Introduction
Inflammatory bowel disease (IBD), comprising Crohn’s
disease (CD) and ulcerative colitis (UC), is considered to be a
chronic relapsing disorder involving inflammation of the
gastrointestinal tract (1).
Numerous IBD patients suffer from a relapse of the disease;
therefore ideal therapeutic strategies are imperative.
Although the precise etiology of IBD is not well
understood, certain substantial advances have been made. Among the
complex pathogenesis of IBD, leukocyte recruitment is a common
event, occurring in the initiation and progression of the disease
(2). This recruitment is regulated
by cell adhesion molecules (CAMs) (3) and chemokines (4), expressed by endothelial cells, which
are activated during the development of IBD. The overexpression of
CAMs [intercellular adhesion molecule (ICAM)-I, vascular cell
adhesion molecule (VCAM)-1 and mucosal vascular addressin cell
adhesion molecule (MAdCAM)-1] (5)
and increasing production of chemokines [interleukin (IL)-8 and
monocyte chemoattractant protein (MCP)-1] (4) have been identified in IBD patients.
As CAMs and chemokines have pivotal roles in the development of
IBD, targeting these molecules appears to be a promising
therapeutic option.
Carnosol is an oxidation product of carnosic acid
(CA), carnosol and CA are phenolic diterpenes and major components
of rosemary and sage extracts (6).
It has been reported that carnosol and CA possess potent
anti-microbial (7),
anti-inflammatory (8),
neuroprotective (9), anti-oxidant
(10) and antitumor properties
(11).
CA has been shown to attenuate the expression of
adhesion molecules in IL-1β stimulated human umbilical vein
endothelial cells (HUVECs) (12).
Further research has demonstrated that carnosol may suppress tumor
necrosis factor (TNF)-α-induced ICAM-1 expression by inhibiting IκB
kinase beta (IKK-β) activity or upregulating heme-oxygenase-1
expression (13). However, whether
carnosol is capable of inhibiting the TNF-α-induced expression of
other CAMs, the production of proinflammatory cytokines in
endothelial cells and the inhibition of other potential signaling
pathways, remains unknown. This study examined the ability of
carnosol to inhibit the expression of CAMs (ICAM-I, VCAM-1 and
E-selectin) and the production of chemokines (IL-8 and MCP-1) in
HUVECs, and also investigated the possible mechanisms underlying
the anti-inflammatory activity of carnosol.
Materials and methods
Cell culture
HUVECs were isolated from human umbilical veins by
treatment with collagenase (0.1%). Subsequently, the cells were
collected and cultured in EGM-2 medium (Lonza Inc., Walkersville,
MD, USA) with 10% foetal bovine serum (FBS, Life Technology,
Carlsbad, CA, USA), 100 IU/ml penicillin and 100 μg/ml streptomycin
(Life Technology). All cells were incubated at 37°C in an
atmosphere of 95% air:5% CO2. The purity of the HUVECs
was 98% and was evaluated by morphology and immunofluorescent
staining for cluster of differentiation (CD)31 and von Willebrand
factor (Santa Cruz Biotechnology, Santa Cruz, CA, USA). HUVECs
achieved confluence in the flasks and cells from between three to
five passages were used for the experiment.
Cell viability assay
Cell viability was determined by using a Cell
Counting kit-8 (CCK-8) assay (Dojindo Molecular, Kumamoto, Japan)
as described previously (14). The
HUVECs were seeded onto a 96-well plate at a density of
1×106 cells/ml followed by preincubation with various
concentrations (1, 5 and 10 μmol/l) of carnosol (Cayman Chemical
Company, Ann Arbor, MI, USA) and TNF-α (Life Technology) for 24 h.
Subsequently 10 μl CCK-8 solution was added to each well for an
additional 2 h, and the absorbance at 450 nm was assessed with a
microplate reader (Multiskan MK3, Thermo Labsystems, Vantaa,
Finland).
Western blotting
HUVECs were washed with pre-chilled
phosphate-buffered saline and lysed in radioimmunoprecipitation
assay buffer. Following sonication (Sonicator Q700; QSonica LLC,
Newtown, CT, USA), the lysate was centrifuged (14,000 × g for 15
min at 4°C) and the supernatant was transferred to a tube. The
protein content was quantified with a bicinchoninic acid protein
assay kit (Keygen Biotech, Nanjing, China). Total proteins were
separated by electrophoresis on SDS-polyacrylamide gels and were
subsequently electroblotted onto polyvinylidene fluoride membranes.
The indicated primary antibodies were incubated, washed and
visualized by incubation with a horseradish peroxidase
(HRP)-conjugated secondary antibody (goat anti-rabbit IgG and goat
anti-mouse IgG; 1:2,500, Santa Cruz Biotechnology) and the enhanced
chemiluminescence plus detection system (Amersham, Arlington
Heights, IL, USA). CAM expression was detected with anti-ICAM-1,
anti-VCAM-1 and anti-E-selectin mouse monoclonal antibodies,
respectively (1:1,000, Santa Cruz Biotechnology). The activation of
NF-κB and mitogen-activated protein kinase (MAPK) was detected with
anti-p-65, anti-phospho-p-65, anti-phospho-IκB-α (mouse
monoclonal), anti-extracellular-signal regulated kinase (ERK) 1/2,
anti-p-38, anti-phospho-ERK1/2 and anti-phospho-p-38 rabbit
monoclonal antibodies (1:1,000, Cell Signaling Technology, Danvers,
MA, USA).
ELISA
HUVECs were plated into the wells of a 24-well
cluster plate at a density of 5×104 cells/ml/well.
Subsequently, the cell culture supernatants were harvested by
centrifugation at 800 × g for 5 min at 4°C to remove cell debris
and were frozen at −80°C. Supernatant samples were thawed once and
assayed for IL-8 and MCP-1 content in duplicate using a
commercially available ELISA kit (R&D systems, Abingdon, UK),
as previously reported (15).
Assay of transcription factor NF-κB
NF-κB activity was measured using the TransAM NF-κB
Family kit, according to the manufacturer’s instructions (Active
Motif, Carlsbad, CA, USA). The samples were analyzed in a 96-well
plate containing the immobilized NF-κB consensus site
(5′-GGGACTTTCC-3′) oligonucleotide. Nuclear extracts were prepared
for analysis of NF-κB activity; the activated form of NF-κB p-65
and p-50 subunits in the nuclear extract bind to this
oligonucleotide, respectively. Using antibodies against the p-65
and p-50 subunits and a HRP-conjugated secondary antibody, the
developing solution of the TransAM NF-κB Family kit was added to
produce a blue color and subsequently quantified with the
microplate reader (Multiskan MK3, Thermo Labsystems) at 450 nm.
Data from triplicate wells were expressed as the mean ± standard
deviation (SD).
Statistical analysis
All experiments were repeated a minimum of three
times. The results were expressed as the mean ± SD using SPSS 13.0
statistical software (SPSS Inc., Chicago, IL, USA). Statistical
analyses were performed using one-way analysis of variance,
followed by Duncan’s multiple range test. P<0.05 was considered
to indicate a statistically significant difference.
Results
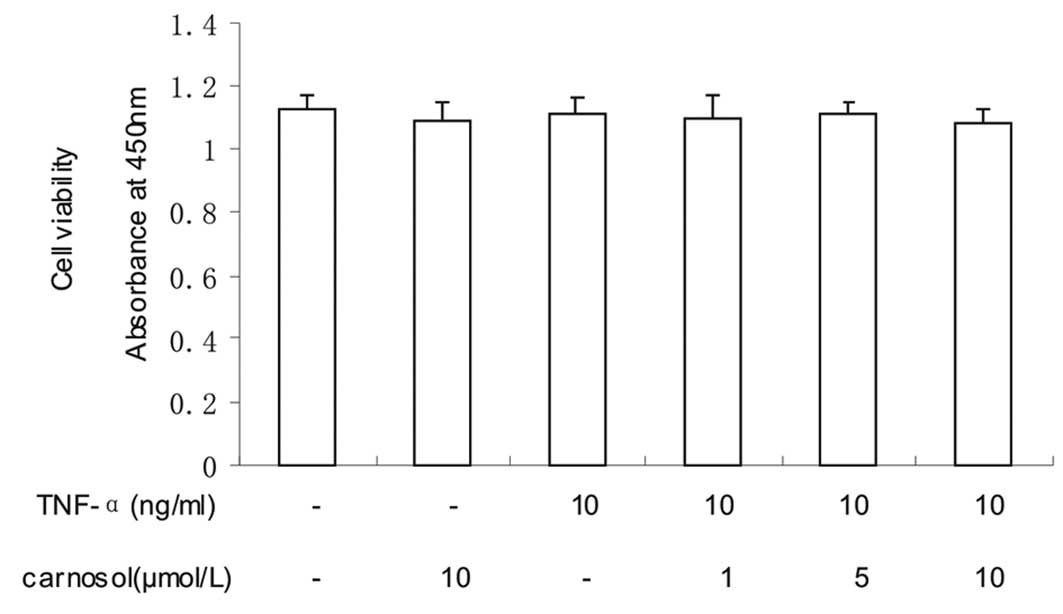
Effect of carnosol on cell viability
The effect of carnosol on cell viability was
investigated using CCK-8. The results revealed that the viability
of the HUVECs was not significantly influenced by carnosol
(Fig. 1).
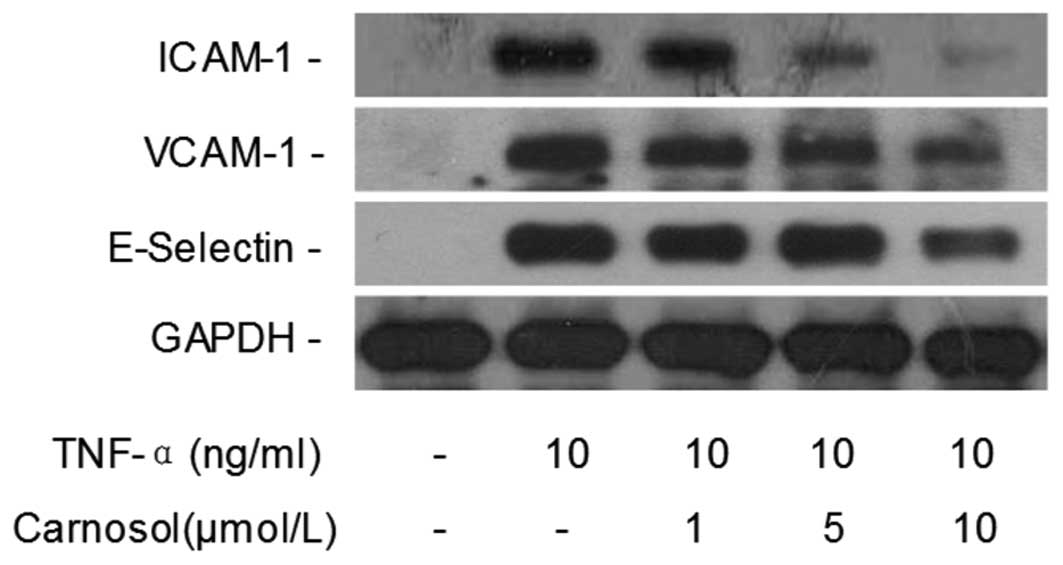
Carnosol inhibits TNF-α-induced CAM
expression levels
Western blotting was performed in order to
investigate whether carnosol affects TNF-α-induced CAM expression
in HUVECs. Compared with the control group, the TNF-α only group
revealed that TNF-α significantly increased the expression of
ICAM-1, VCAM-1 and E-selectin in HUVECs (Fig. 2). However, when the cells were
pretreated with 5 and 10 μmol of carnosol, the expression was
markedly inhibited in a dose-dependent manner.
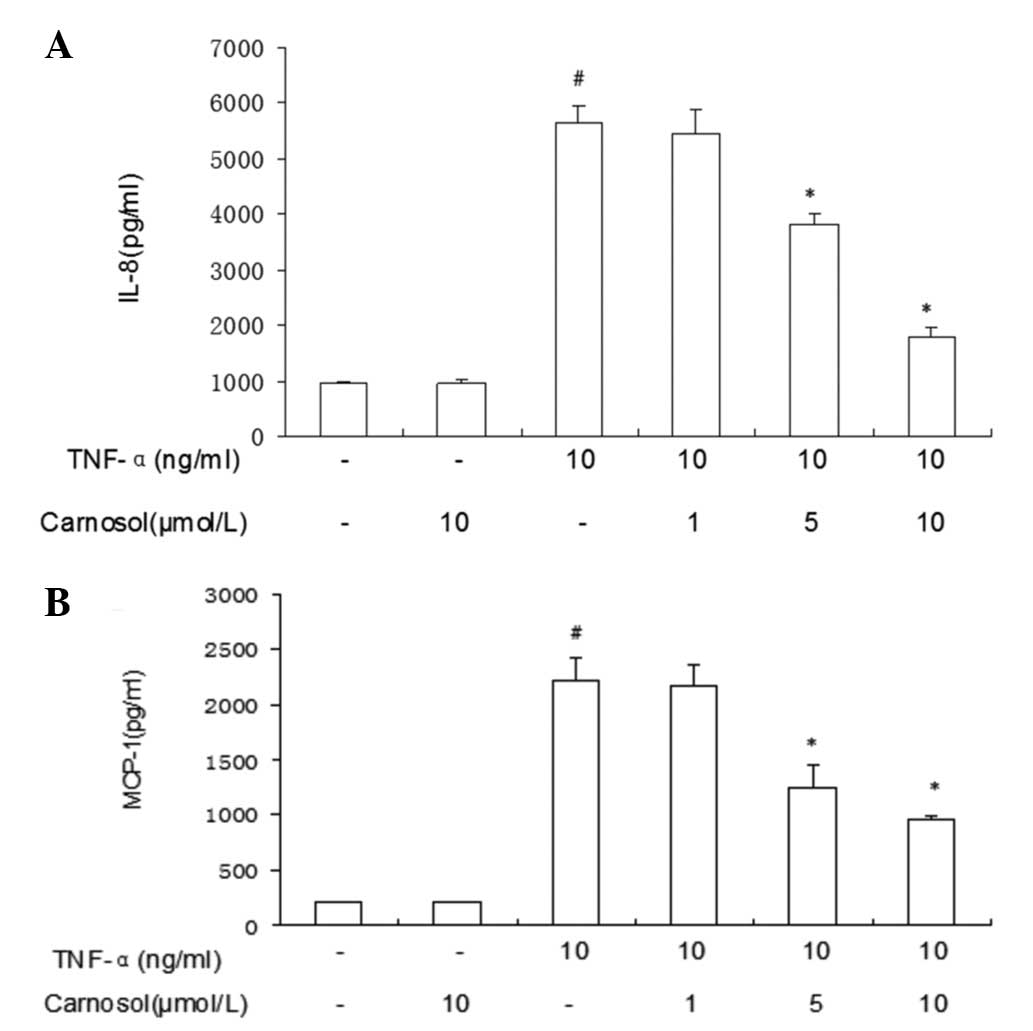
Carnosol inhibits IL-8 and MCP-1
expression in HUVECs induced by TNF-α
The ELISA results revealed that TNF-α enhanced IL-8
expression in HUVECs (Fig. 3).
However, following treatment with carnosol only, IL-8 expression
was not identified to have a significant difference. Pretreatment
with higher doses of carnosol may inhibit IL-8 expression more
markedly. Pretreatment with higher doses (5 μmol/l and 10 μmol/l)
of carnosol may inhibit MCP-1 expression more markedly than a lower
dose (1 μmol/l).
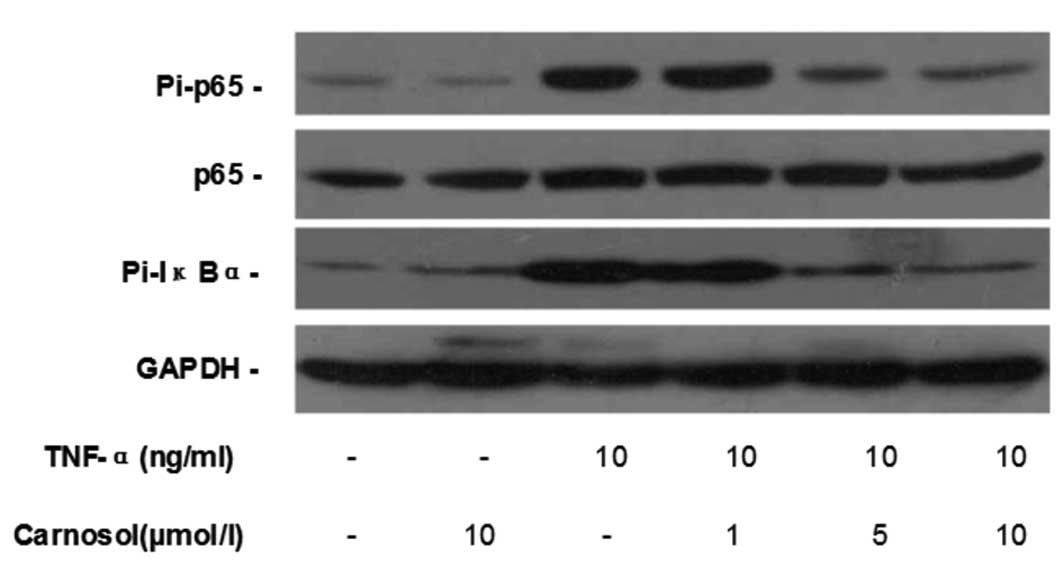
Carnosol inhibits TNF-α-induced NF-κB
activation
Western blotting was used to investigate whether
carnosol affected TNF-α-induced NF-κB activation in HUVECs.
Compared with the control group, carnosol had no impact on the
phosphorylation of p-65 and IκB-α in HUVECs, while TNF-α
significantly increased their phosphorylation. When pretreated with
5 and 10 μmol carnosol, the phosphorylation levels of p-65 and
IκB-α, but not the total p-65 expression, were reduced in a
dose-dependent manner (Fig.
4).
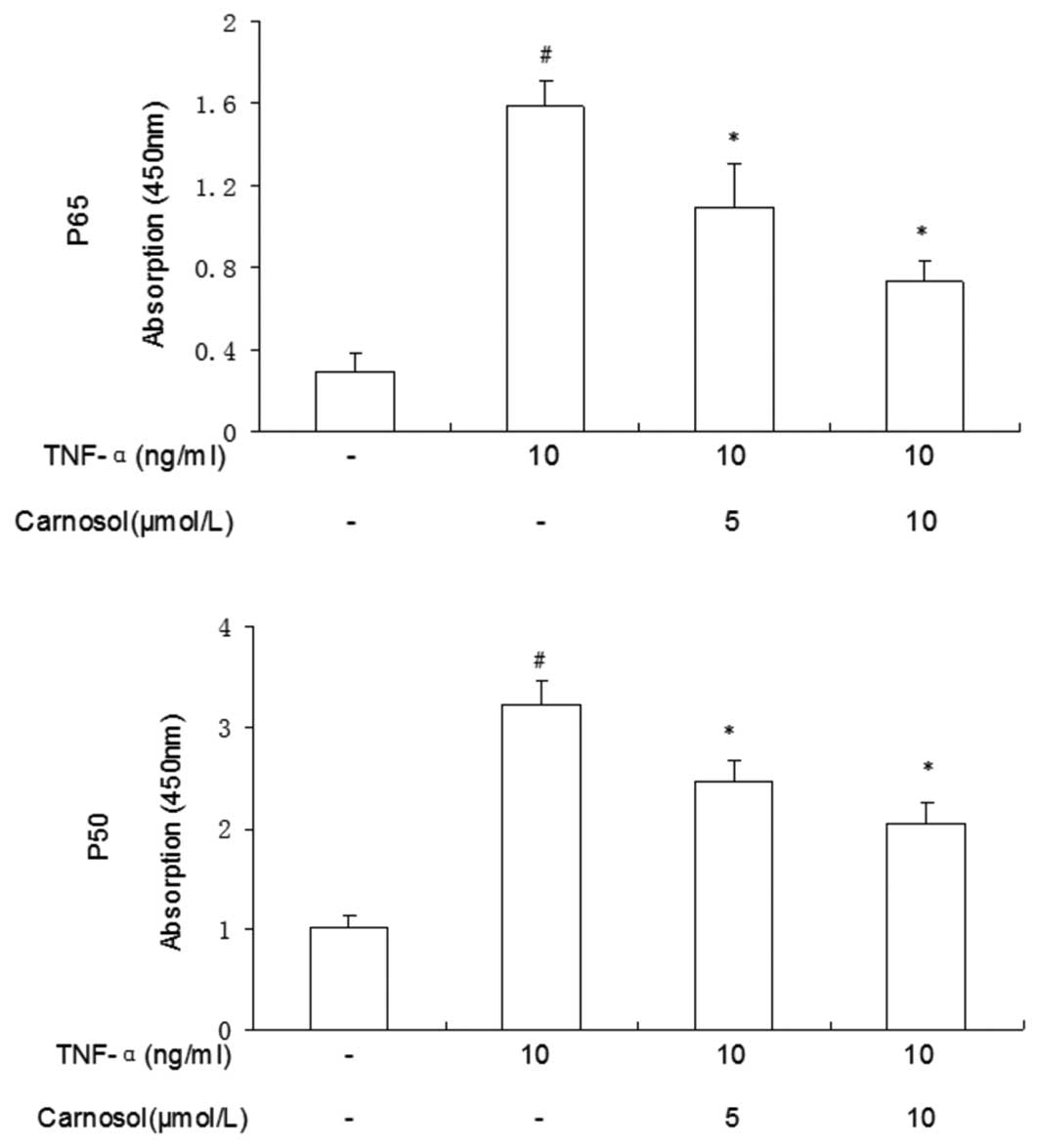
Carnosol reduces TNF-α-induced activation
of NF-κB p-65 and p-50
The nuclear translocation of p-50 and p-65 proteins
of the NF-κB family of transcription factors were measured.
Compared with the control group, a greater number of p-50 and p-65
proteins were located when the cells had been treated with TNF-α
only (Fig. 5). Following
pretreatment with carnosol, the total quantities of the two
proteins were significantly reduced. These results supported the
view that NF-κB-DNA binding activity was inhibited, in a
dose-dependent manner, by the pretreatment of HUVECs with carnosol
prior to TNF-α stimulation.
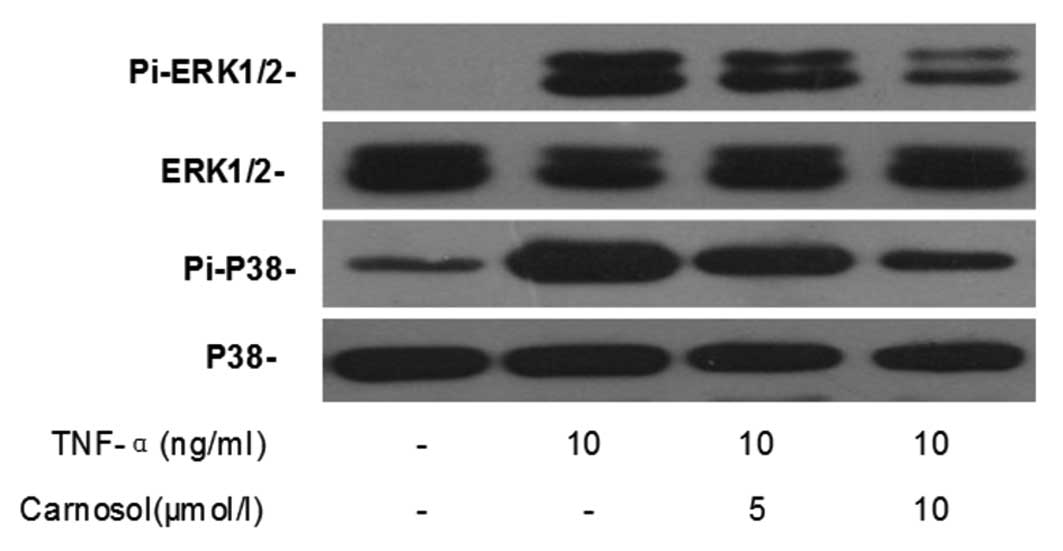
Effects of carnosol on MAPK
signaling
Western blotting was performed in order to
investigate the effect of carnosol on TNF-α-induced MAPK activation
in HUVECs. The results demonstrated that TNF-α significantly
enhanced the phosphorylated expression of ERK1/2 and p38, but not
that of total ERK1/2 and p38, in HUVECs (Fig. 6). Furthermore, pretreatment with
carnosol was shown to decrease their expression in a dose-dependent
manner.
Discussion
Endothelial activation induced by proinflammatory
cytokines is crucial in the inflammation process, as it is directly
responsible for the proinflammatory cytokines recruiting the
leukocytes into the inflamed focus from blood vessels during the
inflammation process. During the inflammatory process, the levels
of several proinflammatory cytokines were elevated, which induced
the expression of adhesion molecules, monocyte adhesion and
chemokine release (16). CAMs,
including VCAM-1, ICAM-1 and E-selectin, were increasingly
expressed by these endothelial cells (16), in addition to the production of
IL-8 and MCP-1 (4).
The stimulatory effect of TNF-α on CAM expression
and chemokine production in HUVECs observed in our study concurs
with that observed in previous reports (13,17,18).
The present study revealed that carnosol reduced the expression of
VCAM-1, ICAM-1 and E-selectin and the production of IL-8 and MCP-1
in TNF-α-stimulated HUVECs in a dose-dependent manner. These
adhesion molecules have been verified as critical for the
recruitment of inflammatory cells to the endothelium (19). Furthermore, previous studies have
demonstrated that the functional blocking of these adhesion
molecules was capable of suppressing T cell adhesion to endothelial
cells (20) and that reducing the
production of chemokines (21) may
be a treatment for IBD. The results of the present study suggested
that carnosol may retard the endothelial inflammatory process by
suppressing the secretion of chemoattractant molecules and the
production of chemokines.
Furthermore, this secretion of chemoattractant
molecules and the production of chemokines is always dependent on
the activities of NF-κB (13) and
MAPK (4). The analysis in the
present study indicated that carnosol suppressed TNF-α-induced
NF-κB activation. Numerous genes involved in the development of
IBDs are regulated by NF-κB. In addition, activated NF-κB has been
identified in the inflamed gut of IBD patients (22). NF-κB is composed of p-65 and p-50
subunits, and inactive NF-κB dimers are sequestered in the cytosol
in association with various inhibitory molecules of the IκB-α
family. In response to TNF-α, IκB-α is phosphorylated, which in
turn is targeted for ubiquitination- and proteasome-dependent
degradation (23). Previous
studies have demonstrated that the active forms of MAPK are
upregulated in patients with IBD (24). The present study demonstrated that
carnosol inhibited the phosphorylation of ERK1/2 and p-38 in
TNF-α-stimulated HUVECs. Due to the fact that the phosphorylation
of ERK1/2 and p-38 is associated with the CAM expression of
endothelial cells (25), it was
hypothesized that the molecular mechanism underlying the
anti-inflammatory activity of carnosol is associated with its
inhibitory effect on the MAPK pathway. As demonstrated in a
previous study, the inhibition of MAPK- and NF-κB-signaling
pathways may suppress IBD (26).
Therefore, it may be assumed that carnosol may have promising
anti-inflammatory ability by its inhibition of the MAPK and NF-κB
pathways, leading to a reduction in CAMs and chemokines.
There are emerging therapeutic agents for IBD
treatment which specifically target the endothelium. Natalizumab,
the first anti-α4-integrin inhibitor, demonstrated
significant therapeutic benefit for the treatment of CD, by
blocking the interaction of α4 expressing leukocytes
with their ligands, including E-selectin and VCAM-1 (27). Another drug, the soluble epoxide
hydrolase hydrolase inhibitor, also demonstrated high potential for
the treatment of IBD by decreasing the levels of IFN-γ, TNF-α,
MCP-1, VCAM-1 and NF-κB signals (28). In the present study, it was
demonstrated that carnosol may also inhibit the expression of CAMs
and chemokines associated with leukocyte recruitment.
In conclusion, the current study demonstrated that
carnosol may provide an effective approach for the treatment of
chronic inflammation, including IBDs. This anti-inflammatory effect
may be due to its ability to inhibit the production of CAMs
(ICAM-I, VCAM-1 and E-selectin) and chemokines (IL-8 and MCP-1),
which increase leukocyte infiltration to the inflammatory tissues.
Furthermore, the molecular mechanism underlying this effect may be
associated with the inhibitory effect of carnosol on the NF-κB and
MAPK pathways.
Acknowledgements
This study was partially sponsored by the National
Natural Science Foundation of China (grant nos. 91029702 and
81072046).
References
|
1
|
Khor B, Gardet A and Xavier RJ: Genetics
and pathogenesis of inflammatory bowel disease. Nature.
474:307–317. 2011. View Article : Google Scholar
|
|
2
|
Danese S: Inflammation and the mucosal
microcirculation in inflammatory bowel disease: the ebb and flow.
Curr Opin Gastroenterol. 23:384–389. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ghosh N, Chaki R and Mandal SC: Inhibition
of selective adhesion molecules in treatment of inflammatory bowel
disease. Int Rev Immunol. 31:410–427. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Cheng Q, McKeown SJ, Santos L, Santiago
FS, Khachigian LM, Morand EF and Hickey MJ: Macrophage migration
inhibitory factor increases leukocyte-endothelial interactions in
human endothelial cells via promotion of expression of adhesion
molecules. J Immunol. 185:1238–1247. 2010. View Article : Google Scholar
|
|
5
|
Danese S and Fiocchi C: Etiopathogenesis
of inflammatory bowel diseases. World J Gastroenterol.
12:4807–4812. 2006.PubMed/NCBI
|
|
6
|
Ninomiya K, Matsuda H, Shimoda H, Nishida
N, Kasajima N, Yoshino T, Morikawa T and Yoshikawa M: Carnosic
acid, a new class of lipid absorption inhibitor from sage. Bioorg
Med Chem Lett. 14:1943–6. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Weckesser S, Engel K, Simon-Haarhaus B,
Wittmer A, Pelz K and Schempp CM: Screening of plant extracts for
antimicrobial activity against bacteria and yeasts with
dermatological relevance. Phytomedicine. 14:508–516. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Poeckel D, Greiner C, Verhoff M, et al:
Carnosic acid and carnosol potently inhibit human 5-lipoxygenase
and suppress pro-inflammatory responses of stimulated human
polymorphonuclear leukocytes. Biochem Pharmacol. 76:91–97. 2008.
View Article : Google Scholar
|
|
9
|
Kim SJ, Kim JS, Cho HS, Lee HJ, Kim SY,
Kim S, Lee SY and Chun HS: Carnosol, a component of rosemary
(Rosmarinus officinalis L.) protects nigral dopaminergic
neuronal cells. Neuroreport. 17:1729–1733. 2006.PubMed/NCBI
|
|
10
|
Satoh T, Izumi M, Inukai Y, et al:
Carnosic acid protects neuronal HT22 cells through activation of
the antioxidant-responsive element in free carboxylic acid- and
catechol hydroxyl moieties-dependent manners. Neurosci Lett.
434:260–265. 2008. View Article : Google Scholar
|
|
11
|
Johnson JJ, Syed DN, Suh Y, Heren CR,
Saleem M, Siddiqui IA and Mukhtar H: Disruption of androgen and
estrogen receptor activity in prostate cancer by a novel dietary
diterpene carnosol: implications for chemoprevention. Cancer Prev
Res (Phila). 3:1112–1123. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Yu YM, Lin CH, Chan HC and Tsai HD:
Carnosic acid reduces cytokine-induced adhesion molecules
expression and monocyte adhesion to endothelial cells. Eur J Nutr.
48:101–106. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lian KC, Chuang JJ, Hsieh CW, Wung BS,
Huang GD, Jian TY and Sun YW: Dual mechanisms of NF-kappaB
inhibition in carnosol-treated endothelial cells. Toxicol Appl
Pharmacol. 245:21–35. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Adachi D, Oda T, Yagasaki H, Nakasato K,
Taniguchi T, D’Andrea AD, Asano S and Yamashita T: Heterogeneous
activation of the Fanconi anemia pathway by patient-derived FANCA
mutants. Hum Mol Genet. 11:3125–3134. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Scaldaferri F, Sans M, Vetrano S, et al:
Crucial role of the protein C pathway in governing microvascular
inflammation in inflammatory bowel disease. J Clin Invest.
117:1951–1960. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
16
|
Vestweber D: Adhesion and signaling
molecules controlling the transmigration of leukocytes through
endothelium. Immunol Rev. 218:178–196. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ley K, Laudanna C, Cybulsky MI and
Nourshargh S: Getting to the site of inflammation: the leukocyte
adhesion cascade updated. Nat Rev Immunol. 7:678–689. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Baggiolini M and Loetscher P: Chemokines
in inflammation and immunity. Immunol Today. 21:418–420. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Lehmann JC, Jablonski-Westrich D, Haubold
U, Gutierrez-Ramos JC, Springer T and Hamann A: Overlapping and
selective roles of endothelial intercellular adhesion molecule-1
(ICAM-1) and ICAM-2 in lymphocyte trafficking. J Immunol.
171:2588–2593. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Reiss Y and Engelhardt B: T cell
interaction with ICAM-1-deficient endothelium in vitro:
transendothelial migration of different T cell populations is
mediated by endothelial ICAM-1 and ICAM-2. Int Immunol.
11:1527–1539. 1999.PubMed/NCBI
|
|
21
|
Funakoshi T, Yamashita K, Ichikawa N, et
al: A novel NF-kappaB inhibitor, dehydroxymethylepoxyquinomicin,
ameliorates inflammatory colonic injury in mice. J Crohns Colitis.
6:215–225. 2002. View Article : Google Scholar
|
|
22
|
Andresen L, Jørgensen VL, Perner A, Hansen
A, Eugen-Olsen J and Rask-Madsen J: Activation of nuclear factor
kappaB in colonic mucosa from patients with collagenous and
ulcerative colitis. Gut. 54:503–509. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Viatour P, Merville MP, Bours V and
Chariot A: Phosphorylation of NF-kappaB and IkappaB proteins:
implications in cancer and inflammation. Trends Biochem Sci.
30:43–52. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Mitsuyama K, Suzuki A, Tomiyasu N, et al:
Pro-inflammatory signaling by Jun-N-terminal kinase in inflammatory
bowel disease. Int J Mol Med. 17:449–455. 2006.PubMed/NCBI
|
|
25
|
Scaldaferri F, Sans M, Vetrano S, et al:
The role of MAPK in governing lymphocyte adhesion to and migration
across the microvasculature in inflammatory bowel disease. Eur J
Immunol. 39:290–300. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Hollenbach E, Neumann M, Vieth M, Roessner
A, Malfertheiner P and Naumann M: Inhibition of p38 MAP kinase- and
RICK/NF-kappaB-signaling suppresses inflammatory bowel disease.
FASEB J. 18:1550–1552. 2004.PubMed/NCBI
|
|
27
|
Ghosh S and Panaccione R: Anti-adhesion
molecule therapy for inflammatory bowel disease. Therap Adv
Gastroenterol. 3:239–258. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zhang W, Yang AL, Liao J, et al: Soluble
epoxide hydrolase gene deficiency or inhibition attenuates chronic
active inflammatory bowel disease in IL-10(−/−) mice. Dig Dis Sci.
57:2580–2591. 2012.
|