Introduction
Acinetobacter baumannii has emerged as a
major causative agent of nosocomial infection in hospitals
worldwide (1,2). According to the data from a national
surveillance study performed in the United States, A.
baumannii was shown to be the etiological agent in 6.9% of
nosocomial pneumonias in 2004, which was an ~72% increase compared
with data collected in 1986 (3).
Pulmonary compartments are the most favored site of A.
baumannii infection, and ventilatory-associated and
community-acquired pneumonia are extremely common. The mortality of
ventilatory-associated pneumonias due to A. baumannii may be
as high as 75% (4). Furthermore,
emergence of multi-drug resistant A. baumannii has become a
significant health concern leading to limited therapeutic options
in recent decades (1,2). The increased incidence of antibiotic
resistance in these bacteria underlines the importance of
understanding the host immune response against A. baumannii
to prevent and control infection.
Innate immune systems provide the first line of
defense against pathogenic bacteria, by recognition of
pathogen-associated molecular patterns. Toll-like receptors (TLRs)
are the best-studied family of pattern recognition receptors (PRRs)
and are comprised of N-terminal leucine-rich repeats, a
transmembrane domain and a C-terminal Toll/interleukin (IL)-1
receptor domain. TLRs 1, 2, 4, 5 and 6 are present on the cell
surface, whereas TLRs 3, 7 and 9 are localized in the endosome
(5). Following the recognition of
specific molecular patterns found in microbial pathogens, TLRs
trigger downstream signaling pathways [myeloid differentiation
primary-response protein 88 (MyD88)-dependent and/or TIR
domain-containing adapter-inducing interferon-β-dependent
pathways], and produce proinflammatory cytokines and antimicrobial
factors to eradicate the invading pathogens. Engagement of TLRs
initiates signaling through intracellular pathways that lead to
activation of nuclear factor-κB (NF-κB), mitogen-activated protein
kinases (MAPKs) and interferon regulatory factors (6,7).
TLR4 is critical for host defense against
Gram-negative bacterial infection as it recognizes
lipopolysaccharide (LPS) (7). TLR4
mediates bacterial clearance and production of proinflammatory
cytokines and chemokines in A. baumannii infection (8). In addition to TLR4, TLR2 is involved
in host innate immunity against microbial infections by recognizing
peptidoglycans of the bacterial cell wall and activating NF-κB and
MAPKs through the MyD88-dependent pathway (9–11).
Although A. baumannii is known to induce TLR2-stimulatory
activity (12,13), the role of TLR2 in A.
baumannii-induced immune responses remains unclear. Previously,
an in vivo study demonstrated that TLR2 deficiency leads to
improved bacterial clearance and increased production of chemokines
and myeloperoxidase in the lungs of mice infected with A.
baumannii (8). By contrast,
TLR2 is essential for optimal immune responses against A.
baumannii in macrophages and lung epithelial cells (12,14).
Therefore, the present study sought to confirm the role of TLR2 in
bacterial colonization, cytokine and chemokine production and lung
histopathology, by A. baumannii infection in mice.
Materials and methods
Mice
TLR2-deficient mice on C57BL/6 background were
purchased from Jackson Laboratory (Bar Harbor, ME, USA) and
wild-type (WT) C57BL/6 mice were from Koatech (Pyeongtaek, Korea).
The animal studies were conducted under approved protocols by the
Institutional Animal Care and Use Committee of Konyang University
(Daejeon, Korea).
Bacterial infection
A. baumannii strain KCCM 35453 (ATCC 15150)
was purchased from Korean Culture Center of Microorganisms (Seoul,
Korea). For bacterial preparation, single colonies were inoculated
into 5 ml Luria Bertani (LB) broth and grown overnight at 37°C
under agitation. A 1:5 dilution of the culture grown overnight was
allowed to grow in fresh medium at 37°C under agitation for a
further 2 h. Bacteria were washed and resuspended with sterile
phosphate-buffered saline (PBS) to 109 colony-forming
units (CFU)/ml. Mice were anesthetized by intraperitoneal injection
of 10 mg/kg rompun (Bayer Korea Ltd., Seoul, Korea) and 50 mg/kg
zoletil (Virbac Korea Co., Ltd., Seoul, Korea), and 30 μl prepared
bacteria was inoculated intranasally. Infected mice were monitored
daily for body weight changes and clinical signs. At 1, 3 and 5
days after inoculation, mice were sacrificed and bronchoalveolar
lavage (BAL) fluid was collected.
Bacterial count in lung tissue
Following BAL fluid collection, the right lobes of
lungs were collected in sterile PBS for bacterial counting. The
extracted lobes were weighed, homogenized, serially diluted and 100
μl homogenate was spread onto LB agar plates. Following overnight
culture in a 37°C incubator, the colony was counted and the number
of bacteria (measured as CFU/g lung tissue) was calculated.
Measurement of cytokines and
chemokines
The levels of IL-6, tumor necrosis factor-α (TNF-α),
chemokine (C-X-C motif) ligand 2 (CXCL2) and chemokine (C-C motif)
ligand 2 (CCL2) from BAL fluid of A. baumannii-infected mice
were determined using commercial ELISA kits (R&D Systems,
Minneapolis, MN, USA).
Histopathological examination
The left lobes of lungs were harvested and fixed in
10% neutral formalin for histopathological observation. The tissues
were routinely processed with alcohol and xylene series and
embedded in paraffin. Subsequently, 3-μm sections were prepared,
stained with hematoxylin and eosin and examined by microscopy.
Statistical analysis
The differences in mean values among different
groups were tested and the values were expressed as the mean ±
standard deviation. All of the statistical calculations were
carried out using Microsoft Excel (Microsoft, Redmond, WA, USA).
P<0.05 was considered to indicate a statistically significant
difference.
Results
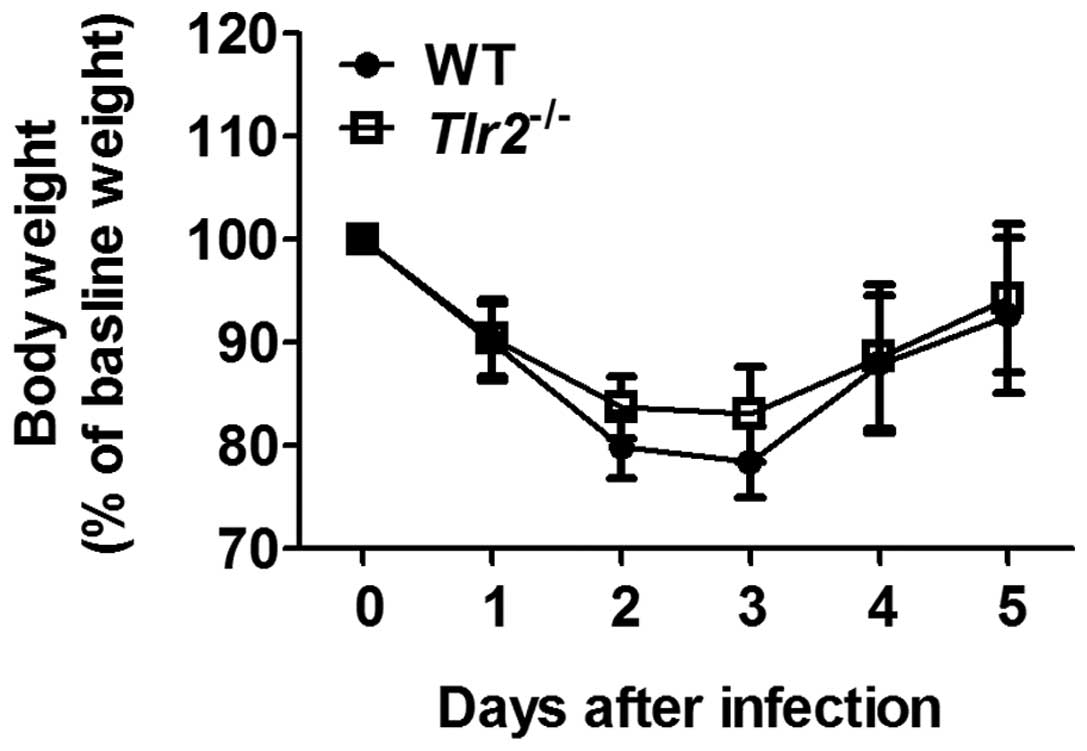
Clinical signs and body weight changes by
A. baumannii infection in mice
A. baumannii infection caused mild physical
signs of ill health in WT and TLR2-deficient mice, manifested as
weight loss, ruffled fur, decreased movement and huddling. These
were without any significant difference 1 day after infection (data
not shown). These clinical signs improved from 3 days after
infection. The weight loss was prominent in the early stages of
infection and continued until 3 days after infection. Following
this, the majority of mice gradually regained body weight and had
recovered to within a normal range by 5 days after infection
(Fig. 1).
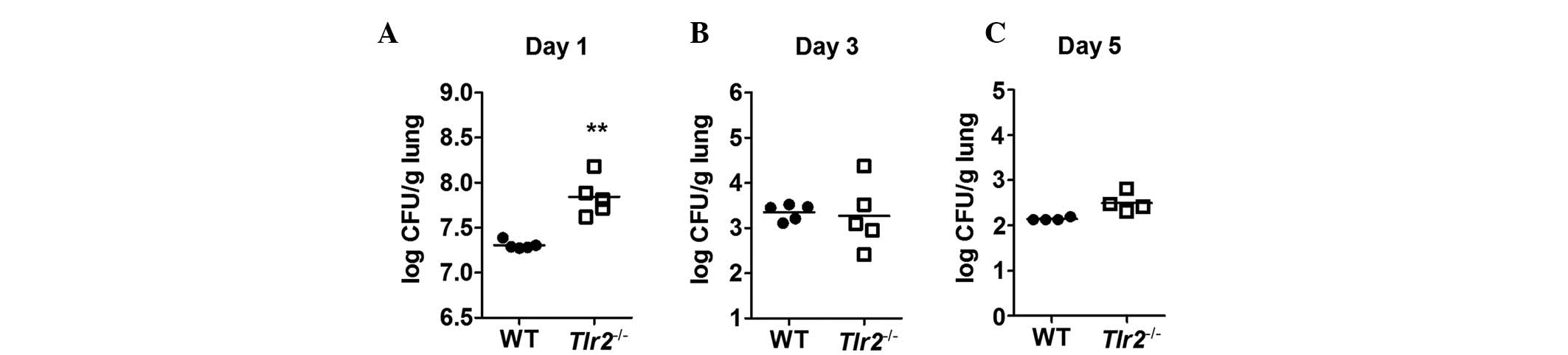
Bacterial clearance in the lungs of mice
infected with A. baumannii
To determine the role of TLR2 in bacterial clearance
in vivo, the lung tissue was extracted from A.
baumannii-infected WT and TLR2-deficient mice and the number of
bacteria was counted. The pulmonary bacterial loads were
significantly higher in TLR2-deficient mice compared with WT mice 1
day after infection (Fig. 2A).
However, at 3 and 5 days after infection, the loads were comparable
in WT and TLR2-deficient mice (Fig. 2B
and C). These results indicate that TLR2 may contribute to
bacterial clearance in A. baumannii-infected mice at the
early stages of infection but not at the later stages.
Production of cytokines and chemokines by
A. baumannii in BAL fluids
To examine whether TLR2 has a role in the production
of cytokines and chemokines following A. baumannii
infection, levels of cytokines (IL-6 and TNF-α) and chemokines
(CXCL2 and CCL2) from BAL fluids of A. baumannii-infected
mice were measured 1, 3 and 5 days after infection. The levels of
IL-6 and CXCL2 were significantly higher in the BAL fluid of
TLR2-deficient mice than in WT mice 1 day after infection (Fig. 3A and B). Although there was no
statistically significant difference, the TNF-α level was also
slightly higher in the BAL fluid of TLR2-deficient mice compared
with WT mice (Fig. 3C).
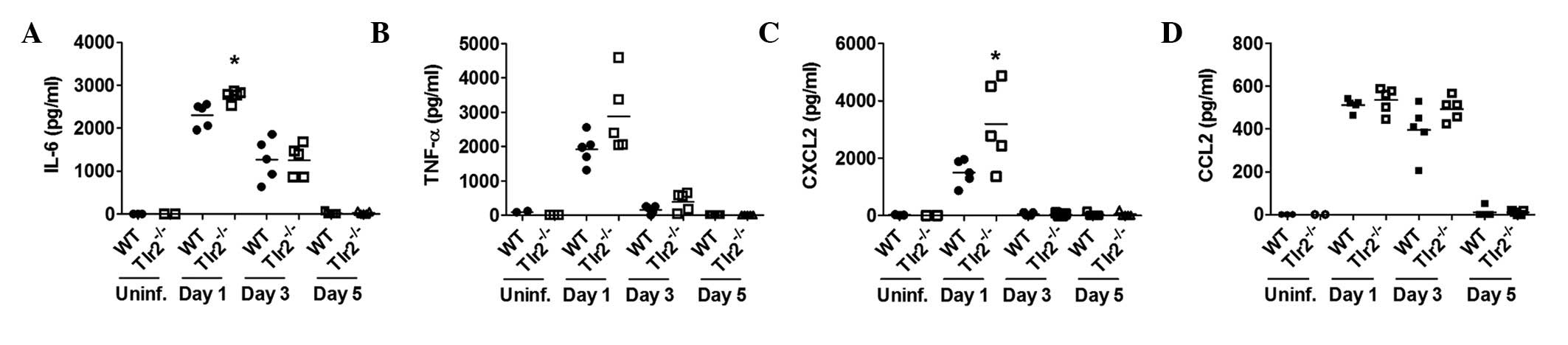 | Figure 3The production of cytokines and
chemokines by A. baumannii in BAL fluids. WT and
TLR2-deficient mice were infected with A. baumannii and BAL
fluids were collected 1, 3 and 5 days after infection. The levels
of (A) IL-6, (B) TNF-α, (C) CXCL2 and (D) CCL2 in BAL fluids were
measured by ELISA. *P<0.01, vs. WT group. BAL,
bronchoalveolar lavage; TLR-2, toll-like receptor 2; IL-6,
interleukin-6; TNF-α, tumor necrosis factor-α; CXCL2, chemokine
(C-X-C motif) ligand 2; CCL2, chemokine (C-C motif) ligand 2; WT,
wild-type. |
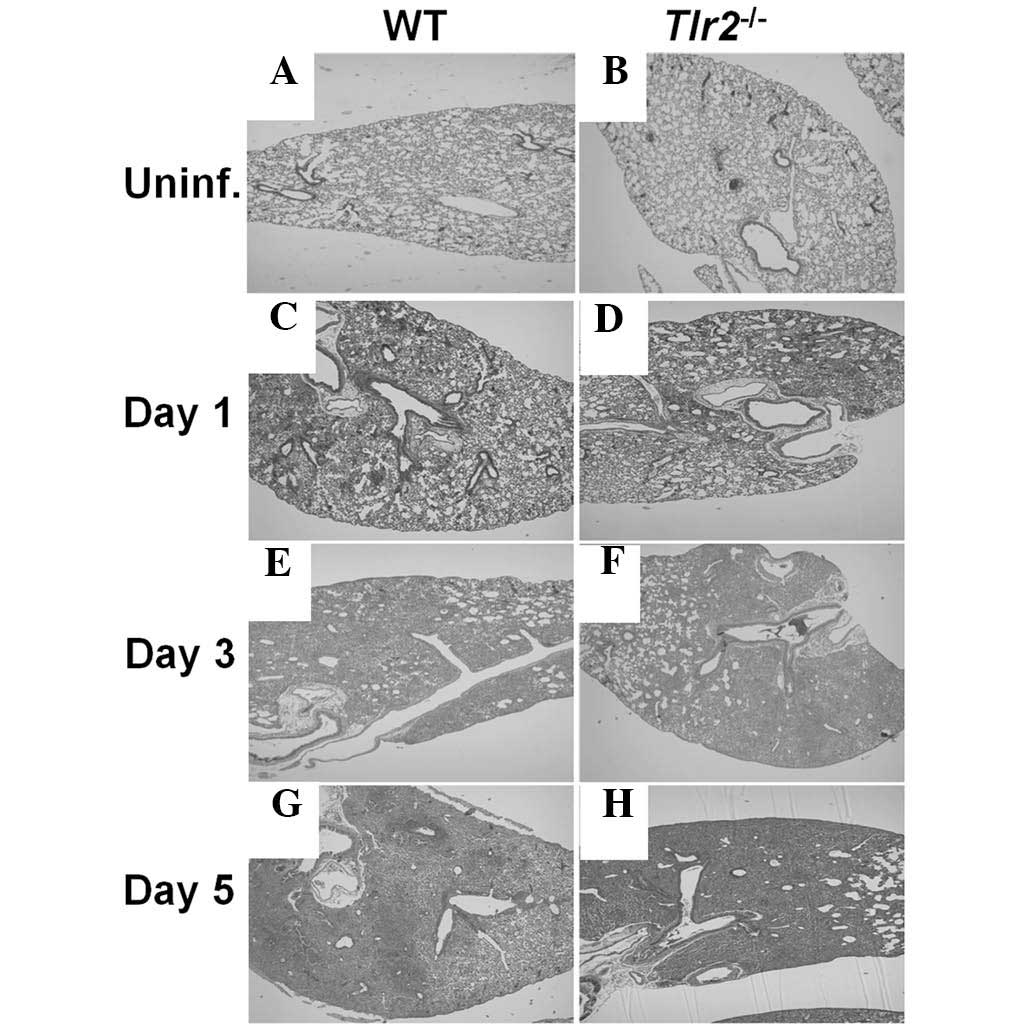
Histopathology of the lung of A.
baumannii-infected mice
Histopathological features of lung tissue from WT
and TLR2-deficient mice was assessed 1, 3 and 5 days after
inoculation with A. baumannii. Whilst no infiltration of
inflammatory cells was observed prior to A. baumannii
infection in the lungs of WT and TLR2-deficient mice (Fig. 4A and B), infection led to
polymorphonuclear (PMN) cell infiltration in the alveolar spaces
and perivascular and peribronchiolar areas in WT and TLR2-deficient
mice from 1 day after infection (Fig.
4C and D). Infiltration of these cells became severe at 3
(Fig. 4E and F) and 5 days
(Fig. 4G and H) after infection,
and no marked difference in inflammatory cell infiltration was
observed between WT and TLR2-deficient mouse lung tissue (Fig. 4). These results indicate that TLR2
may not be involved in the progression of lung inflammation in
mice.
Discussion
Inflammatory responses play a pivotal role in lung
defense against bacterial pathogens, including A. baumannii.
This specific response includes the release of pro-inflammatory
cytokines and chemokines (8,15,16).
IL-6, TNF-α, and IL-1β are representative proinflammatory cytokines
and are associated with the upregulation of cell adhesion molecules
and antigen presentation. IL-8 (keratinocyte-derived protein
chemokine/CXCL1 in mice), monocyte chemotactic protein-1/CCL2, and
macrophage inflammatory protein-2/CXCL2 act as chemoattractants and
are required for recruitment of immune cells from the bloodstream
into the airway (17). In addition
to immune cells, airway epithelial cells contribute to host
resistance against A. baumannii by producing antimicrobial
peptides, for example defensins (18,19).
All of these factors are closely related and cooperate during the
immune response against A. baumannii.
In the present study, mice lacking functional TLR2
exhibited a selective impaired bacterial clearance compared with WT
mice 1 day after infection, although lung infiltration of immune
cells was comparable between the two groups. These results are in
accordance with a previous report demonstrating that TLR2
deficiency impaired the nitric oxide-mediated bacterial killing
capacity of PMN cells and alveolar macrophages, without any defects
in the immune cell influx in pulmonary infection of
Porphyromonas gingivalis (20). In addition, the possible role of
TLR2-dependent local immune responses cannot be excluded. Our
previous study revealed that TLR2 is not required for cytokine
production in macrophages in response to A. baumannii
(21). However, A.
baumannii may induce IL-8 production in human lung epithelial
cells and was inhibited by TLR2 small interfering RNA (12). Although TLR2 deficiency
demonstrated increased CXCL2 production 1 day after infection, no
significant difference was observed between the two groups with
regard to PMN cell infiltration into the lung. It is possible that
TLR2-independent mechanisms may have compensated for this function,
for example stimulation by bacterial-derived formyl peptides of the
formyl-peptide receptor, which plays a major role in PMN
recruitment to infected alveoli (22).
A number of studies have reported that
TLR2-deficient mice exhibit increased resistance to pulmonary
infection with A. baumannii (8) or pilA mutant Pseudomonas
aeruginosa (23), and to
systemic challenge with Yersinia enterocolitica (24) or Candida albicans (25). It is hypothesized that these
results may be due to TLR2 signaling having several inhibitory
effects on the inflammatory response, by inducing IL-10 or
downregulating TLR4 expression. However, the distinct mechanisms
have not been clearly identified. In the present study, no
bacterial clearance defects were observed in the lungs of
TLR2-deficient mice compared with those of WT mice 3 and 5 days
after infection. In addition, TLR2-deficient mice exhibited similar
inflammatory cell infiltration in the lungs as WT mice with A.
baumannii, which was not consistent with the previous report
(8). This discrepancy may be due
to the distinct bacterial origin and different experimental
conditions.
Although TLR4 is regarded as the main PRR of
Gram-negative bacteria, TLR2 has also been reported to participate
in the recognition of some Gram-negative bacteria, including
Helicobacter pylori, Porphyromonas gingivalis and
Bacteroides fragilis (11,26,27).
Therefore, TLR2 serves as a functional receptor for Gram-positive
and -negative bacteria and induces production of proinflammatory
cytokines (28). In conclusion,
results of the present study demonstrated that TLR2 may be involved
in the recognition of A. baumannii, particularly at the
early stages of infection. A previous study revealed that
LPS-deficient A. baumannii exhibited impaired ability to
produce TNF-α in TLR2-deficient macrophages compared with WT cells
(14). Therefore, it is necessary
to re-analyze the in vivo effects of TLR2, using an
LPS-deficient strain of A. baumannii or by comparing
phenotypes between TLR4- and TLR2/4-deficient mice.
Acknowledgements
This study was supported by a program for general
research from the National Research Foundation of Korea (no.
NRF-2012R1A1A2041944).
References
|
1
|
Perez F, Hujer AM, Hujer KM, Decker BK,
Rather PN and Bonomo RA: Global challenge of multidrug-resistant
Acinetobacter baumannii. Antimicrob Agents Chemother.
51:3471–3484. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Van Looveren M and Goossens H; ARPAC
Steering Group. Antimicrobial resistance of Acinetobacter spp. in
Europe. Clin Microbiol Infect. 10:684–704. 2004.
|
|
3
|
Gaynes R and Edwards JR; National
Nosocomial Infectious Surveillance System. Overview of nosocomial
infections caused by gram-negative bacilli. Clin Infect Dis.
41:848–854. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
4
|
Fagon JY, Chastre J, Domart Y, Trouillet
JL and Gibert C: Mortality due to ventilator-associated pneumonia
or colonization with Pseudomonas or Acinetobacter species:
assessment by quantitative culture of samples obtained by a
protected specimen brush. Clin Infect Dis. 23:538–542. 1996.
View Article : Google Scholar
|
|
5
|
Akira S: Innate immunity to pathogens:
diversity in receptors for microbial recognition. Immunol Rev.
227:5–8. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kawai T and Akira S: TLR signaling. Cell
Death Differ. 13:816–825. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Takeda K and Akira S: Toll-like receptors
in innate immunity. Int Immunol. 17:1–14. 2005. View Article : Google Scholar
|
|
8
|
Knapp S, Wieland CW, Florquin S, et al:
Differential roles of CD14 and toll-like receptors 4 and 2 in
murine Acinetobacter pneumonia. Am J Respir Crit Care Med.
173:122–129. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Flo TH, Halaas O, Lien E, et al: Human
toll-like receptor 2 mediates monocyte activation by Listeria
monocytogenes, but not by group B streptococci or
lipopolysaccharide. J Immunol. 164:2064–2069. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Echchannaoui H, Frei K, Schnell C, Leib
SL, Zimmerli W and Landmann R: Toll-like receptor 2-deficient mice
are highly susceptible to Streptococcus pneumoniae meningitis
because of reduced bacterial clearing and enhanced inflammation. J
Infect Dis. 186:798–806. 2002. View
Article : Google Scholar
|
|
11
|
Smith MF Jr, Mitchell A, Li G, et al:
Toll-like receptor (TLR) 2 and TLR5, but not TLR4, are required for
Helicobacter pylori-induced NF-κB activation and chemokine
expression by epithelial cells. J Biol Chem. 278:32552–32560. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
March C, Regueiro V, Llobet E, et al:
Dissection of host cell signal transduction during Acinetobacter
baumannii-triggered inflammatory response. PloS One.
5:e100332010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Erridge C, Moncayo-Nieto OL, Morgan R,
Young M and Poxton IR: Acinetobacter baumannii
lipopolysaccharides are potent stimulators of human monocyte
activation via Toll-like receptor 4 signalling. J Med Microbiol.
56:165–171. 2007. View Article : Google Scholar
|
|
14
|
Moffatt JH, Harper M, Mansell A, et al:
Lipopolysaccharide-deficient Acinetobacter baumannii shows
altered signaling through host Toll-like receptors and increased
susceptibility to the host antimicrobial peptide LL-37. Infect
Immun. 81:684–689. 2013.PubMed/NCBI
|
|
15
|
Qiu H, KuoLee R, Harris G and Chen W: High
susceptibility to respiratory Acinetobacter baumannii
infection in A/J mice is associated with a delay in early pulmonary
recruitment of neutrophils. Microbes Infect. 11:946–955. 2009.
|
|
16
|
Van Faassen H, KuoLee R, Harris G, Zhao X,
Conlan JW and Chen W: Neutrophils play an important role in host
resistance to respiratory infection with Acinetobacter
baumannii in mice. Infect Immun. 75:5597–5608. 2007.PubMed/NCBI
|
|
17
|
Craig A, Mai J, Cai S and Jeyaseelan S:
Neutrophil recruitment to the lungs during bacterial pneumonia.
Infect Immun. 77:568–575. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Routsias JG, Karagounis P, Parvulesku G,
Legakis NJ and Tsakris A: In vitro bactericidal activity of human
beta-defensin 2 against nosocomial strains. Peptides. 31:1654–1660.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Maisetta G, Batoni G, Esin S, et al: In
vitro bactericidal activity of human β-defensin 3 against
multidrug-resistant nosocomial strains. Antimicrob Agents
Chemother. 50:806–809. 2006.
|
|
20
|
Hajishengallis G, Wang M, Bagby GJ and
Nelson S: Importance of TLR2 in early innate immune response to
acute pulmonary infection with Porphyromonas gingivalis in
mice. J Immunol. 181:4141–4149. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kim CH, Jeong YJ, Lee J, et al: Essential
role of toll-like receptor 4 in Acinetobacter
baumannii-induced immune responses in immune cells. Microb
Pathog. 54:20–25. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Fillion I, Ouellet N, Simard M, Bergeron
Y, Sato S and Bergeron MG: Role of chemokines and formyl peptides
in pneumococcal pneumonia-induced monocyte/macrophage recruitment.
J Immunol. 166:7353–7361. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Lorenz E, Chemotti DC, Vandal K and
Tessier PA: Toll-like receptor 2 represses nonpilus adhesin-induced
signaling in acute infections with the Pseudomonas
aeruginosa pilA mutant. Infect Immun. 72:4561–4569. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Sing A, Rost D, Tvardovskaia N, et al:
Yersinia V-antigen exploits Toll-like Receptor 2 and CD14 for
interleukin 10-mediated immunosuppression. J Exp Med.
196:1017–1024. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Netea MG, Sutmuller R, Hermann C, et al:
Toll-like receptor 2 suppresses immunity against Candida
albicans through induction of IL-10 and regulatory T cells. J
Immunol. 172:3712–3718. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Darveau RP, Pham TT, Lemley K, et al:
Porphyromonas gingivalis lipopolysaccharide contains
multiple lipid A species that functionally interact with both
toll-like receptors 2 and 4. Infect Immun. 72:5041–5051. 2004.
View Article : Google Scholar
|
|
27
|
Erridge C, Pridmore A, Eley A, Stewart J
and Poxton IR: Lipopolysaccharides of Bacteroides fragilis,
Chlamydia trachomatis and Pseudomonas aeruginosa
signal via toll-like receptor 2. J Med Microbiol. 53:735–740.
2004.PubMed/NCBI
|
|
28
|
Dziarski R and Gupta D: Role of MD-2 in
TLR2- and TLR4-mediated recognition of Gram-negative and
Gram-positive bacteria and activation of chemokine genes. J
Endotoxin Res. 6:401–405. 2000. View Article : Google Scholar : PubMed/NCBI
|