Introduction
Proliferative vitreoretinopathy (PVR) is essentially
an excessive wound-healing response mediated by the proliferation
of many types of cells inside the vitreous cavity and on the
surface of the retina, resulting in membrane formation and traction
on the retina (1,2). It is one of the most common causes
for failed retinal detachment surgeries, and it develops in 5–10%
of all retinal detachments (3,4). The
management of this situation is complicated further due to the
capability of PVR to result in the detachment of otherwise
successfully reattached retinas or even cause new breaks,
necessitating additional corrective surgeries. The pathogenesis of
PVR is not completely understood, but it is widely accepted that
PVR is a phenomenon involving the migration, proliferation, and
connective tissue production by a variety of cells that gain access
to the vitreous cavity (5,6). In addition, the various types of
cells involved in PVR, immunohistochemical and ultrastructural
studies have consistently reported the presence of retinal pigment
epithelial (RPE) cells in fibrocellular scars (7,8).
Animal model experiments have demonstrated the ability of RPE cells
to cause tractional retinal detachment, which supports the
pathogenic role of RPE cells (9,10).
Previous studies on RPE cell behavior in vitro suggested
numerous growth factors (GF), including platelet-derived GF (PDGF),
vascular endothelial GF (VEGF), transforming GF, insulin-like GF
(IGF), fibroblast GF and epidermal GF, as promoters of key cellular
activities (11–15).
In a previous proteomic study, insulin-like growth
factor-binding protein-6 (IGFBP-6) was one of 24 specific vitreous
proteins shared between moderate and severe PVR samples (16). Experiments were designed to verify
whether a similar correlation could be observed in the PVR rat
models and to investigate the mechanisms of IGFBP-6 expression in
PVR.
Materials and methods
The present study was conducted in compliance with
the ARVO Statement for the Use of Animals in Ophthalmic and Vision
Research. The animal experiments were performed under the protocols
approved by the National Eye Institute Institutional Animal Care
and Use Committee. The present study also followed the tenets of
the Declaration of Helsinki for the use of human subjects.
RPE-J cell preparation and culture
RPE-J cells (CRL-2240, ATCC, Rockville, MD, USA)
were a generous gift from Lian-Fang Du (Department of Medical
Ultrasound, Shanghai Jiaotong University Affiliated First People’s
Hospital, Shanghai, China). The cells were cultured in Dulbecco’s
modified Eagle’s medium with 4% fetal bovine serum and 1%
antibiotic/antimycotic (all from Gibco, Grand Island, NE, USA) at
37°C with 5% CO2. The cells used in the
3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium,
inner salt (MTS) proliferation assay were cultured in a humidified
incubator.
The cells used for rat model induction were
inoculated in a six-well plate at a concentration of
2×104/cm2 and maintained in complete medium
for 24 h. Following adhesion of the cells, the medium was switched
to serum-free medium for 24 h.
Induction of rat models
Seventy-six male adult Wistar rats (Shanghai Slac
laboratory animal Co. Ltd., Shanghai, China; weight, 200±10 g; age,
7 weeks; specific pathogen-free) were included in the present
study, and they were divided into a PVR (n=40) and a control group
(n=36). The PVR Wistar rat models were established as previously
described (17). In brief,
hyaluronidase (1 U) was injected into the vitreous cavity to
liquefy the vitreous, and RPE-J cells (1×106/5 μl) and
platelet-rich plasma (PRP) (1×107/5 μl) were injected
into the vitreous cavity of rats to induce PVR. Sterile
pyrogen-free normal saline was injected into the vitreous cavity in
the control group.
The rats were observed by slit lamp, fundus
examination and photography at 1, 2, 4 and 8 weeks after
intravitreal injection. Rats with any anterior segment
inflammation, vitreous hemorrhage or cataracts were excluded from
the present study. Tissues of the PVR (n=10, 8, 10 and 6) and
control (n=10, 10, 10 and 6) groups were collected at 1, 2, 4 and 8
weeks after model induction, respectively. The proliferative
response was evaluated according to the following grading scale
(18): 0, no proliferative
response; 1, intravitreal proliferation; 2, epiretinal membrane
formation with retinal folds and 3, white dense membrane covering
the retina, with retinal folds and localized retinal detachments
with or without a localized posterior capsular cataract.
Collection of tissues and sample
preparation
Liver
The livers of Wistar rats were isolated immediately
after anesthesia with Napental. In total, 200 mg liver tissue was
stored in a 1.5 ml centrifuge tube at −80°C.
Serum
Venous blood (2 ml) was collected from the rats from
the abdomen cardinal vein and placed in a procoagulant tube at room
temperature for 1 h. The blood was centrifuged at 4°C at 7,000 × g
for 5 min. The supernatant was stored at −80°C.
Vitreous
The vitreous was placed in a 1.5 ml centrifuge tube
following isolation and centrifuged at 4°C at 13,400 × g for 5 min.
The supernatant was stored at −80°C.
Retina
The retina was placed in a 1.5 ml centrifuge tube at
−80°C.
Effect of cytokines on IGFBP-6 mRNA
expression in RPE-J cells
The groups were divided as follows: the control,
which contained no growth factors; the IGF-II, which contained 10,
20 or 50 ng/ml IGF-II; the VEGF, which contained 10, 20 or 40 ng/ml
VEGF and the PDGF, which contained 10, 20 or 40 ng/ml PDGF.
Effect of vitreous and serum on
IGFBP-6 mRNA expression in RPE-J cells
Vitreous and serum samples of PVR patients with
primary rhegmatogenous retinal detachment from the Department of
Ophthalmology, Nantong University Affiliated Hospital, were used in
the present study. Patients with ocular trauma, age-related macular
degeneration, uveitis, glaucoma, diabetes mellitus, a history of
ocular surgery or other systemic diseases were excluded. Informed
consent was obtained from all the patients following verbal and
written explanation of the nature and possible consequences of the
present study. PVR was graded in accordance with the standards of
the Committee TRST in 1983 (19)
and evaluated by at least three associate chief or chief surgeons.
Severe PVR (grade C or D, n=5) and moderate PVR (grade B, n=5)
(20) were included in the present
study. Undiluted 0.3–1.0 ml vitreous humor samples were obtained
from patients with a syringe by aspirating liquefied vitreous from
the center of the vitreous cavity prior to the vitrectomy infusion.
The corresponding serum samples were obtained prior to surgery.
The control group of normal human eyes without any
known ocular diseases (n=5), which were donated for corneal
transplant in accordance with the standardized rules for the
development and applications of organ transplants, was obtained
from the Organ Transplant Center in Shanghai (Shanghai, China). In
total, 0.8–1.0 ml of normal vitreous samples were aspirated with a
syringe from the pars plana. The normal serum samples were obtained
from five healthy volunteers who underwent a physical examination
at the Shanghai Tenth People’s Hospital; these volunteers had no
ocular or systemic diseases.
Harvested vitreous humor samples were collected in
Eppendorf tubes (Axygen, Union City, CA, USA), immediately placed
on ice, centrifuged for 15 min at 12,000 rpm to separate the cell
contents and stored at −80°C until use. The serum samples were
placed at room temperature for 1 h, centrifuged for 15 min at −4°C
and stored at −80°C. The demographic characteristics of the samples
obtained from the donors are shown in Table I. There was no significant
difference among the groups (P>0.05).
 | Table IDemographic characteristics of the
samples. |
Table I
Demographic characteristics of the
samples.
| Characteristics | N | Vitreous | Serum | Age, years | Gender
(male/female) | Eye (right/left) |
|---|
| Moderate PVR | 5 | + | + | 57.8±5.8 | 2/3 | 2/3 |
| Severe PVR | 5 | + | + | 56.4±5.4 | 3/2 | 3/2 |
| Normal donors | 5 | + | − | 58.2±8.3 | 3/2 | 2/3 |
| Healthy
volunteers | 5 | − | + | 56.2±4.3 | 2/3 | − |
Quantitative polymerase chain reaction
(qPCR)
Total RNA was isolated from the retina of PVR rats
and RPE-J cells as per the manufacturer’s instructions
(TRIzol®; Invitrogen, Carlsbad, CA, USA). In total, 2 μg
RNA was converted into cDNA. The primer sequences (5′-3′) were:
IGFBP-6 (NM_013104): forward, 5′-GAAGAGACTACCAAGGAGAGCAAAC-3′ and
reverse, 3′-CTGCAGTACTGAATCCAAGTGTCT-5′; β-actin (NM_031144):
forward, 5′-CCCATCTATGAGGGTTA CGC-3′ and reverse,
3′-TTTAATGTCACGCAGATTTC-5′. The qPCR assays were performed
according to the manufacturer’s instructions.
Enzyme-linked immunosorbent assay for
IGFBP-6 measurement in rats
The IGFBP-6 concentration was measured in the serum
and vitreous of rats with an enzyme-linked immunosorbent assay kit
(Millipore, Billerica, MA, USA) at 8 weeks following intravitreous
injection. All procedures were conducted according to the
manufacturer’s instructions.
MTS proliferation assay
The RPE-J cells were counted by the MTS assay, which
relies on the formation of a colored substrate by mitochondrial
enzyme activity in viable cells. The cells were plated in a 96-well
plate in growth medium and allowed to attach overnight (2,000 per
well). Following washing twice with phosphate-buffered saline, the
cells were switched to serum-free media and left overnight at 37°C.
The cells were incubated in serum-free medium with or without 50
ng/ml IGF-II (R&D Systems, MN, USA) at 37°C for 24 or 48 h.
Variable concentrations of 1, 10, 100, 500 and 1,000 ng/ml
recombinant human IGFBP-6 (R&D Systems) were added to the
cells. MTS (20 μl per well) was then added for 3 h. The absorbance
was measured with a plate reader (Molecular Devices, Sunnyvale, CA,
USA) at 490 nm.
Statistical analysis
Statistical analysis was performed using SPSS
(version 14.0, SPSS, Inc., Chicago, IL, USA). The results were
expressed as the mean ± standard deviation (SD). Multiple
comparisons within the experimental groups were performed using a
one-way analysis of variance, and comparisons between the two
groups were performed using independent group t-tests. P<0.05
was used to indicate a statistically significant difference.
Results
In vivo results of the PVR rat model
induction
Two rats were excluded due to cataracts in the PVR
group in the 1st week after intravitreal injection. The success
rate of the PVR rat models at the 8th week was 89.5% (34/38). In
total, 15 grade one and three grade two PVR rat models were
observed at the 1st week subsequent to intravitreal injection. At
the 2nd week, two grade three PVR rat models were observed. More
grade two and three PVR rat models were observed at the 4th week.
At the 8th week, there were five, eight and 21 rat models at PVR
grades one, two and three, respectively (Table II).
 | Table IIResults of the PVR rat model
induction. |
Table II
Results of the PVR rat model
induction.
| Variables | 1 weeks | 2 weeks | 4 weeks | 8 weeks |
|---|
| Grade 0 | 20 | 13 | 6 | 4 |
| Grade 1 | 15 | 14 | 5 | 5 |
| Grade 2 | 3 | 9 | 10 | 8 |
| Grade 3 | 0 | 2 | 17 | 21 |
| PVR rate, % | 47.4 | 65.8 | 84.2 | 89.5 |
In vivo IGFBP-6 concentration in the
vitreous and serum of rat models
IGFBP-6 in general samples
The IGFBP-6 concentration (225.44±19.36 ng/ml) in
the vitreous of the PVR rat models was significantly higher than
173.25±21.11 ng/ml concentration in the control group (P=0.003).
The concentration of IGFBP-6 in the serum of the PVR group was
higher than that in the control group (P=0.012).
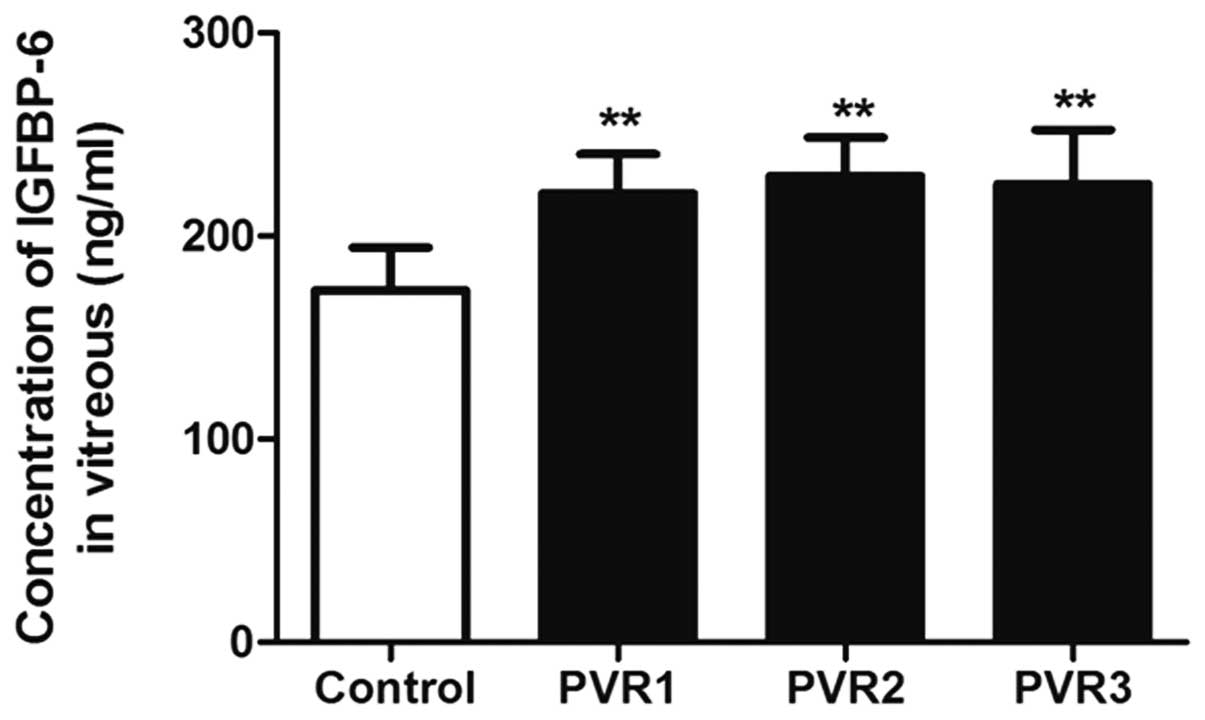
IGFBP-6 in the different PVR
grades
The IGFBP-6 concentrations 221.00±19.3, 229.63±18.89
and 225.70±26.71 ng/ml in the vitreous of PVR grades 1, 2 and 3,
respectively, was significantly higher than that in the control
group (P<0.01). However, there was no significant difference
among the three PVR grade groups (P=0.892) (Fig. 1).
Comparison of IGFBP-6 between vitreous
and serum
In normal rats, the concentration of 173.25±21.11
ng/ml IGFBP-6 was significantly higher in the vitreous compared
with 95.96±17.40 ng/ml in the serum (P=0.000). Similarly, in the
PVR rat models, the concentration of 225.44±19.36 ng/ml IGFBP-6 in
the vitreous was significantly higher compared with 108.48±15.78
ng/ml in the serum (P=0.000). It was increased to a higher extent
in the vitreous than in the serum.
In vivo results of qPCR in rats
After measuring the cycle threshold (CT) value of
each sample, the ΔCT was determined by subtracting the CT value of
β-actin from IGFBP-6. The relative amount of IGFBP-6 expression was
calculated as 2−ΔCT and presented as
2−ΔCT×10−4 due to the low value.
Expression of IGFBP-6 mRNA in the
retina
In general, the expression of IGFBP-6 mRNA in the
retina and of IGFBP-6 of each grade of the PVR group was higher
compared with that of the control group (P=0.000; Table III and P<0.05, respectively).
However, there was no significant difference among the different
grades of PVR (P>0.05; Table
IV).
 | Table IIIThe expression of IGFBP-6 mRNA in
retina of rats (2−ΔCTx10−4) (mean ± SD). |
Table III
The expression of IGFBP-6 mRNA in
retina of rats (2−ΔCTx10−4) (mean ± SD).
| Variables | 1 weeks | 2 weeks | 4 weeks | 8 weeks | In general |
|---|
| Control group | 8.85±2.32 | 8.37±2.59 | 8.32±2.96 | 8.18±1.81 | 8.32±2.41 |
| PVR group | 10.03±2.55 | 11.02±2.92 | 11.62±2.33 | 11.82±2.27 | 11.09±2.57 |
| P-value | 0.293 | 0.058 | 0.013 | 0.009 | 0.000 |
 | Table IVThe expression of IGFBP-6 mRNA in
retina and liver of different grade PVR rat models
(2−ΔCTx10−4). |
Table IV
The expression of IGFBP-6 mRNA in
retina and liver of different grade PVR rat models
(2−ΔCTx10−4).
| Retina | Liver |
|---|
|
|
|
|---|
| Group | Mean ± SD | P-value | Mean ± SD | P-value |
|---|
| Control | 8.32±2.41 | | 25.01±12.04 | |
| PVR 1 | 10.87±2.77 | 0.035 | 27.58±18.98 | 0.999 |
| PVR 2 | 11.35±2.45 | 0.003 | 21.92±11.94 | 0.982 |
| PVR 3 | 11.07±2.67 | 0.000 | 31.41±11.07 | 0.477 |
Expression of IGFBP-6 mRNA in the
liver
In the PVR group, no significant difference was
observed in the expression of IGFBP-6 mRNA in the liver at
different times (P>0.05). No difference was found between the
PVR and control groups at any time points (P=0.443) and there was
no significant difference among the different grades of PVR
(P>0.05; Table IV).
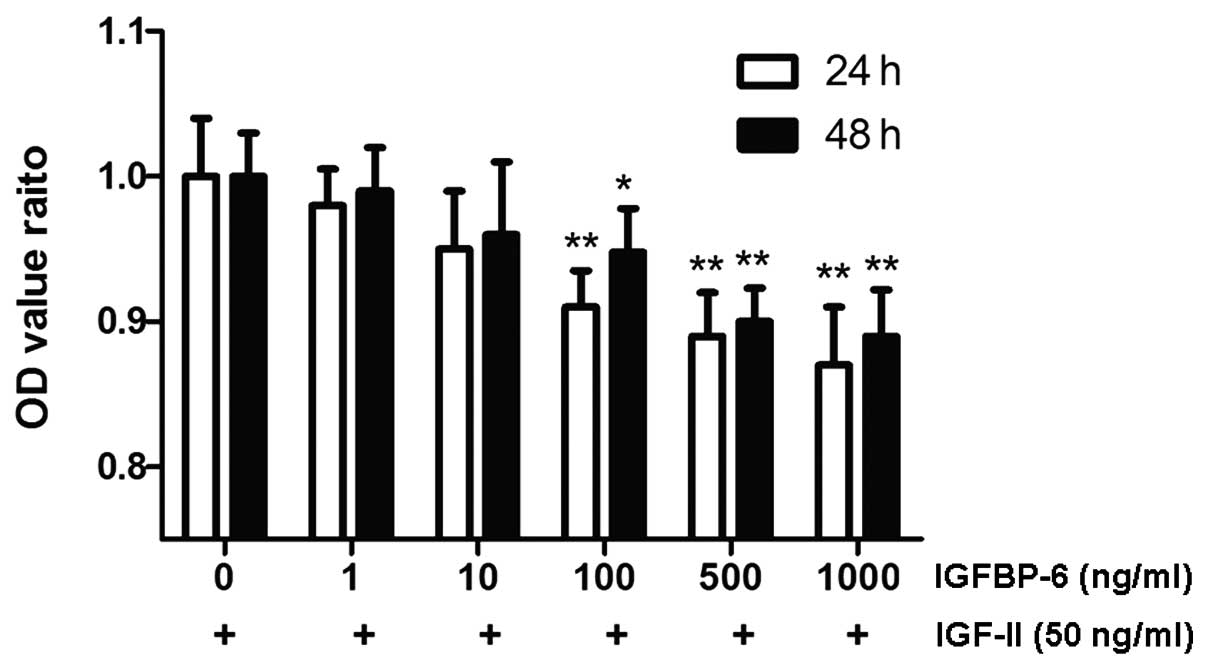
In vitro effects of IGFBP-6 on RPE-J
cell proliferation
After the RPE-J cells were incubated with 50 ng/ml
exogenous IGF-II for 24 or 48 h, the OD value, which reflected the
cell number, increased significantly (24 h: from 1.26±0.05 to
1.38±0.05 and 48 h: from 1.14±0.05 to 1.44±0.06; P<0.05). When
500 ng/ml IGFBP-6 was added to the DMEM plates for 3 h, the OD
value was significantly reduced to 1.23±0.04 and 1.30±0.05,
respectively (P<0.01). However, there was no significant
difference following IGFBP-6 treatment in the VEGF or PDGF groups.
IGFBP-6 alone had no effect on basal proliferation (P>0.05;
Fig. 2).
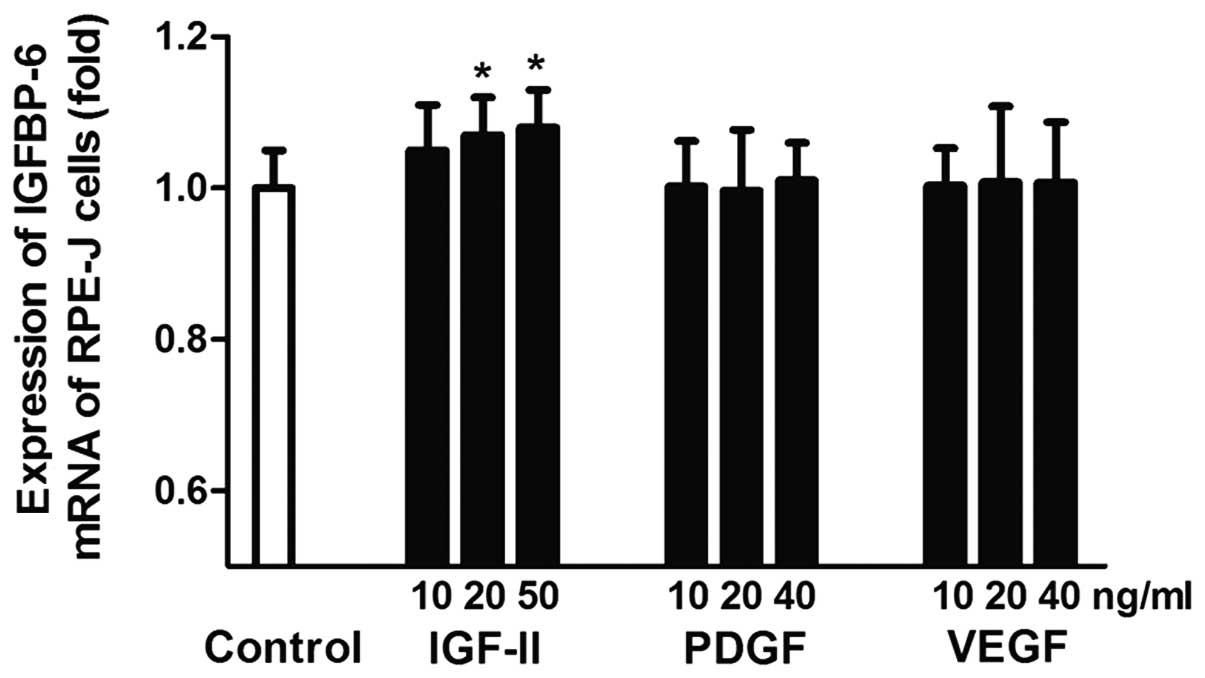
In vitro effects of cytokines on
IGFBP-6 mRNA expression in RPE-J cells
At 20 and 50 ng/ml, IGF-II significantly stimulated
the expression of IGFBP-6 mRNA, which was 1.07±0.08-fold (P=0.036)
and 1.08±0.05-fold (P=0.020) higher compared with the control
group. There was no significant difference between the IGF-II and
control group at 10 ng/ml (P>0.05). (Fig. 3)
Different concentrations of PDGF had no significant
effect on the expression of IGFBP-6 mRNA. The fold changes were
1.002±0.061, 0.997±0.080 and 1.010±0.051 at 10, 20 and 50 ng/ml,
respectively (P>0.05).
As with PDGF, there was no significant difference
between the VEGF and control group at 10, 20 and 50 ng/ml
(P>0.05).
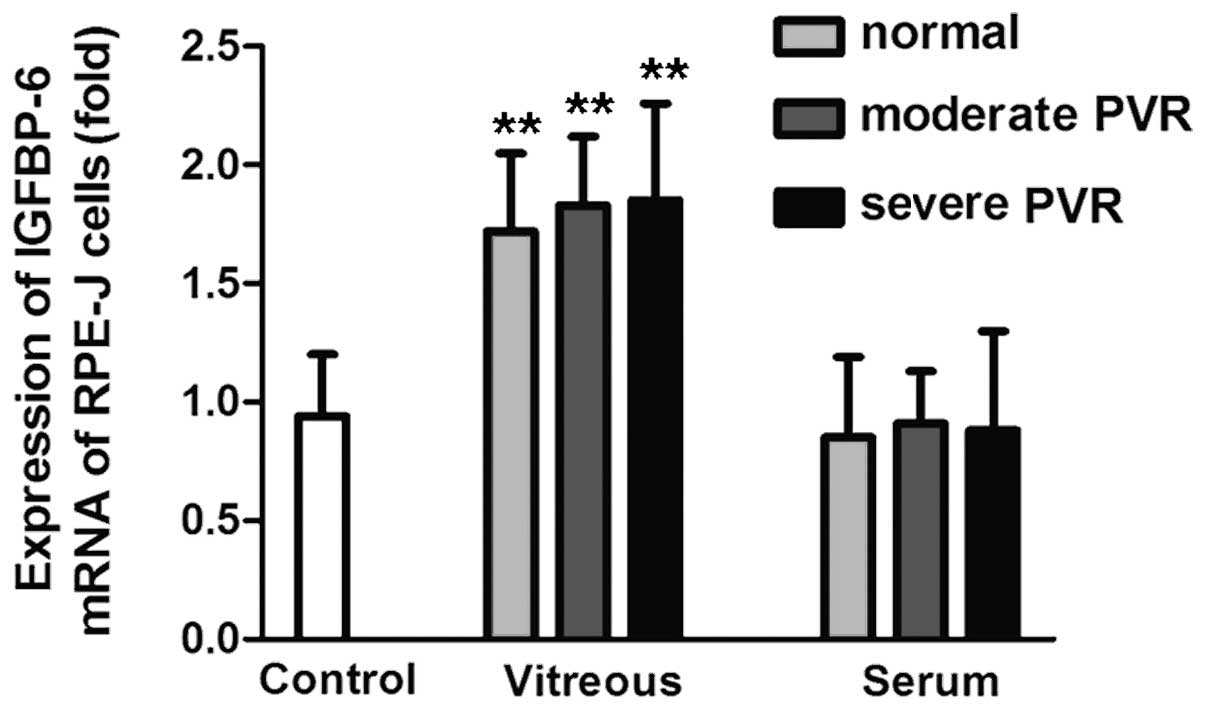
In vitro effects of vitreous or serum
on IGFBP-6 mRNA expression in RPE-J cells
In general, the vitreous from PVR patients and
donors significantly stimulated the expression of IGFBP-6 mRNA. The
IGFBP-6 mRNA expression level in the RPE-J cells stimulated by
vitreous from donors, moderate PVR and severe PVR groups was
1.72±0.33, 1.83±0.29 and 1.85±0.41-fold higher, respectively,
compared with the control group (P<0.01). However, there was no
significant difference between the serum and control groups. The
IGFBP-6 mRNA level stimulated by serum from healthy volunteers,
moderate PVR and severe PVR patient groups was 0.85±0.34, 0.91±0.22
and 0.88±0.42-fold higher, respectively, compared with the control
group (P>0.05) (Fig. 4).
Discussion
In our previous proteomic study, 102 PVR-specific
proteins were identified in the vitreous of PVR patients by
two-dimensional-nano-liquid chromatography coupled with tandem mass
spectrometry (16). Among these,
24 specific vitreous proteins were shared between moderate and
severe PVR samples (16). In the
previous study, IGFBP-6 was identified as a specific protein in the
vitreous and serum of PVR patients. However, the contributions of
IGFBP-6 to the PVR process remain unclear.
In the present study, a significantly higher
concentration of IGFBP-6 was detected in the vitreous, serum and
retina of the PVR rat models compared with the normal control rats.
This result demonstrates that IGFBP-6 is a specific protein in the
PVR process. The concentration of IGFBP-6 in the vitreous was
significantly higher compared with the serum, suggesting that the
upregulated IGFBP-6 in the vitreous was not from the serum. No
significant difference was found in the expression of IGFBP-6 mRNA
in the liver between the PVR and control groups. Due to IGFBP-6
being primarily produced in the liver (21), this result indicates that
upregulated IGFBP-6 in the vitreous and serum was not from the
liver. Additionally, the upregulated IGFBP-6 was produced in a
local autocrine or paracrine manner. Certain studies have indicated
that the choroids (22) and
ciliary body (23) express IGFBP-6
mRNA, however, further investigations should be performed (23).
IGFBP-6 is a relatively novel member of the IGFBP
family that inhibits proliferation and induces apoptosis in
rhabdomyosarcoma cells (24) and
suppresses striated muscle cell migration (25). Unlike other IGFBPs, the affinity of
IGFBP-6 for IGF-II is ~50-fold higher compared with the IGF-I
(26). This characteristic makes
IGFBP-6 a potent inhibitor of IGF-II, which is significant, in
particular in inhibiting the growth of IGF-II-dependent tumors
(27,28), including neuroblastoma (29), rhabdomyosarcoma (29) and colon carcinoma (30). IGF-II, an autocrine tumor growth
factor, is a potent promoter of RPE cell tractional force
generation in vitro(11). A
previous study confirmed that IGF-II was expressed at higher levels
in PVR patients (31). In the
present study, the expression of IGFBP-6 mRNA in RPE-J cells was
significantly upregulated by IGF-II at 20 and 50 ng/ml, which may
have been due to the increased level of IGF-II. Therefore, IGFBP-6
may downregulate RPE-J cell proliferation through inhibiting the
actions of IGF-II.
In this study, IGFBP-6 inhibited the
IGF-II-stimulated proliferation of RPE-J cells but not basal
proliferation, suggesting that the basal growth of RPE-J cells is
IGF-II independent under these conditions. The results indicate
that IGFBP-6 is a potent anti-proliferative agent, and its
anti-proliferative effects depend on its combination with IGF-II.
Therefore, IGFBP-6 may be a novel target to control the PVR
process.
In addition to IGFBP-6, other growth factors were
upregulated in PVR patients, including PDGF (32) and VEGF (33), which are capable of inducing RPE-J
cell proliferation and migration (34,35).
In the current study, these two growth factors were selected to
evaluate the role of IGFBP-6 in the RPE-J cells. The final
concentration used was based on previous studies that revealed
their effect on the RPE-J cells. Neither PDGF nor VEGF had a
significant effect on IGFBP-6 mRNA expression. Additionally,
IGFBP-6 only inhibited IGF-II-stimulated but not PDGF- or
VEGF-stimulated RPE-J cell proliferation, which indicated that the
role of IGFBP-6 in RPE-J cell proliferation was independent of PDGF
and VEGF.
In the present study, the vitreous of PVR patients
and donated eyes significantly stimulated the expression of IGFBP-6
mRNA in the RPE-J cells, while the serum had no effect on this
expression. This result revealed that RPE-J cell proliferation in
the PVR progression was dependent on the vitreous environment. The
proliferation and migration of RPE-J cells are significant during
the development of PVR. RPE-J cells usually remain in the G0 phase,
with no proliferative or migratory activity in the normal state,
until the retina is broken by trauma or surgery; subsequently, they
gain access to the vitreous cavity or subretinal space and begin
proliferating and migrating (36).
Those data are in agreement with the results of the present
study.
In summary, the trends and effects of IGFBP-6 may
provide a possibility of a PVR therapeutic target, with the
vitreous serving as a significant environmental factor in the
progression of PVR.
Acknowledgements
This study was supported in whole or in part, by the
National Nature Science Foundation Project (grant no. 30901643),
Shanghai Science Committee Biology Department Pilot Project (grant
no. 10411964900) and The New Excellence Project of Shanghai Health
Bureau (grant no. XYQ2011067).
References
|
1
|
Pastor JC: Proliferative
vitreoretinopathy: an overview. Surv Ophthalmol. 43:3–18. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Weller M, Wiedemann P and Heimann K:
Proliferative vitreoretinopathy - is it anything more than wound
healing at the wrong place? Int Ophthalmol. 14:105–117. 1990.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Girard P, Mimoun G, Karpouzas I and
Montefiore G: Clinical risk factors for proliferative
vitreoretinopathy after retinal detachment surgery. Retina.
14:417–424. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Pastor JC, de la Rúa ER and Martín F:
Proliferative vitreoretinopathy: risk factors and pathobiology.
Prog Retin Eye Res. 21:127–144. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ryan SJ: The pathophysiology of
proliferative vitreoretinopathy in its management. Am J Ophthalmol.
100:188–193. 1985. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wiedemann P and Weller M: The
pathophysiology of proliferative vitreoretinopathy. Acta Ophthalmol
Suppl. 189:3–15. 1988.
|
|
7
|
Baudouin C, Hofman P, Brignole F, Bayle J,
Loubière R and Gastaud P: Immunocytology of cellular components in
vitreous and subretinal fluid from patients with proliferative
vitreoretinopathy. Ophthalmologica. 203:38–46. 1991. View Article : Google Scholar
|
|
8
|
Vinores SA, Campochiaro PA and Conway BP:
Ultrastructural and electron-immunocytochemical characterization of
cells in epiretinal membranes. Invest Ophthalmol Vis Sci. 31:14–28.
1990.PubMed/NCBI
|
|
9
|
Wong CA, Potter MJ, Cui JZ, et al:
Induction of proliferative vitreoretinopathy by a unique line of
human retinal pigment epithelial cells. Can J Ophthalmol.
37:211–220. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yang CH, Huang TF, Liu KR, Chen MS and
Hung PT: Inhibition of retinal pigment epithelial cell-induced
tractional retinal detachment by disintegrins, a group of
Arg-Gly-Asp-containing peptides from viper venom. Invest Ophthalmol
Vis Sci. 37:843–854. 1996.PubMed/NCBI
|
|
11
|
Mukherjee S and Guidry C: The insulin-like
growth factor system modulates retinal pigment epithelial cell
tractional force generation. Invest Ophthalmol Vis Sci.
48:1892–1899. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Spraul CW, Kaven C, Amann J, Lang GK and
Lang GE: Effect of insulin-like growth factors 1 and 2, and glucose
on the migration and proliferation of bovine retinal pigment
epithelial cells in vitro. Ophthalmic Res. 32:244–248. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Mitsuhiro MR, Eguchi S and Yamashita H:
Regulation mechanisms of retinal pigment epithelial cell migration
by the TGF-beta superfamily. Acta Ophthalmol Scand. 81:630–638.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Spraul CW, Kaven C, Lang GK and Lang GE:
Effect of growth factors on bovine retinal pigment epithelial cell
migration and proliferation. Ophthalmic Res. 36:166–171. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Hollborn M, Bringmann A, Faude F,
Wiedemann P and Kohen L: Signaling pathways involved in PDGF-evoked
cellular responses in human RPE cells. Biochem Biophys Res Commun.
344:912–919. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yu J, Liu F, Cui SJ, et al: Vitreous
proteomic analysis of proliferative vitreoretinopathy. Proteomics.
8:3667–3678. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zheng XZ, Du LF and Wang HP: An
immunohistochemical analysis of a rat model of proliferative
vitreoretinopathy and a comparison of the expression of TGF-β and
PDGF among the induction methods. Bosn J Basic Med Sci. 10:204–209.
2010.PubMed/NCBI
|
|
18
|
Behar-Cohen FF, Thillaye-Goldenberg B, de
Bizemont T, Savoldelli M, Chauvaud D and de Kozak Y: EIU in the rat
promotes the potential of syngeneic retinal cells injected into the
vitreous cavity to induce PVR. Invest Ophthalmol Vis Sci.
41:3915–3924. 2000.PubMed/NCBI
|
|
19
|
No authors listed. The classification of
retinal detachment with proliferative vitreoretinopathy.
Ophthalmology. 90:121–125. 1983. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Yanyali A and Bonnet M: Risk factors of
postoperative proliferative vitreoretinopathy in giant tears. J Fr
Ophtalmol. 19:175–180. 1996.(In French).
|
|
21
|
Jones JI and Clemmons DR: Insulin-like
growth factors and their binding proteins: biological actions.
Endocr Rev. 16:3–34. 1995.PubMed/NCBI
|
|
22
|
Burren CP, Berka JL, Edmondson SR, Werther
GA and Batch JA: Localization of mRNAs for insulin-like growth
factor-I (IGF-I), IGF-I receptor, and IGF binding proteins in rat
eye. Invest Ophthalmol Vis Sci. 37:1459–1468. 1996.PubMed/NCBI
|
|
23
|
Bergman PB, Moravski CJ, Edmondson SR, et
al: Expression of the IGF system in normal and diabetic transgenic
(mRen-2)27 rat eye. Invest Ophthalmol Vis Sci. 46:2708–2715. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Gallicchio MA, Kaun C, Wojta J, Binder B
and Bach LA: Urokinase type plasminogen activator receptor is
involved in insulin-like growth factor-induced migration of
rhabdomyosarcoma cells in vitro. J Cell Physiol. 197:131–138. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Gallicchio MA, Kneen M, Hall C, Scott AM
and Bach LA: Overexpression of insulin-like growth factor binding
protein-6 inhibits rhabdomyosarcoma growth in vivo. Int J Cancer.
94:645–651. 2001. View
Article : Google Scholar : PubMed/NCBI
|
|
26
|
Bach LA: Insulin-like growth factor
binding protein-6: the ‘forgotten’ binding protein? Horm Metab Res.
31:226–234. 1999.
|
|
27
|
Chaves J and Saif MW: IGF system in
cancer: from bench to clinic. Anticancer Drugs. 22:206–212. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kuo YS, Tang YB, Lu TY, Wu HC and Lin CT:
IGFBP-6 plays a role as an oncosuppressor gene in NPC pathogenesis
through regulating EGR-1 expression. J Pathol. 222:299–309. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Seurin D, Lassarre C, Bienvenu G and
Babajko S: Insulin-like growth factor binding protein-6 inhibits
neuroblastoma cell proliferation and tumour development. Eur J
Cancer. 38:2058–2065. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Leng SL, Leeding KS, Whitehead RH and Bach
LA: Insulin-like growth factor (IGF)-binding protein-6 inhibits
IGF-II-induced but not basal proliferation and adhesion of LIM 1215
colon cancer cells. Mol Cell Endocrinol. 174:121–127. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Ricker LJ, Kijlstra A, Kessels AG, et al:
Interleukin and growth factor levels in subretinal fluid in
rhegmatogenous retinal detachment: a case-control study. PLoS One.
6:e191412011. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Pennock S and Kazlauskas A: Vascular
endothelial growth factor A competitively inhibits platelet-derived
growth factor (PDGF)-dependent activation of PDGF receptor and
subsequent signaling events and cellular responses. Mol Cell Biol.
32:1955–1966. 2012. View Article : Google Scholar
|
|
33
|
Ricker LJ, Dieudonné SC, Kessels AG, et
al: Antiangiogenic isoforms of vascular endothelial growth factor
predominate in subretinal fluid of patients with rhegmatogenous
retinal detachment and proliferative vitreoretinopathy. Retina.
32:54–59. 2012. View Article : Google Scholar
|
|
34
|
Liang CM, Tai MC, Chang YH, et al:
Glucosamine inhibits epithelial-to-mesenchymal transition and
migration of retinal pigment epithelium cells in culture and
morphologic changes in a mouse model of proliferative
vitreoretinopathy. Acta Ophthalmol. 89:e505–e514. 2011. View Article : Google Scholar
|
|
35
|
Karthikeyan B, Kalishwaralal K,
Sheikpranbabu S, Deepak V, Haribalaganesh R and Gurunathan S: Gold
nanoparticles downregulate VEGF-and IL-1β-induced cell
proliferation through Src kinase in retinal pigment epithelial
cells. Exp Eye Res. 91:769–778. 2010.PubMed/NCBI
|
|
36
|
Parrales A, López E and López-Colomé AM:
Thrombin activation of PI3K/PDK1/Akt signaling promotes cyclin D1
upregulation and RPE cell proliferation. Biochim Biophys Acta.
1813:1758–1766. 2011. View Article : Google Scholar : PubMed/NCBI
|