Introduction
Mesenchymal stem cells (MSCs) have been extensively
investigated as potential therapeutic cells for heart failure and
myocardial infarction (MI) (1,2);
however, their inherent weakness, including poor survival of donor
cells (3) and lack of functional
coupling (4) with the host tissue
recount the efficacy of cell therapy. Numerous methods, including
gene modification (5), cytokine
stimulation (6) and hypoxic
preconditioning (7), appear to
augment the survival of MSCs and the upregulation of connexin 43
(Cx43).
Cx43 forms intracellular communication channels,
known as gap junctions (GJ) and facilitates electrical coupling
between cells (8). GJs are also
involved in the pathophysiology of cell death in injury (9). Mitochondrial Cx43 may also regulate
apoptosis (10,11). Generally, Cx43 expression in MSCs
is extremely low, although it may increase progressively in the
cardiac microenvironment (12).
Previously, studies have shown that high Cx43 expression in MSCs by
gene modification or insulin-like growth factor-1 (IGF-1)
preconditioning promotes cell survival, cardiomyogenesis and heart
function following transplantation (13,14).
However, to the best of our knowledge, the role of Cx43 in cell
therapy remains to be fully determined.
Previous studies have shown that cardiac protection
of cell therapy attributes to paracrine factors released from MSCs,
including angiogenic cytokines [such as vascular endothelial growth
factor (VEGF) and basic fibroblast growth factor (bFGF)] and
antiapoptotic factors (15,16).
In the current study, an in vitro model with deprivation of
serum and oxygen, and a murine MI model with permanent ligation of
the left anterior-descending (LAD) coronary artery were used to
investigate whether Cx43 or gap junctions promote angiogenesis and
enhance the therapeutic efficacy of MSCs.
Materials and methods
Animal care
Sprague-Dawley rats were obtained from the
Experimental Animal Center of Nanjing Medical University (Nanjing,
China). All experiments were approved by the Institutional Animal
Care and Use Committee of Nanjing Medical University.
Isolation and culture of bone marrow
MSCs
The MSCs were isolated from the femurs of 4-week-old
male Sprague-Dawley rats as described previously (3). Bone marrow was suspended in 5 ml low
glucose Dulbecco’s modified Eagle’s medium (DMEM) containing 100
U/ml penicillin and 100 μg/ml streptomycin. Mononuclear cells were
recovered from the interface following centrifugation (4,000 × g, 5
min) in Ficoll and were washed twice, resuspended in 10% fetal
bovine serum-DMEM and plated in flasks at 1×106 cells
per 100 cm2. Cultures were maintained at 37°C in a
humidified atmosphere containing 5% CO2. After 48 or 72
h, non-adherent cells were discarded and the adherent cells were
thoroughly washed twice with phosphate-buffered saline (PBS). Fresh
complete medium was added and replaced every three or four days for
~10 days. The second passage cells were used in the in vitro
and in vivo experiments.
Cx43 genetic modification of MSCs
The full-length cDNA of rat Cx43 was cloned into
pIRES2-eGFP, a eukaryotic expression plasmid, (Clontech, Mountain
View, CA, USA). The siRNA sequence 5′-CGT GGA GAT GCA CCT GAA GTT
CAA GAG ACT TCA GGT GCA TCT CCA CG TTT TTT-3′ was cloned into the
pRNA-U6-neo-vector (GenScript Co., Piscataway, NJ, USA). Plasmids
were delivered using Fugene HD transfection reagent (Roche
Diagnostics GmbH, Mannheim, Germany) according to the
manufacturer’s instructions. The genetically modified MSCs were
termed MSCs-vector, MSCs-Cx43 and
MSCs-siCx43.
Gap junction alterations between MSCs by
synthetic peptides
Connexin mimetic peptides (MP) for Cx43
(SRPTEKTIFII, specific and reversible inhibitors of gap-junctional
communication), antiarrhythmic peptide (AAP,
H2N-Gly-Ala-Gly-4Hyp-Pro-Tyr-CONH2) and
control peptide (CP) were synthesized by ChinaPeptides Co., Ltd.
(Shanghai, China). MSCs were incubated with connexin mimetic
peptides (50 μmol) and antiarrhythmic peptide (50 μmol) for 30 min
to inhibit or enhance gap-junctional communication between cells
and were subjected to hypoxic intervention.
Scrape loading/dye transfer (SL/DT)
method
Levels of gap junctional intercellular communication
(GJIC) were determined by the sSL/DT technique using a fluorescent
dye, Lucifer Yellow (LY; Sigma-Aldrich, St. Louis, MO, USA) as
previously described (17).
Cardiomyocytes were washed thoroughly with Ca2+-free
Tyrode’s solution and sliced with a razor blade. A total of 0.5% LY
dye was added to the cells for 5 min and the cells were washed
three times with PBS and fixed with 4% paraformaldehyde. Cells
stained with LY dye were detected by fluorescence emission with an
inverted fluorescent microscope (Olympus, Tokyo, Japan) equipped
with a camera.
Hypoxic model of MSCs in vitro
Hypoxic conditions were generated by incubating the
cells at 37°C using an AnearoPack system (http://www.mgc-a.com; Mitsubishi Gas Chemical Co.,
Inc. Tokyo, Japan) to scavenge free oxygen. The oxygen content in
the medium was <1% within 1 h and maintained over the
experimental time. Conditioned medium was generated as described
previously (18). In brief, 90%
confluent third passage MSCs were fed with serum-free
high-glucose-DMEM and incubated for 8 h under hypoxia. The medium
was then collected and subsequently assayed.
Detection of cytokine release from MSCs
by enzyme-linked immunosorbent assay (ELISA)
The concentrations of VEGF and bFGF were measured in
the culture supernatant with ELISA kits (VEGF and bFGF; R&D
Systems, Minneapolis, MN, USA), according to the manufacturer’s
instructions. All samples and standards were measured in
duplicate.
Myocardial infarction model and MSC
transplantation
Female Sprague-Dawley rats underwent left anterior
descending (LAD) coronary artery ligation to create MI, as
previously described (19). Rats
were anesthetized by intraperitoneal injection of pentobarbital (50
mg/kg body weight; Roche Diagnostics GmbH), endotracheally
intubated and mechanically ventilated. Following thoracotomy, the
LAD was ligated with a 6-0 silk suture 3–4 mm from the tip of the
left atrium. Successful ligation of the LAD coronary artery was
verified by visual inspection of the left ventricular apex, which
showed a pale discoloration. Electrocardiography indicated an
elevated S-T. Seven days following ligation, surviving animals were
injected with a 30 μl volume containing 5×105 MSCs or
PBS in three separate peri-infarct regions of the heart, using a
31-gauge needle. Two weeks following transplantation, surviving
animals were used for assay.
Western blotting
The protein concentration of the samples was
determined by a bicinchoninic acid protein assay (Sigma-Aldrich).
Total proteins (20 μg) were resolved using 14% SDS-PAGE prior to
transferral to a PVDF membrane. The membrane was blocked in PBS
containing 0.2% Tween-20 and 5% skimmed milk for 2 h at 37°C, and
incubated overnight at 4°C with primary monoclonal antibodies
(mouse monoclonal anti-Cx43 antibody, rabbit anti-VEGF and
anti-bFGF polyclonal antibodies, and mouse anti-GAPDH polyclonal
antibody; Abcam, Cambridge, UK). The housekeeping protein GAPDH
served as a loading control. Antibody binding was detected with
polyclonal horseradish peroxidase-conjugated goat anti-mouse/rabbit
IgG secondary antibodies (Chemicon, Temecula, CA, USA) and
visualized using an enhanced chemiluminescence kit
(Sigma-Aldrich).
Global cardiac function by direct
hemodynamic evaluation
Two weeks following MI, invasive hemodynamic
investigations were performed following anesthesia (50 mg/kg) and
mechanical ventilation. A left ventricle catheter was inserted from
the right carotid artery to the LV cavity to access
intraventricular pressure. Hemodynamic data were monitored by a
biological signal recording system (RM6240, Chengyi, China) to
measure systolic (LVSP) and end-diastolic left ventricle pressures
(LVEDP), ±dp/dtmax and heart rate (HR).
Infarct size measurement
The hearts were removed from the rats, washed,
weighed and embedded in paraffin. Sections were cut into 5-μm
slices, processed and stained with Trichrome-Masson. The extent of
fibrosis in the infarcted region of each heart was measured as
previously described (6). The
percentage of blue staining, indicative of fibrosis, was measured
from the infarct area on two sections from each heart and averaged.
The value is expressed as the ratio of Trichrome-stained fibrosis
area to total infarct area.
Quantitative assessment of blood vessel
density
Blood vessel density in the heart two weeks
following cell therapy, was assessed as previously described
(20). Briefly, cryosections (8-μm
thickness) were quantified by dual fluorescent immunostaining for
von Willebrand Factor-VIII (vWF-VIII) and smooth muscle actin (SMA)
expression. vWF-VIII and SMA were detected with fluorescein
isothiocyanate (FITC)- or rhodamine-labeled (TRITC) secondary
antibodies (Roche Applied Science, Rotkreuz, Switzerland). Blood
vessel density is expressed as the number of vessels per
microscopic field. Blood vessel maturation was assessed by
calculating the number of SMA-positive blood vessels in relation to
the vWF-VIII-positive vessels.
Statistical analysis
The data are expressed as the mean ± standard error
of the mean. Two-way analysis of variance and Student’s t-test were
performed to analyze statistical differences in each response
variable. P<0.05 was considered to indicate a statistically
significant difference.
Results
Cx43 promotes the release of VEGF and
bFGF from MSCs in vitro
VEGF and bFGF are the most well-studied paracrine
cytokines. To illustrate the effects of Cx43 on the production of
angiogenic cytokines, the Cx43 gene was regulated by genetic
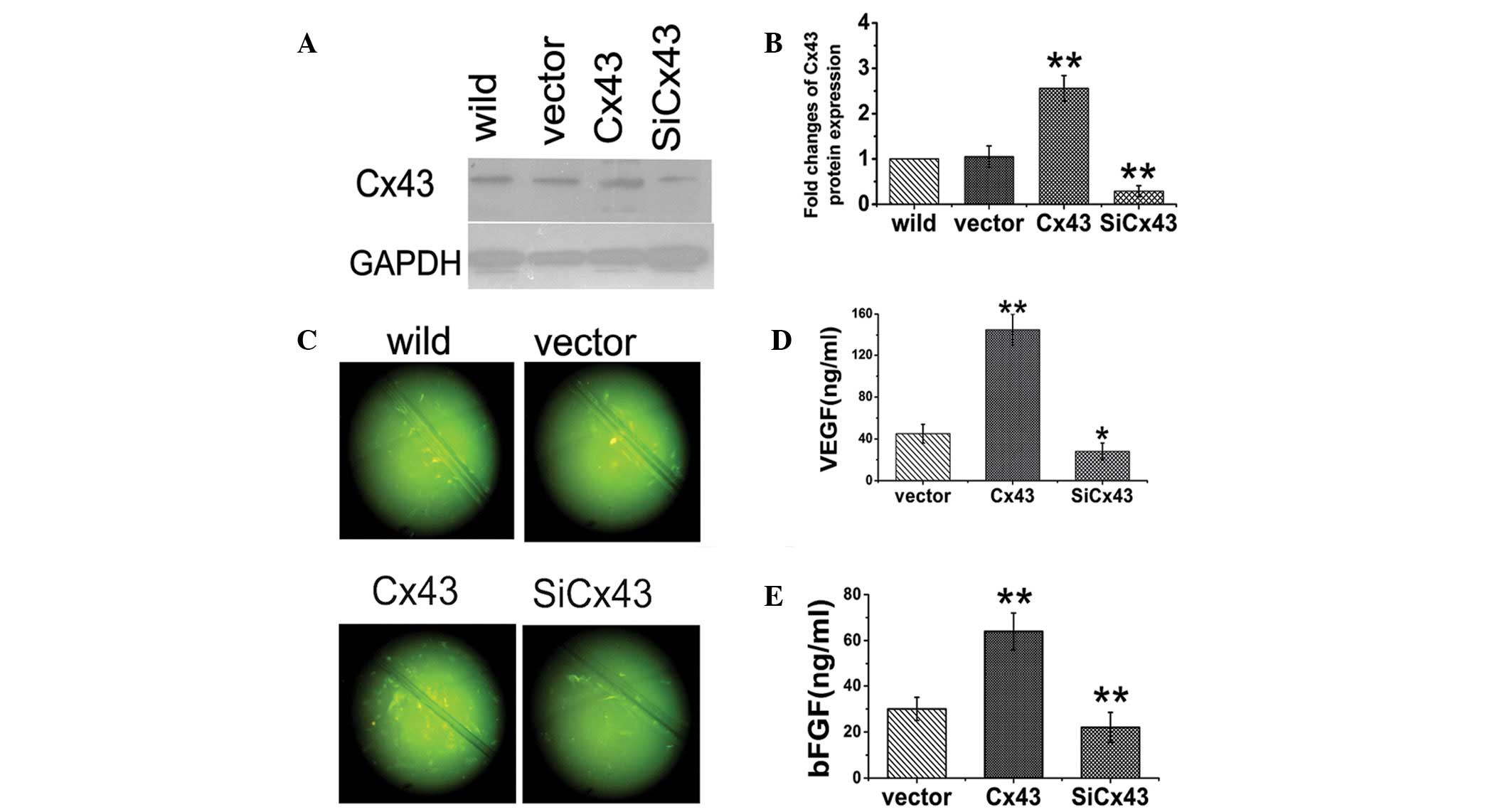
modification. Two days following transfection, Cx43 expression was
increased two-fold in MSCs-Cx43 and 30% in
MSCs-siCx43 compared with MSCs-vector and
wild-type MSCs (Fig. 1A and B).
SL/DT showed that LY transferred to an increased number of
neighboring cells in MSCs-Cx43, but a decreased number
of neighboring cells in MSCs-siCx43 (Fig. 1C) compared with
MSCs-vector. After 8 h of hypoxia, the levels of VEGF
and bFGF were increased in MSCs-Cx43 and decreased in
MSCs-siCx43 compared with MSCs-vector
(Fig. 1D and E).
Gap junctions do not affect VEGF and bFGF
secreted from MSCs in vitro
Synthesized peptides specifically recognize and
reversibly bind to outer domains of proteins without entering cells
(21). To confirm the role of gap
junctions formed by Cx43 in angiogenic cytokine production,
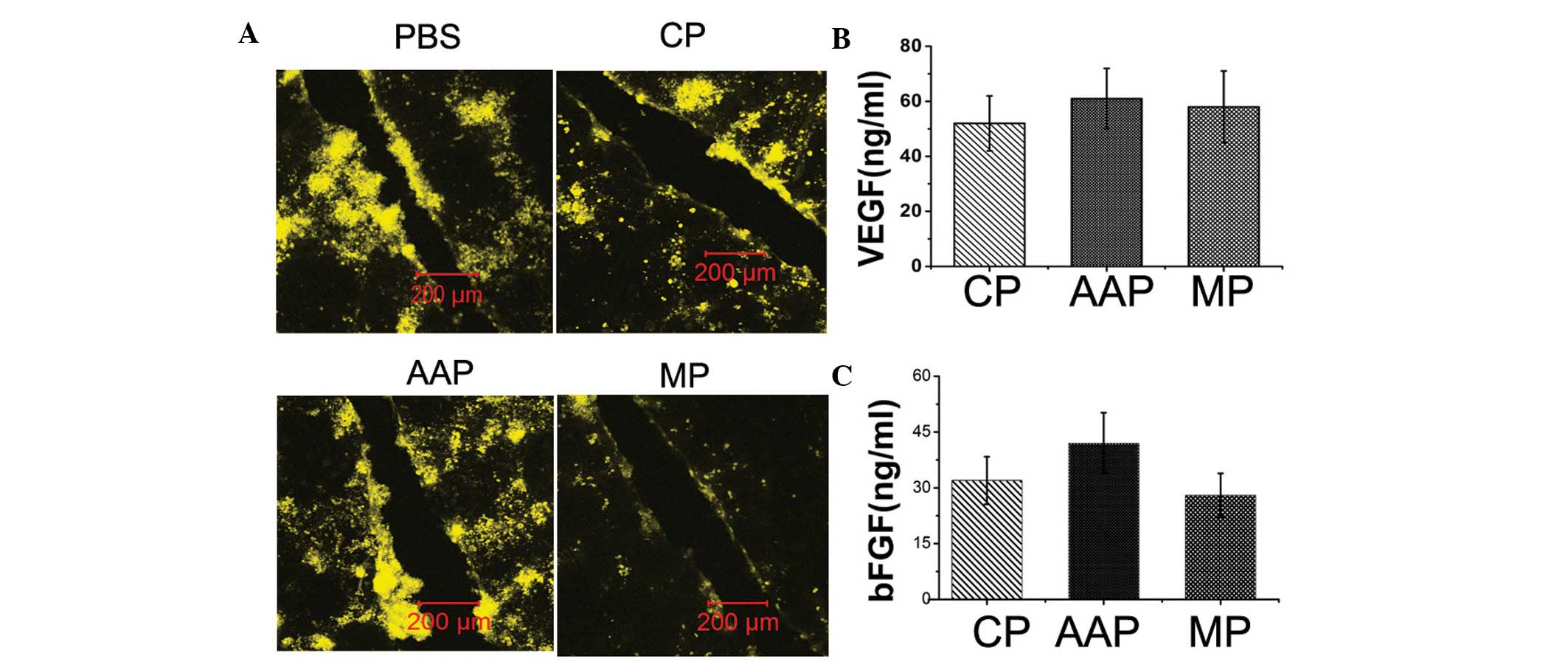
specific and reversible gap-junction closers (22) or openers (23) were used. The efficacy of the
synthesized peptides in modulating gap junction was confirmed in
cultured cardiomyocytes using scrape loading/LY transfer
experiments (Fig. 2A). Following 8
h hypoxia-conditioning medium from MSCs exhibited increased VEGF
and bFGF secretion. Connexin mimetic peptides, as well as the
antiarrhythmic peptide, exhibited no significant effects on the
production of VEGF and bFGF (Fig. 2B
and 2C).
Cx43 promotes the production of VEGF and
bFGF following transplantation
To identify the mechanism of Cx43 in the angiogenic
effect of MSCs following myocardial injury, protein of multiple
paracrine factors responsible for angiogenesis from cardiac samples
of the peri-infarct LV area was evaluated two weeks following
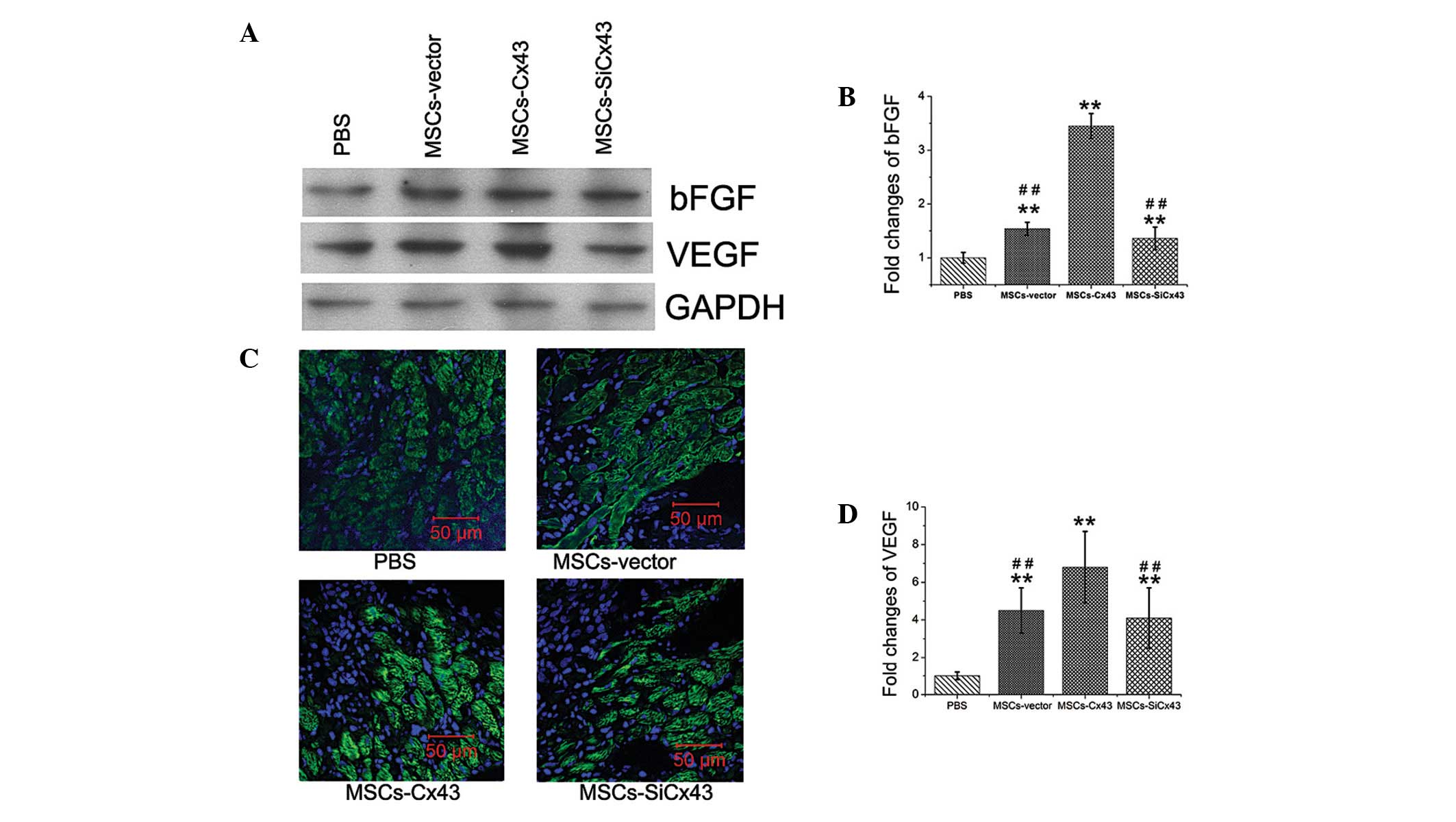
transplantation (n=4). Two weeks following MI, immunofluororence
showed VEGF expression in the infarct border zone of the heart
(Fig. 3B). Western blotting showed
that MSC-transplanted hearts had elevated levels of VEGF (4.5±1.2
fold) and bFGF (1.54±0.12 fold) compared with the PBS group
(Fig. 3A, C and D). There were
significantly elevated VEGF and bFGF tissue concentrations
following MSCs-Cx43 transplantation, respectively (VEGF,
6.8±1.9 vs. 4.5±1.2; bFGF, 3.45±0.23 vs. 1.54±0.12; P<0.01).
However, no significant difference of the two paracrine factors was
detected between the MSCs-vector (14.8±1.3) and
MSCs-siCx43 groups.
Cx43 promotes angiogenesis in MI in
vivo
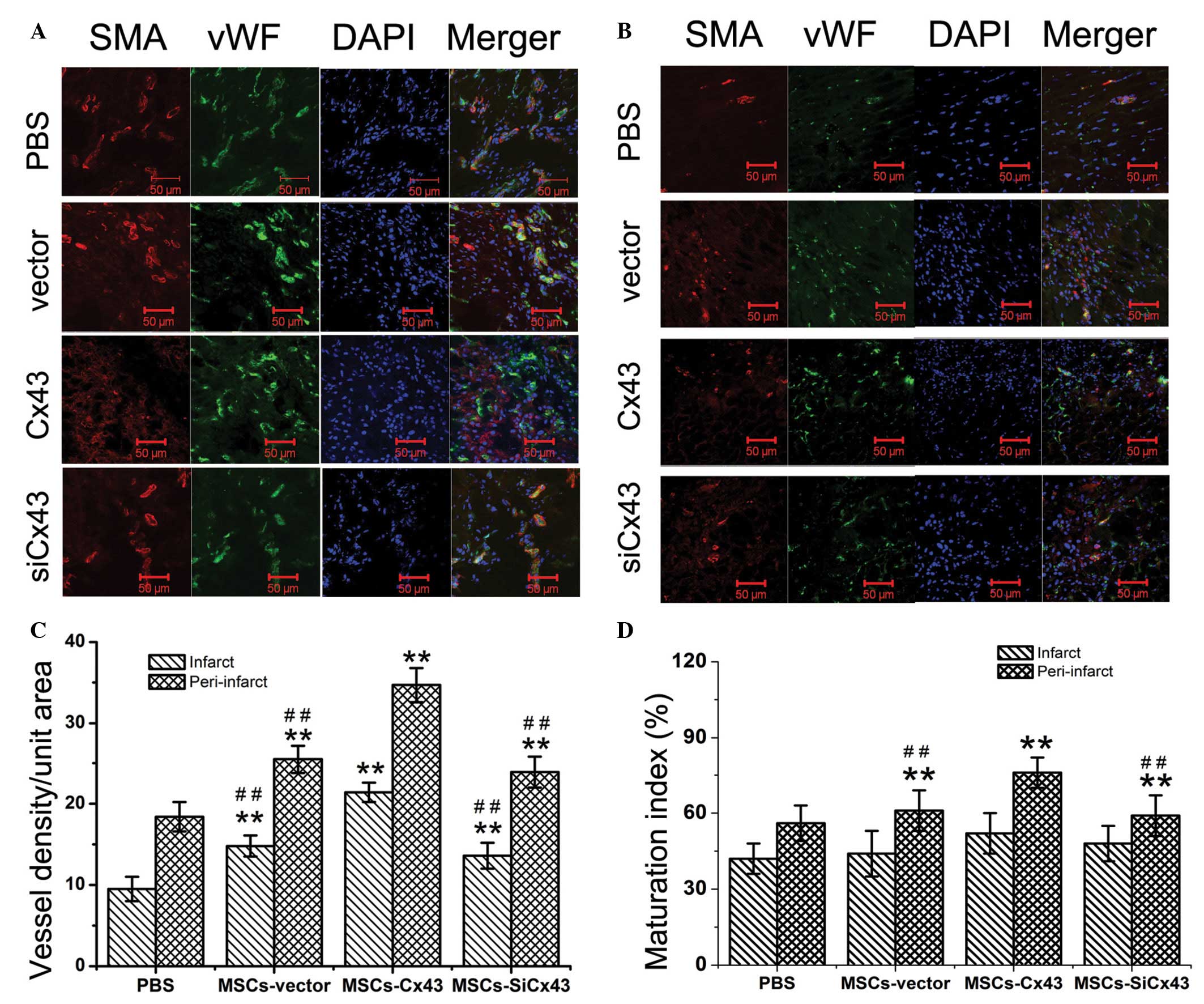
To determine the impact of Cx43 on angiogenesis of
MSCs in the infarcted myocardium, vessel density was quantified
following vWFactor-VIII (vWF) and smooth muscle actin (SMA)
staining, respectively. Two weeks following MI, vessel density was
higher in the cell-transplanted groups of animals compared with the
PBS-injected rats (Fig. 4A and B).
Blood vessel density per surface area in the infarct was
significantly greater in the MSCs-Cx43 group (21.4±1.2;
P<0.01) compared with the MSCs-vector (14.8±1.3) and
MSCs-siCx43 groups (13.6±1.6). There was a greater
vessel density in the MSCs-Cx43 group (34.7±2.1;
P<0.01) compared with the MSCs-vector (25.5±1.7) and
MSCs-siCx43 groups (23.9±1) in the peri-infarcted area.
However, no significant differences were identified of blood vessel
density between MSCs-vector (14.8±1.3) and
MSCs-siCx43 groups in the infarcted (P=0.154) and in the
peri-infarcted areas (P=0.107) (Fig.
4C).
Double-fluorescent immunostaining for vWF and SMA
expression showed the newly formed vascular structures matured to
develop smooth muscle covering the infarcted and the peri-infarcted
areas (Figs. 4 and 5). The maturation index (based on
positivity of vessels for vWF and SMA/total number of blood
vessels) was higher in the MSCs-Cx43 group (81.1±4.1%)in
the peri-infarct region compared with the MSCs-vector
group (75.8±4.5%) and MSCs-siCx43 group (62.4±3.5%).
However, no significant differences were identified in maturation
index between all groups in the infarcted areas (43.3±4.6, 45±3.8,
52±5.5 and 47±7.1%, respectively; P=0.23) (Fig. 4D).
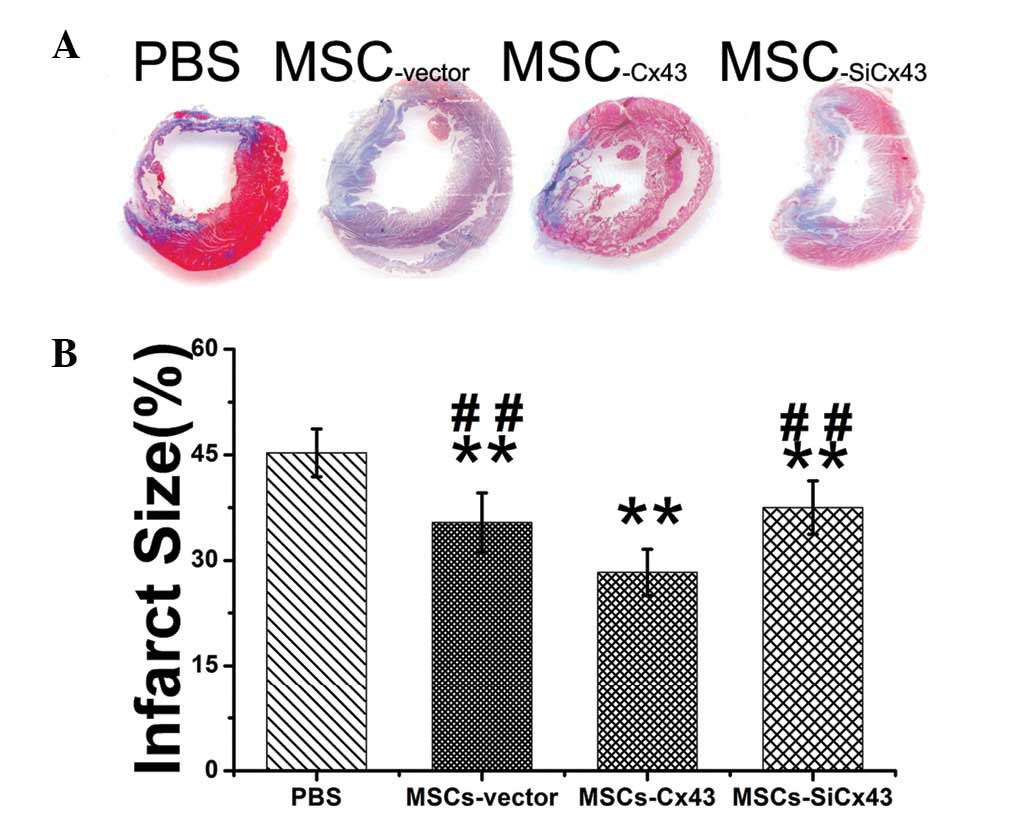
Cx43-enhanced MSCs reduce infarct size
and preserve cardiac hemodynamics
Myocardial infarction by LAD coronary artery
ligation exhibits typical histological changes, including extensive
collagen deposition (which was stained blue) and a minor viable
island of myocardium stained red (Fig.
5A). Quantitative analysis showed ~50% infarcted areas in the
PBS group (45.3±3.4%, n=5; Fig.
5B). Animals in the MSCs-Cx43 group (28.3±3.3%, n=5)
had smaller infarct size than those treated with
MSCs-siCx43 (37.5±3.8%, n=5) as well as
MSCs-vector (35.4±4.2%, n=5).
MSC-injected rats had significant hemodynamic
preservation compared with PBS groups showing increased left
ventricular systolic pressure (LVSP), +dP/dtmax and
decreased left ventricular end-diastolic pressure (LVEDP) and
-dP/dtmax. However, the improvement of LVSP, LVEDP and
±dP/dtmax was greater in the MSCs-Cx43 group
compared with the MSCs-vector and MSCs-siCx43
groups, although the differences did not reach statistical
significance (Table I).
 | Table IHemodynamics and cardiac function
(mean ± standard error of the mean). |
Table I
Hemodynamics and cardiac function
(mean ± standard error of the mean).
| Group | n | LVSP, kPa | LVEDP, kPa |
+dP/dtmax, +kPa/s |
−dP/dtmax, −kPa/s | HR, bpm |
|---|
| Control | 7 | 12.9±0.78 | 0.89±0.15 | 740±71 | 568±64 | 363±64 |
| PBS | 8 | 6.14±1.46b | 1.36±0.36a | 464±49b | 376±32b | 334±31 |
|
MSCs-vector | 9 | 7.87±1.61bc | 1.06±0.09 | 549±144b | 467±52bd | 336±48 |
|
MSCs-Cx43 | 8 | 9.79±2.42bd | 0.97±0.42c | 599±112a | 471±46bc | 356±51 |
|
MSCs-siCx43 | 8 | 8.04±2.12bc | 1.14±0.36 | 520±89b | 438±56bc | 342±46 |
Discussion
In the current study, three major findings were
observed: i) Transplantation of Cx43-enriched MSCs was more
effective in reducing infarct size, promoting neovascularization
and preserving cardiac hemodynamics in the ischemic heart. ii) MSCs
overexpressing Cx43 secreted more VEGF and bFGF in the hypoxic
condition and iii) formation of gap junctional communication by
Cx43 was not required for the production of VEGF and bFGF.
Cx43 is an essential protein in the formation of
hemichannels and gap junctions responsible for electrical and
mechanical coupling between cells in the myocardium (24). Previous studies demonstrated that
Cx43 may be used to reduce proarrhythmogeny of myoblast
transplantation (25,26). MSCs from the bone marrow,
expressing a specific amount of Cx43 (27), resulted in 4-fold reduction of
arrhythmias following transplantation in ischemic heart disease in
a clinical trial (28). MSCs
expressing more Cx43 by growth factor (GF) stimulation exhibited
improved preservation of ischemic hearts (6,13).
In addition, Cx43 knockdown was observed to reduce cell survival
under oxygen and glucose deprivation and following transplantation
into an infarcted heart (13).
These studies suggest that Cx43 may contribute to the therapeutic
efficacy of MSCs. Our previous study directly showed that Cx43
overexpression by genetic modification promoted survival of MSCs
and cardiac function preservation in the ischemic heart (14). In the current study,
transplantation of MSCs overexpressing Cx43 was observed to result
in smaller infarct size and greater functional improvement.
Notably, Cx43 was confirmed to promote neovascularization in
infarcted hearts.
The therapeutic mechanism of MSCs on the ischemic
heart has been extensively studied and repeatedly renewed from
myocardial regeneration through transdifferentiation to cell fusion
(29,30). Currently, the paracrine actions of
MSCs have been known as the major mechanism for the functional
recovery of the infarcted heart (31). Therefore, Cx43 is hypothesized to
affect the production of angiogenic cytokines and this may explain
the improved neovascularization observed in vivo in the
present study. In the current study, VEGF and bFGF concentrations
were assayed in culture medium, two representative angiogenic
cytokines as confirmed by previous studies (32,33).
The results showed that Cx43 genetic modification modulates VEGF
and bFGF secretion from MSCs. Furthermore, VEGF was observed to be
expressed in myocardial tissue of peri-infarcted areas of hearts.
VEGF and bFGF protein expression increased in MSCs-Cx43
and decreased in MSCs-siCx43, compared with control
MSCs. Cx43 in MSCs is hypothesized to improve neovascularization in
infarcted hearts through secreting more angiogenic cytokines.
Cx43 is primarily distributed in membranes and forms
gap junctions between cells for signal communication. However, a
previous study observed that the majority of Cx43 is expressed in
the cytoplasm of MSCs (13,14).
Lu et al (34) reported
that Cx43 is located in the mitochondria and involved in protecting
MSCs from apoptosis. To confirm whether Cx43 formed gap junctions
in the membrane involved in the secretion of angiogenic cytokines
from MSCs, specific synthesized peptides were used to open or close
the gap junctions. Since Cx43 mimetic peptides are not capable of
directly entering cells, they selectively bind to extracellular
loops of Cx43 and specifically inhibit gap junction functionality
(21). Known as a gap junction
opener, AAP has been used as an antiarrhythmic treatment. (23). Therefore, the current study
differed from a study by Hahn et al, which observed that
heptanol, a non-selective blocker of gap junctions, partially
inhibited the antiapoptotic effects of GF treated MSCs on myocytes
using co-culture with MSCs and cardiac myocytes (6). Our data showed that both AAP and MP
did not affect the secretions of VEGF and bFGF from MSCs. The
results suggested that overexpression of Cx43 in MSCs improves the
production of angiogenic factors production, which is not dependent
on membrane gap junctional communication.
It should be noted that this study has several
limitations. Firstly, whether other connexins, e.g. Cx40 and Cx45,
affect angiogenic cytokine production was not investigated. A
previous study confirmed that MSCs express Cx43, Cx40 and Cx45
(27). Furthermore, antiarrhythmic
peptides also open Cx45 formed gap junctions. Therefore, further
study is required to determine the role of Cx45. Secondly, in the
in vivo experiment, VEGF and bFGF arising from MSCs or
myocytes themselves were not identified. Thirdly, we were unable to
confirm whether the promotion of angiogenesis could be ascribed to
increased cellular survival in hearts (14). Finally, neovascularization in
infarcted hearts should be observed for a longer time in future
studies.
In conclusion, the present study directly
demonstrates that MSCs overexpressing Cx43 promote
neovascularization, improve heart function and reduce infarct size.
Cx43 is involved in the production of VEGF and bFGF from MSCs in a
gap junction-independent manner. Therefore, Cx43 may act as a
potential target for improving the therapeutic efficacy of MSC
transplantation in ischemic heart disease, regardless of whether it
forms gap junctions.
Acknowledgements
This study was supported by grants from the National
Natural Science Foundation of China (grant nos. 30800464, 30871077
and 81200142), the National Natural Science of Anhui province
(grant nos. 1208085QH156 and 1208085MH129) and the Research
Foundation for Advanced Talents of Yijishan Hospital (grant no.
YR201001).
References
|
1
|
Nagaya N, Kangawa K, Itoh T, et al:
Transplantation of mesenchymal stem cells improves cardiac function
in a rat model of dilated cardiomyopathy. Circulation.
112:1128–1135. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Tse HF, Thambar S, Kwong YL, et al:
Prospective randomized trial of direct endomyocardial implantation
of bone marrow cells for treatment of severe coronary artery
diseases (PROTECT-CAD trial). Eur Heart J. 28:2998–3005. 2007.
View Article : Google Scholar
|
|
3
|
Müller-Ehmsen J, Krausgrill B, Burst V, et
al: Effective engraftment but poor mid-term persistence of
mononuclear and mesenchymal bone marrow cells in acute and chronic
rat myocardial infarction. J Mol Cell Cardiol. 41:876–884.
2006.PubMed/NCBI
|
|
4
|
Leobon B, Garcin I, Menasche P, Vilquin
JT, Audinat E and Charpak S: Myoblasts transplanted into rat
infarcted myocardium are functionally isolated from their host.
Proc Natl Acad Sci USA. 100:7808–7811. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Grauss RW, van Tuyn J, Steendijk P, et al:
Forced myocardin expression enhances the therapeutic effect of
human mesenchymal stem cells after transplantation in ischemic
mouse hearts. Stem Cells. 26:1083–1093. 2008. View Article : Google Scholar
|
|
6
|
Hahn JY, Cho HJ, Kang HJ, et al:
Pre-treatment of mesenchymal stem cells with a combination of
growth factors enhances gap junction formation, cytoprotective
effect on cardiomyocytes, and therapeutic efficacy for myocardial
infarction. J Am Coll Cardiol. 51:933–943. 2008. View Article : Google Scholar
|
|
7
|
Rosová I, Dao M, Capoccia B, Link D and
Nolta JA: Hypoxic preconditioning results in increased motility and
improved therapeutic potential of human mesenchymal stem cells.
Stem Cells. 26:2173–2182. 2008.PubMed/NCBI
|
|
8
|
Contreras JE, Sánchez HA, Véliz LP,
Bukauskas FF, Bennett MV and Saéz JC: Role of connexin-based gap
junction channels and hemichannels in ischemia-induced cell death
in nervous tissue. Brain Res Brain Res Rev. 47:290–303. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Yasui K, Kada K, Hojo M, et al:
Cell-to-cell interaction prevents cell death in cultured neonatal
rat ventricular myocytes. Cardiovasc Res. 48:68–76. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Giardina SF, Mikami M, Goubaeva F and Yang
J: Connexin 43 confers resistance to hydrogen peroxide-mediated
apoptosis. Biochem Biophys Res Commun. 362:747–752. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Goubaeva F, Mikami M, Giardina S, Ding B,
Abe J and Yang J: Cardiac mitochondrial connexin 43 regulates
apoptosis. Biochem Biophys Res Commun. 352:97–103. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Pijnappels DA, Schalij MJ, van Tuyn J, et
al: Progressive increase in conduction velocity across human
mesenchymal stem cells is mediated by enhanced electrical coupling.
Cardiovasc Res. 72:282–291. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lu G, Haider HK, Jiang S and Ashraf M:
Sca-1+ stem cell survival and engraftment in the infarcted heart:
dual role for preconditioning-induced connexin-43. Circulation.
119:2587–2596. 2009.
|
|
14
|
Wang D, Shen W, Zhang F, Chen M, Chen H
and Cao K: Connexin43 promotes survival of mesenchymal stem cells
in ischemic heart. Cell Biol Int. 34:415–423. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Crisostomo PR, Wang Y, Markel TA, Wang M,
Lahm T and Meldrum DR: Human mesenchymal stem cells stimulated by
TNF-alpha, LPS, or hypoxia produce growth factors by an NF kappa B-
but not JNK-dependent mechanism. Am J Physiol Cell Physiol.
294:C675–C682. 2008. View Article : Google Scholar
|
|
16
|
Yoon YS, Wecker A, Heyd L, et al: Clonally
expanded novel multipotent stem cells from human bone marrow
regenerate myocardium after myocardial infarction. J Clin Invest.
115:326–338. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Jia G, Cheng G, Gangahar DM and Agrawal
DK: Involvement of connexin 43 in angiotensin II-induced migration
and proliferation of saphenous vein smooth muscle cells via the
MAPK-AP-1 signaling pathway. J Mol Cell Cardiol. 44:882–890. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Gnecchi M, He H, Noiseux N, et al:
Evidence supporting paracrine hypothesis for Akt-modified
mesenchymal stem cell-mediated cardiac protection and functional
improvement. FASEB J. 20:661–669. 2006. View Article : Google Scholar
|
|
19
|
Dai W, Hale SL, Martin BJ, et al:
Allogeneic mesenchymal stem cell transplantation in postinfarcted
rat myocardium: short- and long-term effects. Circulation.
112:214–223. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Jiang S, Haider H, Idris NM, Salim A and
Ashraf M: Supportive interaction between cell survival signaling
and angiocompetent factors enhances donor cell survival and
promotes angiomyogenesis for cardiac repair. Circ Res. 99:776–784.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Evans WH and Leybaert L: Mimetic peptides
as blockers of connexin channel-facilitated intercellular
communication. Cell Commun Adhes. 14:265–273. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Martin PE, Wall C and Griffith TM: Effects
of connexin-mimetic peptides on gap junction functionality and
connexin expression in cultured vascular cells. Br J Pharmacol.
144:617–627. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Hagen A, Dietze A and Dhein S: Human
cardiac gap-junction coupling: effects of antiarrhythmic peptide
AAP10. Cardiovasc Res. 83:405–415. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Jansen JA, van Veen TA, de Bakker JM and
van Rijen HV: Cardiac connexins and impulse propagation. J Mol Cell
Cardiol. 48:76–82. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Roell W, Lewalter T, Sasse P, et al:
Engraftment of connexin 43-expressing cells prevents post-infarct
arrhythmia. Nature. 450:819–824. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Abraham MR, Henrikson CA, Tung L, et al:
Antiarrhythmic engineering of skeletal myoblasts for cardiac
transplantation. Circ Res. 97:159–167. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Valiunas V, Doronin S, Valiuniene L, et
al: Human mesenchymal stem cells make cardiac connexins and form
functional gap junctions. J Physiol. 555:617–626. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Hare JM, Traverse JH, Henry TD, et al: A
randomized, double-blind, placebo-controlled, dose-escalation study
of intravenous adult human mesenchymal stem cells (prochymal) after
acute myocardial infarction. J Am Coll Cardiol. 54:2277–2286. 2009.
View Article : Google Scholar
|
|
29
|
Balsam LB, Wagers AJ, Christensen JL,
Kofidis T, Weissman IL and Robbins RC: Haematopoietic stem cells
adopt mature haematopoietic fates in ischaemic myocardium. Nature.
428:668–673. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Nygren JM, Jovinge S, Breitbach M, et al:
Bone marrow-derived hematopoietic cells generate cardiomyocytes at
a low frequency through cell fusion, but not transdifferentiation.
Nat Med. 10:494–501. 2004. View
Article : Google Scholar : PubMed/NCBI
|
|
31
|
Gnecchi M, He H, Liang OD, et al:
Paracrine action accounts for marked protection of ischemic heart
by Akt-modified mesenchymal stem cells. Nat Med. 11:367–368. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Grunewald M, Avraham I, Dor Y, et al:
VEGF-induced adult neovascularization: recruitment, retention, and
role of accessory cells. Cell. 124:175–189. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Langer HF, Stellos K, Steingen C, et al:
Platelet derived bFGF mediates vascular integrative mechanisms of
mesenchymal stem cells in vitro. J Mol Cell Cardiol. 47:315–325.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Lu G, Haider HKh, Porollo A and Ashraf M:
Mitochondria-specific transgenic overexpression of connexin-43
simulates preconditioning-induced cytoprotection of stem cells.
Cardiovasc Res. 88:277–286. 2010. View Article : Google Scholar : PubMed/NCBI
|