Introduction
Pancreatic cancer is one of the most aggressive
types of human malignancy with the lowest 5-year survival rate
(1,2). At the time of diagnosis, the majority
of patients have locally advanced disease and/or distant metastatic
lesions precluding radical operational resection (3,4).
Perineural invasion is considered to be an important factor of
aggressive tumor behavior and is associated with local recurrence
and poor outcome in patients with pancreatic cancer (5). Perineural invasion is defined as the
presence of cancer cells within the epineural, perineural and
endoneurial spaces of the neuronal sheet, and around nerves
(5,6). This infiltration results in severe
pain and nerve damage (7). However
it remains unknown why pancreatic cancer exhibits a high frequency
of perineural invasion.
Pleiotrophin (PTN) is a type of neurotrophic factor,
which is also known as a neurite growth-promoting factor. PTN can
promote neurite outgrowth and neuronal survival in primarily
cultured cortical neurons (8). PTN
is mainly expressed during early embryogenesis. In adult tissues,
PTN is markedly downregulated and is present only at minimal levels
in certain tissues. It is not expressed in normal pancreatic
tissues; however, it is expressed in pancreatic cancer cells
(9). A high expression level of
PTN has been observed in 78% of tumor samples from pancreatic
cancer patients (10). PTN and
N-syndecan act as a receptor-ligand pair during the outgrowth of
neurites. Anti-N-syndecan antibodies added to culture media
exhibited an inhibitory effect on the PTN-induced outgrowth of
neurites (11). In a previous
study, we have confirmed that PTN and its receptor N-syndecan can
promote perineural invasion of pancreatic cancer cells (12). Therefore, PTN may be important in
neurite outgrowth, thus promoting the perineural invasion of
pancreatic cancer.
Chemically synthesized RNA interference (RNAi)
molecules have been used to silence the expression of various genes
in mammalian cell lines. The development of small interfering
(si)RNAs to specifically inhibit gene expression by triggering RNAi
pathways may be a potential strategy for the treatment of cancer
(13). Recently, the development
of more effective and stable gene nuclear silencing RNA-mediating
systems has been of interest (14). In the present study, a lentiviral
vector expressing short hairpin (sh)RNA against PTN was used to
transduce MIA PaCa-2 cells. The silencing effect of primate
lentivirus (pLV)-shRNA-PTN on the PTN gene in MIA PaCa-2
cells was investigated and the inhibition of neurite outgrowth from
dorsal root ganglion (DRG) neurons in vitro was also
examined.
Materials and methods
Chemicals and reagents
All chemicals were purchased from Sigma-Aldrich (St.
Louis, MO, USA). Tris/glycine-buffered saline (TBS; 10X) was
purchased from Bio-Rad (Hercules, CA, USA). Ethanol was obtained
from IBI Scientific (Peosta, IA, USA). Monoclonal mouse anti-human
PTN, anti-glyceraldehyde-3-phosphate dehydrogenase (GAPDH)
antibodies and peroxidase-coupled goat anti-mouse IgG secondary
antibody were purchased from Santa Cruz Biotechnology, Inc. (Santa
Cruz, CA, USA).
Design of shRNA-PTN and construction of
pLV-shRNA-PTN
Four lentiviral transduction particles encoding
shRNA against PTN (shRNA/PTN) were purchased from Sigma-Aldrich.
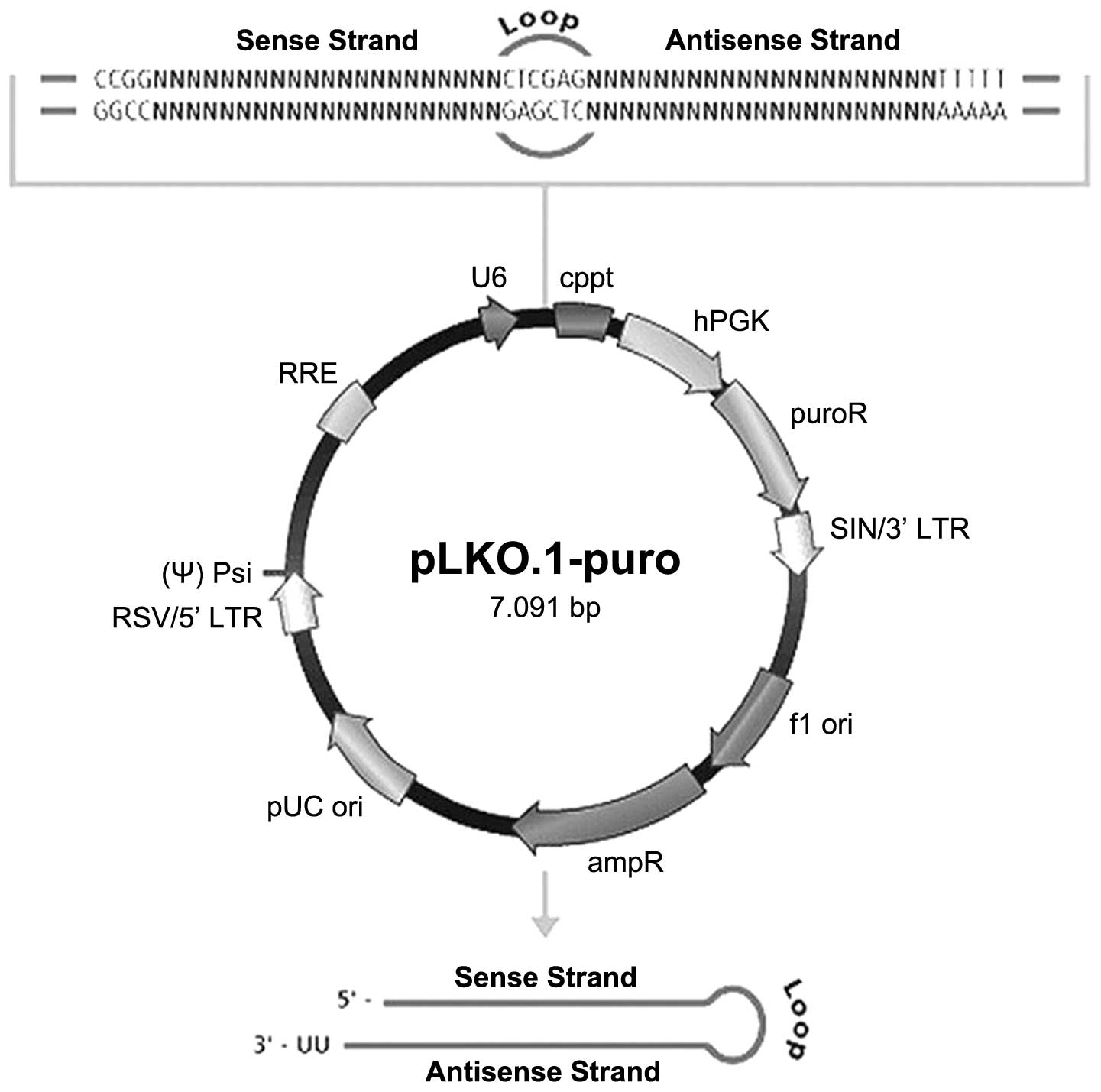
The lentiviral shRNA vector map is shown in Fig. 1. Four 21-nucleotide shRNA duplexes
from different sections of human PTN mRNA (GenBank accession
no. NM002825) were designed using the MISSION search database
(www.sigma-aldrich.com/missionsearch),
which is produced and distributed under license by the
Massachusetts Institute of Technology (Cambridge, MA, USA). The
shRNA sequences analyzed are listed in Table I.
 | Table ISequences of shRNA/PTN clones. |
Table I
Sequences of shRNA/PTN clones.
| shRNA/PTN clones | Sequence |
|---|
| A |
CCGGGCAGCTGTGGATACTGCTGAACTCGAGTTCAGCAGTATCCACAGCTGC
TTTTTG |
| B |
CCGGGCAACTGGAAGAAGCAATTTGCTCGAGCAAATTGCTTCTTCCAGTTGC
TTTTTG |
| C |
CCGGGCCAGAAGACTGTCACCATCTCTCGAGAGATGGTGACAGTCTTCTGGC
TTTTTG |
| D |
CCGGGCAAACCATGAAGACCCAGAGCTCGAGCTCTGGGTCTTCATGGTTTGC
TTTTTG |
Transduction of pLV-shRNA-PTN into MIA
PaCa-2 cells
The MIA PaCa-2 cell line, collected from a poorly
differentiated human pancreatic adenocarcinoma, was transduced with
pLV-shRNA-PTN. The cells were plated at a density of
5×106 cells/well and transduced with 1 μg pLV-shRNA-PTN
(multiplicity of infection, 1) in 96-well tissue culture plates. As
a control, MIA PaCa-2 cells were also transduced with the same
quantity of viral vector containing noncoding shRNA
(Sigma-Aldrich). The transduced cells were selected in cell culture
medium containing 10 μg/ml puromycin. Visible clones were picked
from 96-well plates, expanded in 24-well tissue culture plates and
finally transferred to regular cell culture flasks. All the clones
were screened using molecular biology tools, using the following
stages: i) cell selection and pre-treatment; ii) cell disruption;
iii) extraction; iv) purification (including salting out, organic
solvent precipitation, organic solvent extraction,
ultracentrifugation and crystallization); and v) condensation and
preservation. The expression of PTN protein was determined by
western blotting using the PTN-specific antibody.
Western blotting analysis of PTN
protein
Monolayer MIA PaCa-2 cells were grown to confluence
in 90% Dulbecco’s modified Eagle’s medium (DMEM) with 10% fetal
bovine serum (FBS). Upon reaching confluence, the medium was
removed and the monolayer cells were washed with cold
phosphate-buffered saline and lysed in ice-cold lysis buffer (Santa
Cruz Biotechnology, Inc.) for the radioimmunoprecipitation assay.
Protein isolation was followed according to the manufacturer’s
instructions. The concentration of protein was measured according
to the manufacturer’s instructions of the Quant-it Protein Assay
kit (Invitrogen Life Technologies, Carlsbad, CA, USA) using the
Qubit fluorometer (Invitrogen Life Technologies). For protein
expression analysis, 45 μg protein was separated on 4–20%
SDS-polyacrylamide gels. Following electrophoresis, the proteins
were transferred to polyvinylidene difluoride membranes following
Bio-Rad instructions. After the proteins were transferred, the
membranes were blocked for 1 h using 0.2% I-block solution (Applied
Biosystems, Foster City, CA, USA) in TBS containing 0.05% Tween-20.
The blocked membranes were probed sequentially with anti-PTN and
anti-GAPDH antibodies, followed by incubation with the secondary
antibodies. Unbound secondary antibody was removed by washing the
membrane three times with TBS containing 0.05% Tween-20. The bands
were visualized using an enhanced chemiluminescence system (UVP,
Upland, CA, USA).
Separation and culture of DRG
neurons
Newborn mice were euthanized with carbon dioxide and
sterilized using 75% ethanol. The study was approved by the Ethics
Committee of First Affiliated Hospital, Henan University of Science
and Technology (Luoyang, China). The spines were cut open and the
DRG neurons were isolated from the vertebrae. The spinal cords with
the attached DRG neurons were removed from the vertebrae. DRG
neurons from the cervical and thoracic areas were dissected under
sterile conditions and were transferred to a 15 ml tube with 5 ml
D-Hanks’ solution then centrifuged at 70 × g for 5 min. The
supernatant was removed and 20 μl of 2.5% trypsin was added to the
tube, which was incubated for 20–25 min at 37°C, followed by
centrifugation at 800 rpm for 5 min. The supernatant was removed
and 2 ml serum-supplemented medium (DMEM/F12; Gibco-BRL, Grand
Island, NY, USA) containing 10% heat-inactivated FBS (Sigma, St.
Louis, MO, USA) was added to the tube and mixed. The neurons were
counted and seeded in 6-well culture plates at 105 cells
per well.
Cell culture
The MIA PaCa-2 cell line was obtained from the
American Type Culture Collection (Manassas, VA, USA). The model was
established by co-culturing DRG neurons with MIA PaCa-2 cells as
previously described (15).
Dissociated DRG neurons were seeded on coverglasses treated with
polylysine (10%, Sigma-Aldrich) and placed in 12-well culture
plates. A metal rack was placed in each well after the cells had
adhered to the coverglasses. The MIA PaCa-2 cells were also seeded
onto coverglasses, which were placed in 12-well plates, and 2 ml
DMEM/F12 medium with 10% FCS was added into each well. On the day
of infection, the previous culture medium was removed from the
cells, 50 μl recombinant pLV-shRNA-PTN and 2 ml fresh DMEM/F12
medium containing 2% FCS (at an MOI of 50) were added to the
infection group, and 2 ml fresh DMEM/F12 medium containing 2% FCS
without lentiviral stock was added to the control (uninfected)
group. Following incubation at 37°C for 24 h, the coverglasses were
placed on the metal racks. The MIA PaCa-2/DRG neurons were
routinely cultured in a serum-supplemented medium at 37°C
(DMEM/F12; GIBCO) containing 10% heat-inactivated FBS in a
humidified atmosphere of 95% O2 and 5% CO2.
DRG neurons were also cultured alone as a control.
Image analysis
Following cell culture for 1, 3, 5 and 7 days, the
cell growth was observed, and the cyton size and axon length were
photographed by an inverted imaging microscope at ×200
magnification (Nikon, Tokyo, Japan). Ten random visual fields in
the infected and control groups were observed and analyzed using
Motic software (Macao, China). Neurite outgrowth was evaluated by
measuring the vertical length between the spots where the neurite
grew from the pericaryon and its terminus. The major and minor axis
were denoted as a and b, respectively. The diameter of the cell
body was measured by multiplying a and b and extracting its square
root. All units are presented in micrometers.
Statistical analysis
The images obtained from western blotting were
scanned and analyzed using the Quantity One software (Bio-Rad). The
ratios of PTN to GAPDH were calculated. The statistical
significance between the control and infected groups was calculated
using one-way analysis of variance, and P<0.05 was considered to
indicate a statistically significant difference.
Results
Suppression of PTN protein expression by
recombinant lentivirus pLV-shRNA-PTN
The PLV-shRNA-PTN vectors were successfully
constructed with the viral titer up to 4.4×108 TU/ml.
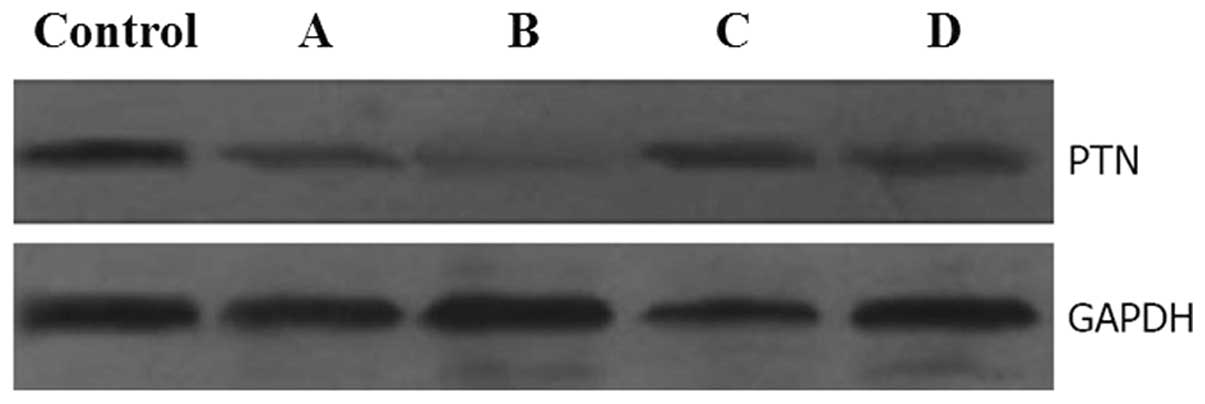
Western blot analysis using anti-PTN-specific antibodies on day 7
of culture revealed that PTN protein expression in MIA PaCa-2 cells
infected with pLV-shRNA-PTN was suppressed when compared with the
control. The inhibition rates of PTN protein expression following
pLV-shRNA-PTN-A, -B, -C and -D infection were 46, 80, 20 and 21%,
respectively, (Fig. 2).
pLV-shRNA-PTN-B exhibited the highest knockdown efficiency. These
results indicate that although all pLV-shRNA-PTN plasmids inhibited
the production of PTN protein in MIA PaCa-2 cells, pLV-shRNA-PTN-B
was the more potent inhibitor for PTN protein expression in MIA
PaCa-2 cells. In follow-up experiments, the pLV-shRNA-PTN-B plasmid
was used in the experimental condition groups.
Dynamic observation of neuron growth
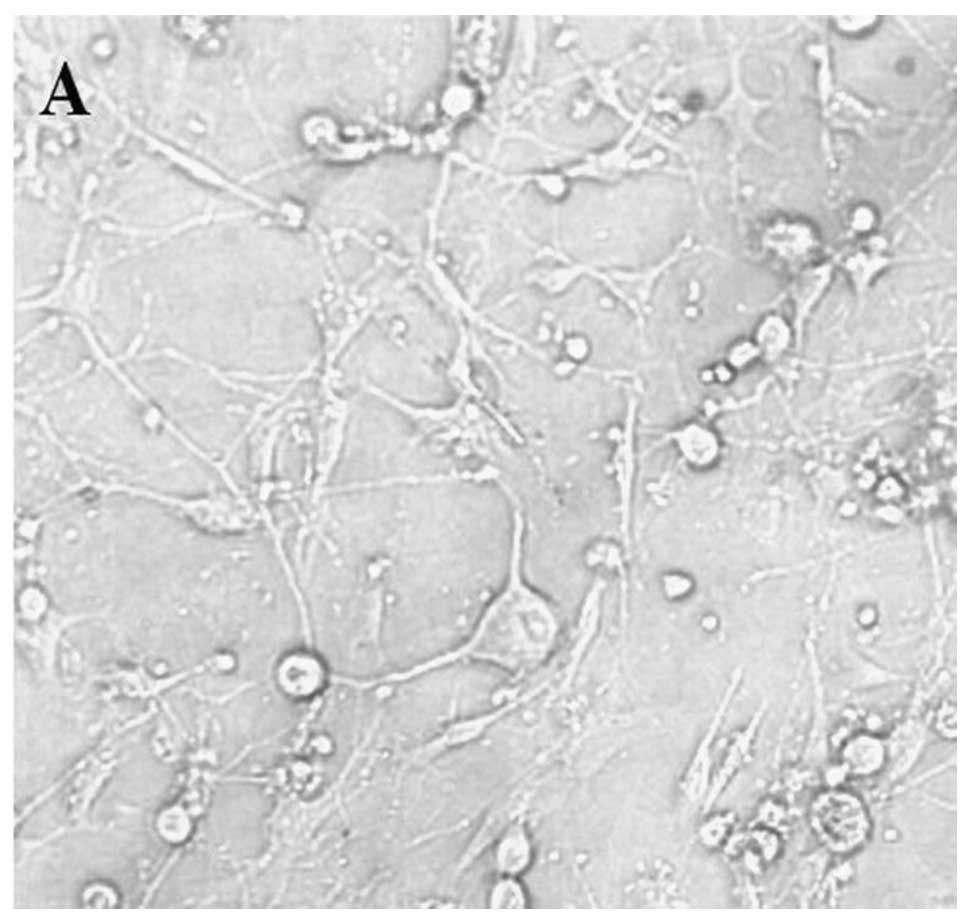
On day 1 of monoculture (cells grown alone), the
cells were large, lucent and smooth, and growth cones around the
cell bodies were observed. During the cell culture, no obvious
morphological changes in the DRG neurons were identified. On day 7,
the number of monocultured neurons decreased and a few neurons
exhibited extended neurites. In the co-cultures of DRG neurons and
MIA PaCa-2 cells, the cytons gradually became fusiform. On day 7,
the neurites in the control group had emerged and increased in
length when compared with the group infected with pLV-shRNA-PTN.
The length of neurites in the control group progressively increased
with time and a cancellous association among neurites emerged
(Fig. 3A), which was not observed
in the infected group (Fig. 3B).
The cells in thetwo groups schizolysed markedly and the cell
envelopes were not smooth.
Measurement of neuron cyton size and
neurite outgrowth
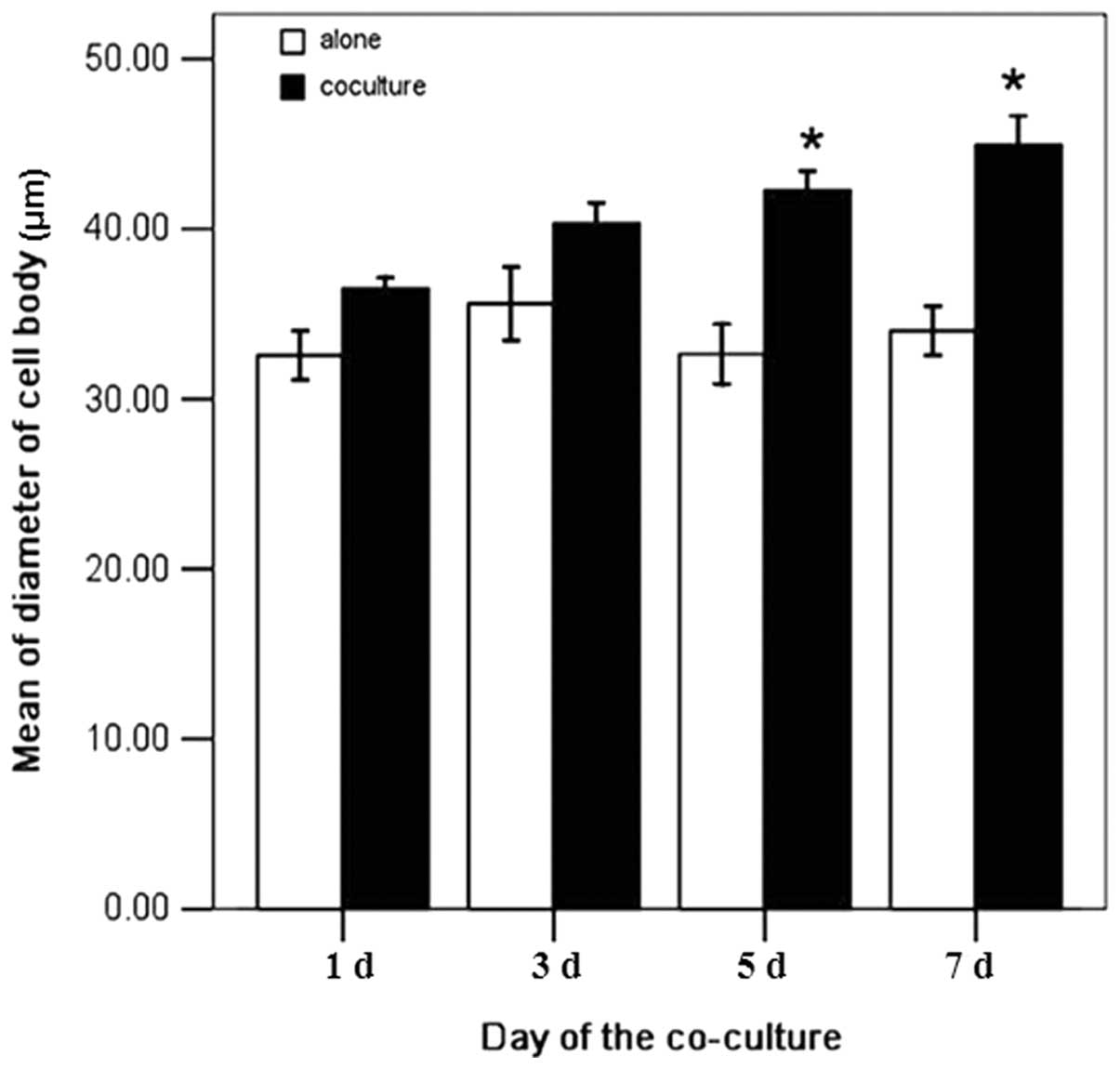
The diameters of the cell bodies from the two groups
were measured on days 1, 3, 5 and 7. In the control group, the
diameters of the cell bodies on these co-culture days were 35.7,
39.0, 41.1 and 43.0 mm, respectively. In the infected group, the
diameters of outgrown neurites on the same days were 31.2, 34.9,
31.6 and 32.1 mm, respectively. The diameters of the cell bodies in
the control group were 1.14, 1.13, 1.30, and 1.34 times larger than
those in the infected group with a significant difference
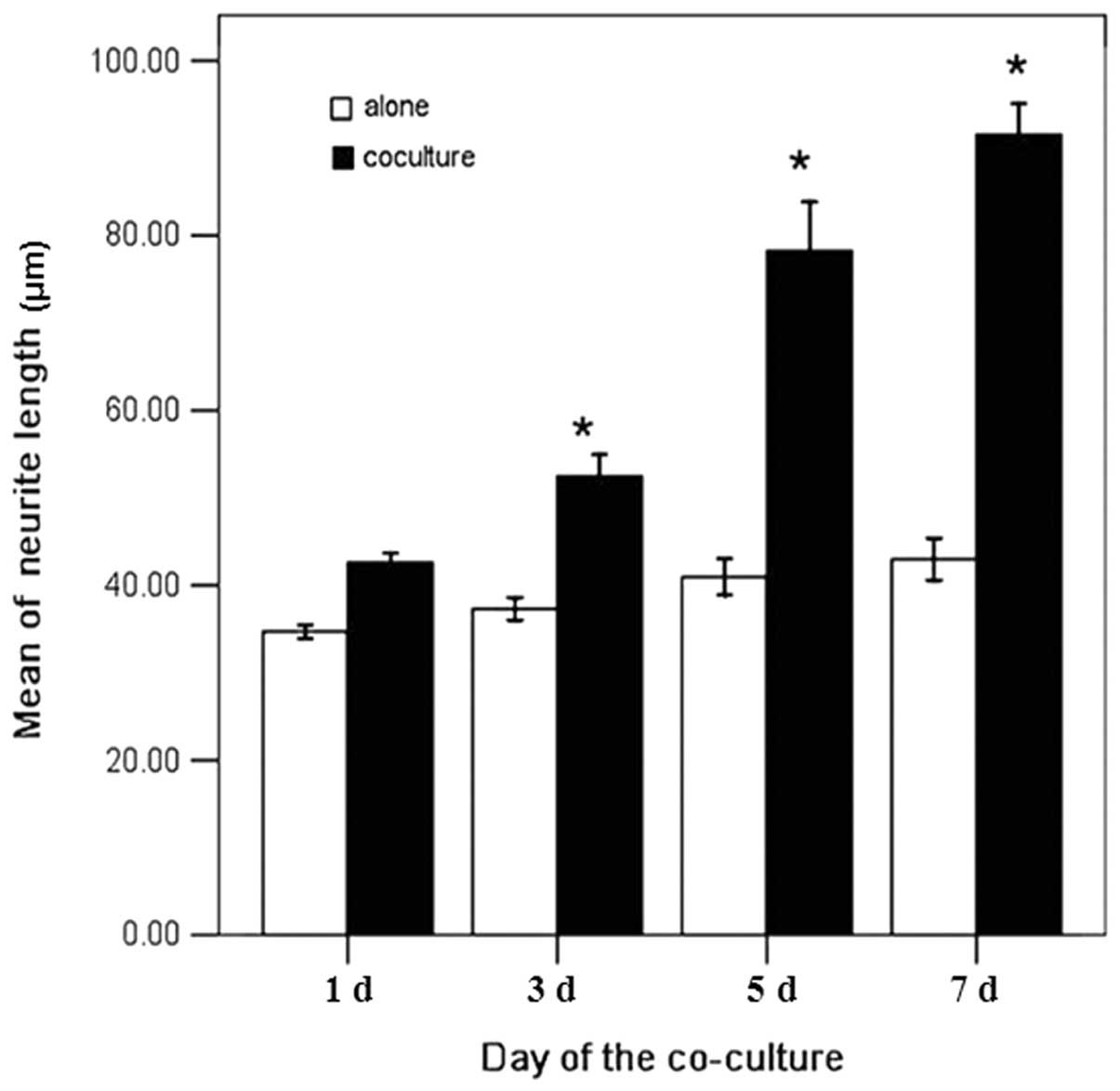
(P<0.05, Fig. 4). The neurite
outgrowths of the two groups were also measured on days 1, 3, 5,
and 7. In the control group, the neurite outgrowths were 43.2,
52.5, 79.6, and 89.3 mm, respectively. In the infected group, the
neurite outgrowths were 33.7, 35.1, 40.8, and 42.4 mm,
respectively. The neurite outgrowths of the control group were
1.28, 1.50, 1.95 and 2.11 times larger than in the infected group
with a significant difference (P<0.05, Fig. 5).
Discussion
Perineural invasion is defined as the presence of
cancer cells along nerves and/or within the epineurial,
perineurial, and endoneurial spaces of the neuronal sheath
(6). Perineural invasion is a
multifactorial process involving various signaling molecules from
different signaling pathways (5,16,17).
These signaling molecules include nerve growth factors,
neurotrophic factors, chemokines, and cell-surface ligands and
receptors (18,19). Pancreatic ductal adenocarcinomas
cells exhibit marked neurotrophic effects and the pancreas is in
close proximity to several neural plexuses, which may partially
explain the high incidence of perineural invasion in pancreatic
cancer (20).
RNAi-triggered mRNA destruction is widely used for
the modulation of gene expression. This technique is under
extensive investigation and has been recently applied to the
inhibition of PTN (21). In the
present study, pLV-shRNA-PTN was used to establish an MIA PaCa-2
cell line with stable suppression of the PTN gene. It was
confirmed that transduction by shRNA/PTN lentiviral vector
constructs produced a pronounced PTN gene-silencing at the
protein level. However, not all positive synthetic shRNA oligos can
exhibit effective functions in vector-based RNAi (22). The silencing may depend on the
transduction efficiency and the half-life of shRNA/PTN. The
accuracy with which the efficiency of an individual siRNA can be
predicted is also unclear (23).
The results of the present study confirm that not all shRNAs were
capable of knocking down PTN expression to the same extent when the
shRNAs were introduced into cells. Therefore, shRNAs exhibit
variable capabilities in mediating RNAi knockdown. The
pLV-shRNA-PTN knockdown cells were subsequently examined, and Clone
B exhibited the highest knockdown efficiency on protein expression
(80% downregulation when compared with the vector control).
The previous mechanisms regarding perineural
invasion have demonstrated that cancer cells exhibit an initiative
effect whilst nerve fibers exhibit a passive effect. However,
whether cancer cells can promote the functions of nerve cells and
nerve fiber effects in the perineural invasion of pancreatic cancer
has not been widely reported, as the potential mechanisms are not
clear. The activity of neurons can be evaluated by observing
neurite outgrowth and cyton size. In the in vitro model
system used in the present study, enhanced cyton size, neurite
outgrowth and neuron activity were observed in the presence of MIA
PaCa-2 cells from the control group, supporting the hypothesis that
cancer cells can promote the functions of nerve cells. These
results indicate that cancer cells also have tropism towards the
growth of nerve cells. This effect may be involved in mediating the
invasion of cancer cells along nerves. In the control group, the
neurites grew significantly on days 5–7 of the culture, suggesting
that certain factors mediate the growth of neurites. It was
hypothesized that specific growth factors produced by nerve cells
or cancer cells may promote the growth and survival of nerve cells,
and exhibit neurotropism. As a neurite growth-promoting factor, PTN
can synergistically promote the development of perineural invasion
in pancreatic cancer.
PTN is also involved in the regeneration of
peripheral nerves following nerve injury and functional recovery
following neural transplantation in rats (24,25).
The available evidence suggests that PTN bound to N-syndecan
promotes neurite outgrowth from DRG neurons. In the present study,
DRG neurons were selected due to their involvement in the
perineural invasion of pancreatic cancer (26). DRG neurons were dissociated from
newborn mice for monoculture or co-culture with MIA PaCa-2 cells.
The results indicate that pLV-shRNA-PTN can be used to infect MIA
PaCa-2 cells and suppress PTN gene expression efficiently.
Compared with the number of control cells, the number of DRG
neurons co-cultured with infected MIA PaCa-2 cells and the number
of extended neurites was significantly decreased. These results are
concurrent with those of our previous study (21).
In conclusion, the lentiviral construct
pLV-shRNA-PTN can efficiently and specifically knockdown PTN
in the MIA PaCa-2 pancreatic cancer cell line, and also inhibit the
neurite outgrowth from DRG neurons in vitro. Therefore, the
PTN gene and pLV-shRNA-PTN are important for investigating
perineural invasion in pancreatic cancer. We will establish a model
of pancreatic cancer in situ in nude mice in future studies.
By injecting pLV-shRNA-PTN plasmid, the effects of PTN gene
and lentiviral construct pLV-shRNA-PTN on perineural invasion of
pancreatic cancer may be further investigated.
Acknowledgements
This study was supported by the National Natural
Science Foundation of China (grant no. U1204819) and by the Health
Science and Technology Innovation Talents Program of Henan Province
(grant no. 4203).
References
|
1
|
Siegel R, Naishadham D and Jemal A: Cancer
statistics, 2012. CA Cancer J Clin. 62:10–29. 2012. View Article : Google Scholar
|
|
2
|
Mössner J: What’s new in therapy of
pancreatic cancer. Dig Dis. 28:679–683. 2010.
|
|
3
|
Han SL, Zhang WJ, Zheng XF, Shen X, Zeng
QQ and Ke QH: Radical resection and outcome for malignant tumors of
the pancreatic body and tail. World J Gastroenterol. 15:5346–5351.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Chiang KC, Yeh CN, Lee WC, Jan YY and
Hwang TL: Prognostic analysis of patients with pancreatic head
adenocarcinoma less than 2 cm undergoing resection. World J
Gastroenterol. 15:4305–4310. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Bapat AA, Hostetter G, Von Hoff DD and Han
H: Perineural invasion and associated pain in pancreatic cancer.
Nat Rev Cancer. 11:695–707. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
6
|
Liebig C, Ayala G, Wilks JA, Berger DH and
Albo D: Perineural invasion in cancer: a review of the literature.
Cancer. 115:3379–3391. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ceyhan GO, Michalski CW, Demir IE, Müller
MW and Friess H: Pancreatic pain. Best Pract Res Clin
Gastroenterol. 22:31–44. 2008. View Article : Google Scholar
|
|
8
|
Takamatsu H, Itoh M, Kimura M,
Gospodarowicz D and Amann E: Expression and purification of
biologically active human OSF-1 in Escherichia coli. Biochem
Biophys Res Commun. 185:224–230. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Weber D, Klomp HJ, Czubayko F, Wellstein A
and Juhl H: Pleiotrophin can be rate-limiting for pancreatic cancer
cell growth. Cancer Res. 60:5284–5288. 2000.PubMed/NCBI
|
|
10
|
Souttou B, Juhl H, Hackenbruck J, et al:
Relationship between serum concentrations of the growth factor
pleiotrophin and pleiotrophin-positive tumors. J Natl Cancer Inst.
90:1468–1473. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Raulo E, Chernousov MA, Carey DJ, Nolo R
and Rauvala H: Isolation of a neuronal cell surface receptor of
heparin binding growth-associated molecule (HB-GAM). Identification
as N-syndecan (syndecan-3). J Biol Chem. 269:12999–13004.
1994.PubMed/NCBI
|
|
12
|
Yao J, Ma Q, Wang L and Zhang M:
Pleiotrophin expression in human pancreatic cancer and its
correlation with clinicopathological features, perineural invasion,
and prognosis. Dig Dis Sci. 54:895–901. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lage H: Potential applications of RNA
interference technology in the treatment of cancer. Future Oncol.
1:103–113. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Song J, Cao L and Li Y: RNA
interference-mediated inhibition of survivin and VEGF in pancreatic
cancer cells in vitro. Mol Med Rep. 7:1651–1655. 2013.PubMed/NCBI
|
|
15
|
Fukuda J: Nerve cells of adult and aged
mice grown in a monolayer culture: age-associated changes in
morphological and physiological properties of dorsal root ganglion
cells in vitro. Dev Neurosci. 7:374–394. 1985. View Article : Google Scholar
|
|
16
|
Ceyhan GO, Demir IE, Altintas B, et al:
Neural invasion in pancreatic cancer: a mutual tropism between
neurons and cancer cells. Biochem Biophys Res Commun. 374:442–447.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Gil Z, Cavel O, Kelly K, et al: Paracrine
regulation of pancreatic cancer cell invasion by peripheral nerves.
J Natl Cancer Inst. 102:107–118. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ma J, Jiang Y, Jiang Y, Sun Y and Zhao X:
Expression of nerve growth factor and tyrosine kinase receptor A
and correlation with perineural invasion in pancreatic cancer. J
Gastroenterol Hepatol. 23:1852–1859. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Marchesi F, Piemonti L, Fedele G, et al:
The chemokine receptor CX3CR1 is involved in the neural tropism and
malignant behavior of pancreatic ductal adenocarcinoma. Cancer Res.
68:9060–9069. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ceyhan GO, Schäfer KH, Kerscher AG, et al:
Nerve growth factor and artemin are paracrine mediators of
pancreatic neuropathy in pancreatic adenocarcinoma. Ann Surg.
251:923–931. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Yao J, Zhang M, Ma QY, Wang Z, Wang LC and
Zhang D: PAd-shRNA-PTN reduces pleiotrophin of pancreatic cancer
cells and inhibits neurite outgrowth of DRG. World J Gastroenterol.
17:2667–2673. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Celius T, Garberg P and Lundgren B: Stable
suppression of MDR1 gene expression and function by RNAi in Caco-2
cells. Biochem Biophys Res Commun. 324:365–371. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Schuck S, Manninen A, Honsho M, Füllekrug
J and Simons K: Generation of single and double knockdowns in
polarized epithelial cells by retrovirus-mediated RNA interference.
Proc Natl Acad Sci USA. 101:4912–4917. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Blondet B, Carpentier G, Lafdil F and
Courty J: Pleiotrophin cellular localization in nerve regeneration
after peripheral nerve injury. J Histochem Cytochem. 53:971–977.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Hida H, Masuda T, Sato T, Kim TS, Misumi S
and Nishino H: Pleiotrophin promotes functional recovery after
neural transplantation in rats. Neuroreport. 18:179–183. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Dai H, Li R, Wheeler T, et al: Enhanced
survival in perineural invasion of pancreatic cancer: an in vitro
approach. Hum Pathol. 38:299–307. 2007. View Article : Google Scholar : PubMed/NCBI
|