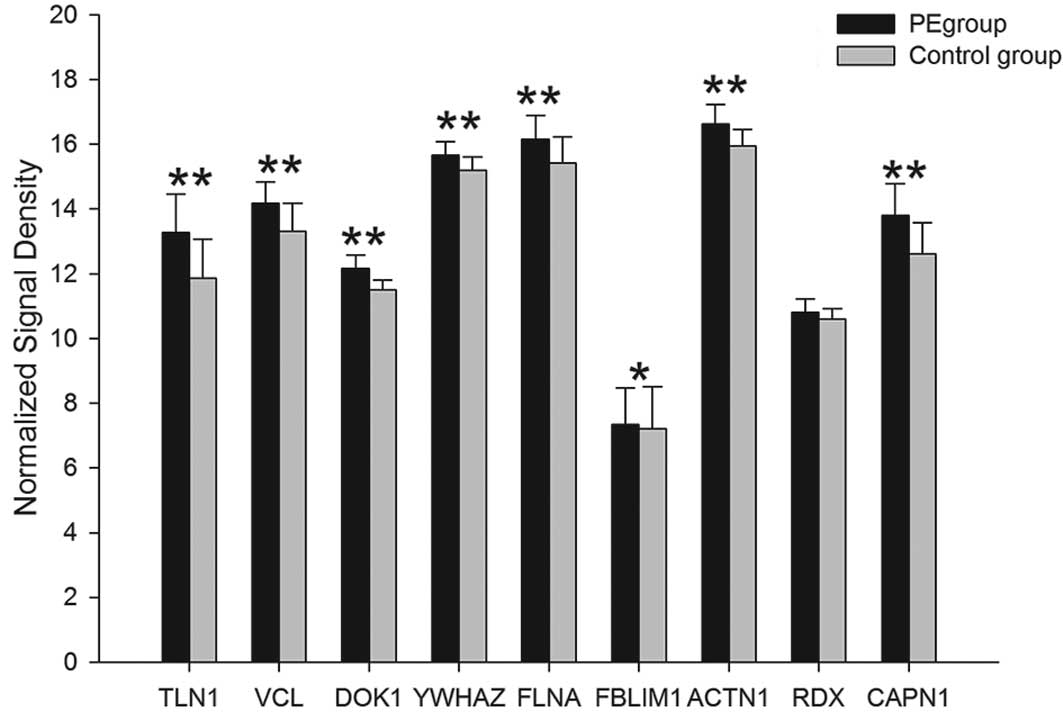
of 15 proteins, PLCγ, SPA1, SLP-76, ADAP, RIAM, RAPL, Talin,
Vinculin, Dok1, 14-3-3ζ, Filamin A, Migfilin, α-actinin, Radixin
and Calpain, were upregulated in the PE group. The differences in
PLCG2, SIPA1, LCP2, FYB, APBB1IP, RASSF5, TLN1, TLN2, VCL, DOK1,
YWHAZ, FLNA, FBLIM1, ACTN1 and CAPN1 mRNA were observed to be
statistically significant (P<0.05). Compared with the controls,
the mRNA expression of DalDAG-GEFI and Mst1 in the PE group were
downregulated. The difference of DalDAG-GEFI encoded by RASGRP1
mRNA exhibited statistical significance (P<0.05), however there
was no significant difference for Mst1 mRNA (P>0.05) (Tables IV and V; Figs.
4 and 5).
 | Table IVmRNA expression of Rap1-related
signals in inside-out activation. |
Table IV
mRNA expression of Rap1-related
signals in inside-out activation.
| Protein | Gene | PE | Control | P-value | P-value |
|---|
| PLCγ | PLCG1 | 10.23±0.96 | 10.1±0.55 | - | 0.48225 |
| PLCG2 | 15.36±0.44 | 14.91±0.39 | <0.01 | 0.00142 |
| SLP-76 | LCP2 | 16.49±0.34 | 16.16±0.26 | <0.01 | 0.00142 |
| ADAP | FYB | 16.68±0.35 | 16.44±0.37 | <0.05 | 0.04653 |
| DalDAG-GEFI | RASGRP1 | 12.31±0.53 | 12.39±0.38 | <0.05 | 0.03376 |
| SPA1 | SIPA1 | 16.23±0.46 | 15.75±0.38 | <0.01 | 0.00089 |
| RIAM | APBB1IP | 15.83±0.44 | 15.32±0.38 | <0.01 | 0.00068 |
| RAPL | RASSF5 | 14.16±0.95 | 13.28±0.77 | <0.01 | 0.00039 |
| Mst1 | MST1 | 11.35±0.51 | 11.55±0.49 | - | 0.22278 |
 | Table VmRNA expression of other proteins in
inside-out activation. |
Table V
mRNA expression of other proteins in
inside-out activation.
| Protein | Gene | PE | Control | P-value | P-value |
|---|
| Talin | TLN1 | 13.28±1.18 | 11.87±1.19 | <0.01 | 0.00056 |
| TLN2 | 6.166±0.43 | 5.667±0.64 | <0.05 | 0.01142 |
| Vinculin | VCL | 14.18±0.66 | 13.32±0.85 | <0.01 | 0.00109 |
| Dok1 | DOK1 | 12.17±0.4 | 11.5±0.3 | <0.01 | 0.00208 |
| 14-3-3ζ | YWHAZ | 15.67±0.41 | 15.19±0.41 | <0.01 | 0.00080 |
| Filamin A | FLNA | 16.16±0.74 | 15.42±0.82 | <0.01 | 0.00447 |
| Migfilin | FBLIM1 | 7.334±1.13 | 7.206±1.3 | <0.05 | 0.01134 |
| α-actinin | ACTN1 | 16.62±0.61 | 15.95±0.51 | <0.01 | 0.00059 |
| Radixin | RDX | 10.81±0.41 | 10.59±0.33 | - | 0.07200 |
| Calpain | CAPN1 | 13.8±0.98 | 12.62±0.96 | <0.01 | 0.00044 |
| CAPN3 | 12.45±0.48 | 11.96±0.52 | <0.01 | 0.00421 |
| CAPN10 | 12.7±0.62 | 12.42±0.46 | <0.05 | 0.04066 |
| CAPN11 | 6.494±1.13 | 5.344±1.05 | <0.01 | 0.00184 |
| CAPN13 | 6.303±1.58 | 5.59±1.13 | <0.05 | 0.01328 |
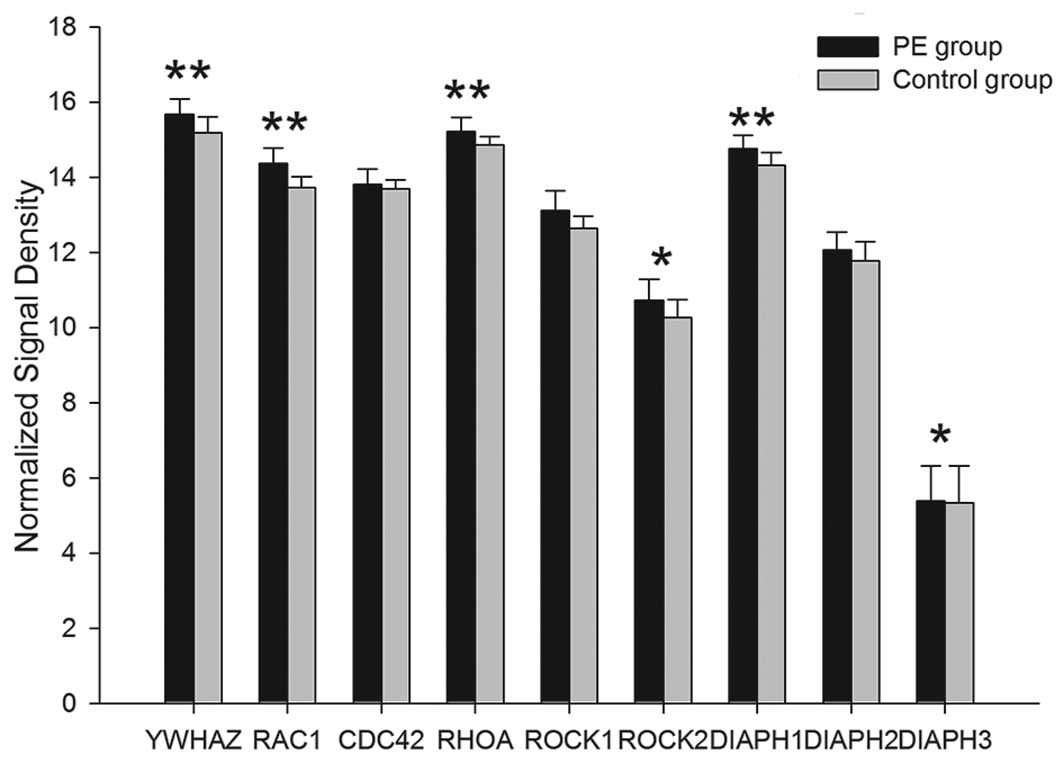
ii) Outside-in activation of the β2
integrins
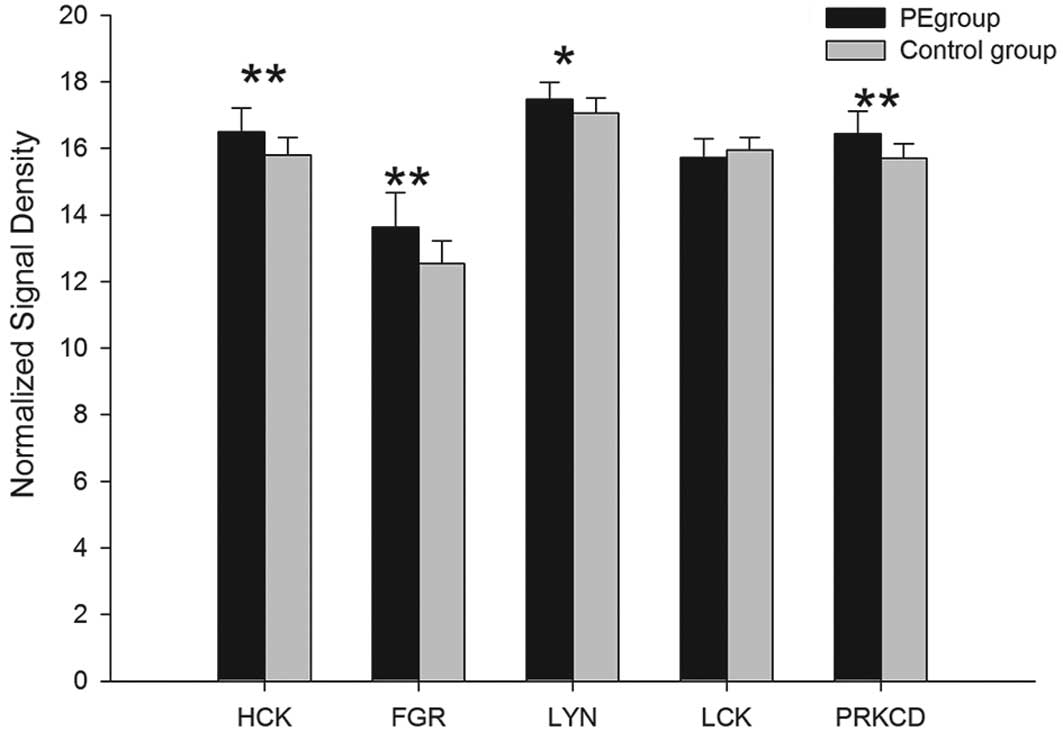
Among the 32 associated mRNAs of 16 outside-in
signal proteins which are related to β2 integrins, the gene
expression of 14 proteins, Hck, Lyn, PKC, Syk, Vav1/3, c-ab1,
c-cb1, FAK, 14-3-3ζ, Rac, cdc42, RhoA, ROCK1/2 and mDia, were
upregulated in the PE group and the differences, with the exception
of cdc42, exhibited statistical significance (P<0.05). Compared
with the controls, the mRNA expression of Fgr and Lck1 in the PE
group was downregulated. The difference of Fgr encoded by FGR mRNA
exhibited statistical significance (P<0.01), however,
alterations in LCK mRNA were not observed to be statistically
significant (P>0.05) (Tables
VI–VIII; Figs. 6–8).
 | Table VImRNA expression of SFK and PKC in
outside-in activation. |
Table VI
mRNA expression of SFK and PKC in
outside-in activation.
| SFK | Gene | PE | Control | P-value | P-value |
|---|
| Hck | HCK | 16.49±0.73 | 15.79±0.54 | <0.01 | 0.00128 |
| Fgr | FGR | 13.63±1.05 | 12.55±0.68 | <0.01 | 0.00044 |
| Lyn | LYN | 17.47±0.52 | 17.06±0.45 | <0.05 | 0.01608 |
| Lck | LCK | 15.72±0.56 | 15.95±0.37 | - | 0.34016 |
| PKC | PRKCA | 7.127±0.67 | 6.268±0.59 | <0.01 | 0.00011 |
| PRKCB1 | 15.82±0.58 | 15.36±0.38 | <0.01 | 0.00511 |
| PRKCBP1 | 7.599±0.48 | 6.902±0.39 | <0.01 | 0.00473 |
| PRKCD | 16.44±0.67 | 15.71±0.44 | <0.01 | 0.00015 |
| PRKCDBP | 8.591±0.84 | 8.226±0.68 | <0.01 | 0.00653 |
| PRKCE | 6.308±0.41 | 6.212±0.22 | - | 0.3829 |
| PRKCG | 6.209±1.11 | 6.179±1.16 | - | 0.93199 |
| PRKCH | 8.351±0.5 | 8.237±0.34 | <0.05 | 0.01567 |
| PRKCI | 11.39±0.46 | 11.51±0.3 | - | 0.18020 |
| PRKCQ | 12.53±0.53 | 12.69±0.35 | - | 0.26876 |
| PRKCSH | 12.4±0.82 | 11.55±0.69 | <0.01 | 0.00111 |
| PRKCZ | 9.199±0.44 | 9.379±0.41 | - | 0.70588 |
Discussion
Neutrophils and monocytes are indispensable parts of
innate immunity. During an inflammatory response, neutrophils and
monocytes destroy invading pathogens by phagocytosis. In 2009, it
was observed (3) that the
associated mRNA expression of neutrophils and monocytes in PE
patients were upregulated significantly (P<0.01). β2 integrins
are the primary adhesion molecules for leukocyte adhesion in the
inflammatory response and are also significant in the phagocytosis
of neutrophils and monocytes.
The leukocyte-restricted β2 integrins include four
members: αLβ2, αMβ2, αXβ2 and αDβ2 (Table IX) (7–13).
The exudation of neutrophils or monocytes includes margination,
rolling, stable adhesion and transendothelial migration. The stable
adhesion occurs mainly through the interaction between αLβ2 and
αMβ2 and their ligands on the surface of cytomembrane. When
inflammation occurs, the expression of chemokines is upregulated.
The inside-out signal transduction is activated following chemokine
binding with the receptors on the surface of neutrophils and
monocytes. Following G-protein signaling, activated phospholipase C
(PLC) mediates the activation of DalDAG-GEFI. Ras-related protein 1
(Rap1) is a small GTPase, which is a key protein in the inside-out
signaling. SPA1 may convert activated Rap1 to inactive Rap1.
DalDAG-GEFI may convert inactive Rap1 to activated Rap1 and
subsequently activate the downstream effectors of Rap1, including
RIAM, RAPL and Mst1. The effectors may regulate the ligand-binding
affinity of the β2 integrins via cytoskeletal proteins which bind
to the integrin β cytoplasmic tail, including Talin, Dok1, 14-3-3ζ,
Filamin A, Migfilin and Radixin, eventually improving the affinity
of the β2 integrins and promoting the adhesion of leukocytes.
 | Table IXThe expression, ligands and functions
of β2 integrins. |
Table IX
The expression, ligands and functions
of β2 integrins.
| Integrin | Other names | Expression | Ligands | Function | Ref. |
|---|
| αLβ2 |
CD11a/CD18
LFA-1 | All leukocytes | ICAM-1, ICAM-2,
ICAM-3, ICAM-4, ICAM-5, JAM-1 | Mediates leukocyte
infiltration; involved in immune
synapse formation | 8
9,10 |
| αMβ2 |
CD11b/CD18
Mac-1 | Monocytes,
neutrophils macrophages, NK cells and γ δ T-cells | ICAM-1, ICAM-2,
ICAM-4, JAM-3, RAGE, laminin 8, fibrinogen and more | Mediates leukocyte
phagocytosis; mediates leukocyte adhesion; involved in immune
tolerance | 11
12,13 |
| αXβ2 |
CD11c/CD18
p150,95 | Monocytes, NK
cells, macrophages and dendritic cells | ICAM-1, ICAM-4,
fibrinogen, LPS and more | Mediates the
adhesion between monocytes and endotheliocytes | |
| αDβ2 | CD11d/CD18 | Macrophages and
eosinophils | ICAM-3, VCAM-1 and
more | Involved in the
phagocytosis of senescent erythrocyte; promotes eosinophil
infiltration | 14 |
The results of the current study showed that the
mRNA expression of associated chemokines were upregulated in the PE
group, compared with the controls. The differences of SDF-1α and
PAF1 mRNA were statistically significant (P<0.01). Among the 23
associated mRNAs of the 17 inside-out signal proteins which related
to the β2 integrins, the mRNA expression of 15 proteins was
upregulated significantly in the PE group (P<0.05). Among the 5
subunit-associated mRNAs, the mRNA expression of ITGAL, ITGAM,
ITGAX and ITGB2, which encode for the subunits of αL, αM, αX and
β2, were upregulated in the PE group and the differences, with the
exception of ITGB2, were observed to be statistically significant
(P<0.05).
The current study suggested that the inside-out
signal transduction pathway of the β2 integrins was activated in
the neutrophils and monocytes of PE patients. Given the
overexpression of activated Rap1, the expression of DalDAG-GEFI was
downregulated as the result of negative feedback. Filamin may
compete with Talin to bind the integrin tail (6). As a negative regulator of integrin
activation, the mRNA expression of Filamin A was upregulated
significantly for the overexpression of activated Rap1. The
associated chemokines SDF-1α, RANTES, fMLP, IL-8 and PAF promote
the migration of leukocytes through the endothelium (7). The differences of SDF-1α and PAF1
mRNA were statistically significant (P<0.01), suggesting that
the exudation of neutrophils and monocytes is enhanced. The gene
expression of Talin, Dok1, 14-3-3ζ, Filamin A, Migfilin and
Skelemin were upregulated significantly (P<0.01). It suggested
that the β2 integrins were activated, neutrophils and monocytes had
high-affinity to the endothelium and the adhesive attraction was
enhanced.
Neutrophils and monocytes associated with the
phagocyte. The phagocytosis of neutrophils or monocytes includes
recognition, adhesion, ingestion and degradation. Depending on the
Fc receptor and C3b receptor (C3bi or αMβ2), neutrophils and
monocytes may recognize and adhere the bacteria which are coated
with the antibody or complement and ingest the phagosome via the
cytoskeletal reorganization.
Phagocytosis mediated by β2 integrins primarily
depends on the outside-in signal transduction pathway of αMβ2. When
the β2 integrins of neutrophils and monocytes bind with the ligand,
the outside-in signaling events are initiated. Non-receptor
tyrosine kinase and the PTK (receptor tyrosine kinase), including
spleen tyrosine kinase (SYK) and FAK, are activated through the
phosphorylation of Src family kinase (SFKs), including Hck, Fgr and
Lyn. SFKs and SYK are required for the regulation of adhesion,
spread and cytoskeletal reorganization in neutrophils and
monocytes. Under the effect of Vav (a Rac GEF), cross-linking of
the β2 integrins may induce the activation of RhoA. The activated
RhoA regulates the cytoskeletal structures and promotes the
phagocytosis of leukocytes. Protein kinase C (PKC) promotes the
phagocytosis of leukocytes. uPAR is required for the adhesion
reaction of endothelial cells with monocytes (14).
The results of the current study showed that among
the 14 associated mRNAs of 12 ligands, the gene expression of 10
ligands was upregulated in the PE group. The differences of ICAM-1,
ICAM-3, JAM-3, AGER, PLAUR and LAMC1 was statistically significant
(P<0.05). Compared with the controls, the mRNA expression of
ICAM-2 and Fibrinogen were downregulated. There were significant
differences of FGA, FGB and FGG mRNA expression (P<0.05). Among
the 32 associated mRNAs of 16 outside-in signaling proteins which
related to the β2 integrins, the mRNA expression of 14 proteins
were upregulated in the PE group and the differences, with the
exception of cdc42, were statistically significant (P<0.05).
Compared with the controls, the mRNA expression of Fgr and Lck1 in
the PE group were downregulated. The difference of FGR mRNA was
statistically significant (P<0.01).
It is hypothesized that the outside-in signal
transduction pathway of β2 integrins was activated in the
neutrophils and monocytes of PE patients. The mRNA expression of
Hck, PKC, FAK and RhoA were upregulated significantly (P<0.01).
The mRNA expression of Lyn and SYK were markedly upregulated
(P<0.05). These observations suggest that the phagocytosis of
neutrophils and monocytes is enhanced. Fgr negatively regulates the
migration and adhesion via the signal transduction pathway of the
β2 integrins. Compared with the controls, there was no negative
feedback rise in the mRNA expression of Fgr, instead, it was
downregulated significantly (P<0.01). Although the β2 integrins
of leukocytes in PE patients were activated, the associated mRNA
expression of Fgr is hypothesized to be inhibited.
The analysis of mRNA differential expression in
leukocyte β2 integrins signal transduction suggested that the
bilateral signal transduction pathways of β2 integrins were
activated. The innate immune response in PE patients was enhanced.
From the perspective of genomics, it is indicated that the
occurrence of PE was apparently associated with the activation of
β2 integrins in neutrophils and monocytes. The occurrence of PE and
the inflammatory reaction are closely related.
References
|
1
|
Smeeth L, Cook C, Thomas S, Hall AJ,
Hubbard R and Vallance P: Risk of deep vein thrombosis and
pulmonary embolism after acute infection in a community setting.
Lancet. 367:1075–1079. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Haoming S, Lemin W, Zhu G, et al: T cell
mediated immune deficiency or compromise in patients with CTEPH. Am
J Respir Crit Care Med. 183:417–418. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Gong Z, Liang AB, Wang LM, et al: The
expression and significance of immunity associated genes mRNA in
patients with pulmonary embolism. Zhonghua Nei Ke Za Zhi.
48:666–669. 2009.(In Chinese).
|
|
4
|
Plow EF, Haas TA, Zhang L, Loftus J and
Smith JW: Ligand binding to integrins. J Biol Chem.
275:21785–21788. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Xie Y, Duan Q, Wang L, Gong Z, Wang Q,
Song H and Wang H: Genomic characteristics of adhesion molecules in
patients with symptomatic pulmonary embolism. Mol Med Rep.
6:585–590. 2012.PubMed/NCBI
|
|
6
|
Kiema T, Lad Y, Jiang P, et al: The
molecular basis of filamin binding to integrins and competition
with talin. Mol Cell. 21:337–347. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Schenkel AR, Mamdouh Z and Muller WA:
Locomotion of monocytes on endothelium is a critical step during
extravasation. Nat Immunol. 5:393–400. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Iezzi G, Karjalainen K and Lanzavecchia A:
The duration of antigenic stimulation determines the fate of naive
and effector T cells. Immunity. 8:89–95. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Perez OD, Mitchell D, Jager GC, et al:
Leukocyte functional antigen 1 lowers T cell activation thresholds
and signaling through cytohesin-1 and Jun-activating binding
protein 1. Nat Immunol. 4:1083–1092. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ehlers MR: CR3: a general purpose
adhesion-recognition receptor essential for innate immunity.
Microbes Infect. 2:289–294. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Varga G, Balkow S, Wild MK, et al: Active
MAC-1 (CD11b/CD18) on DCs inhibits full T-cell activation. Blood.
109:661–669. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Han C, Jin J, Xu S, Liu H, Li N and Cao X:
Integrin CD11b negatively regulates TLR-triggered inflammatory
responses by activating Syk and promoting degradation of MyD88 and
TRIF via Cbl-b. Nat Immunol. 11:734–742. 2010. View Article : Google Scholar
|
|
13
|
Danilenko DM, Rossitto PV, Van der Vieren
M, Le Trong H, McDonough SP, Affolter VK and Moore PF: A novel
canine leukointegrin, alpha d beta 2, is expressed by specific
macrophage subpopulations in tissue and a minor CD8+
lymphocyte subpopulation in peripheral blood. J Immunol. 155:35–44.
1995.PubMed/NCBI
|
|
14
|
Andreas EM: Urokinase receptor surface
expression regulates monocyte adhesion in acute myocardial
infarction. Blood. 100:3611–3617. 2002. View Article : Google Scholar : PubMed/NCBI
|