Introduction
Alveolar echinococcosis (AE) is a rare but fatal
disease caused by infection with the larvae (metacestodes) of
Echinococcus multilocularis (Em). The disease has been fatal
in >95% of cases in the past 10 years. As a chronically
progressive hepatic infestation, AE is characterised by a long
asymptomatic period in which development of an invasive
tumour-like, multi-vesiculated and exogenously budding lesion
occurs (1).
In certain parasitic infection models, T helper (Th)
cells develop gradually to a certain polarised direction of a T
subset, which participates in multiple pathological injuries and is
able to cause tissue damage (2).
With parasitic antigen stimulation, native T cells selectively
differentiate into Th1 or Th2 cells (3,4). Th1
cells play an important role in the defence against intracellular
parasitic infections by secreting interleukin 2 (IL-2), interferon
γ (IFN-γ) and tumour necrosis factor-β (TNF-β), which mediate
cytotoxic effects by promoting cytotoxic lymphocytes, natural
killer cells and macrophages into activation and proliferation.
Since the secreted TNF-β and IFN-γ can recruit and activate
inflammatory cells, the Th1 immune response has often been
associated with inflammation and tissue damage, resulting in
delayed hypersensitivity. Th2 cells are involved in humoral immune
responses and primarily provide efficient help for B-cell
proliferation and antibody production. The Th1/Th2 imbalance is the
most important immunological change in response to pathogenesis,
and the immune response often gradually shifts towards a Th2
response at later stages of infection to prevent Th1-mediated
damage (5).
Identified as a new subset of T helper cells not
associated with Th1 or Th2 cells, Th17 cells are not only involved
in the host defence against extracellular pathogens, but are also
associated with the induction of autoimmunity and inflammatory
responses (6). Previous studies
have demonstrated that Th17 cells and IL-17 play an important role
in parasitic schistosomiasis and toxoplasmosis (7–9);
however, their role in AE has yet to be reported. In order to
investigate the immunopathological regulation in Em infection,
levels of different cytokines were assessed following infection
with Em. The identification of the role of Th17 cells in AE
development may be beneficial in explaining the cellular immunity
observed in the Th1/Th2 axis.
Materials and methods
Animals and grouping
Twenty-four female BALB/c mice were provided by
Xinjiang Medical University (Urumqi, China) and randomly divided
into eight groups: piD2 (2 days post-infection, n=3), piD8 (8 days
post-infection, n=3), piM1 (1 month post-infection, n=3), piM2 (2
months post-infection, n=3), piM3 (3 months post-infection, n=3),
piM6 (6 months post-infection, n=3), piM10 (10 months
post-infection, n=3) and a control (n=3, uninfected group).
Experimental mice were infected by intraperitoneal injection of a
metacestode suspension. Serum and liver samples were collected 2
days, 8 days and 1, 2, 3, 6 and 10 months post-infection,
respectively. The care of the animals and all procedures were
approved by the Institutional Animal Care and Use Committee, and
mice were used in accordance with the ethical guidelines of the
First Affiliated Hospital of Xinjiang Medical University.
Immunohistochemical staining
Formalin-fixed and paraffin-embedded liver biopsy
specimens were cut into 4-μm sections and unwaxed in xylene.
Following washing with ethanol several times, sections were stained
with anti-IL-17 antibodies (Wuhan Boster Biological Technology,
Ltd., Wuhan, China) at a dilution of 1:100.
ELISA
Serum levels of cytokines IL-17, TGF-β1, IL-6, IFN-γ
and IL-4 were determined using ELISA. Cytokine assays were
performed with ELISA kits (Ebioscience, San Diego, CA, USA) for
each individual mouse, in accordance with the manufacturer’s
instructions.
Statistical analysis
All experiments were performed in triplicate. Data
are expressed as the mean ± standard deviation. One-way analysis of
variance was applied to compare the differences among the different
groups. A value of P<0.05 was considered to indicate a
significant difference between values.
Results
IL-17 expression in the livers of BALB/c
mice infected with Em
To assess IL-17 expression in the livers of BALB/c
mice infected with Em, immunohistochemical staining was performed.
Typical pathological characteristics of the Em-infected mice
included various vesicles with a diameter of 0.1 mm-3 cm and
tumour-like growth in the liver with invasion of neighbouring
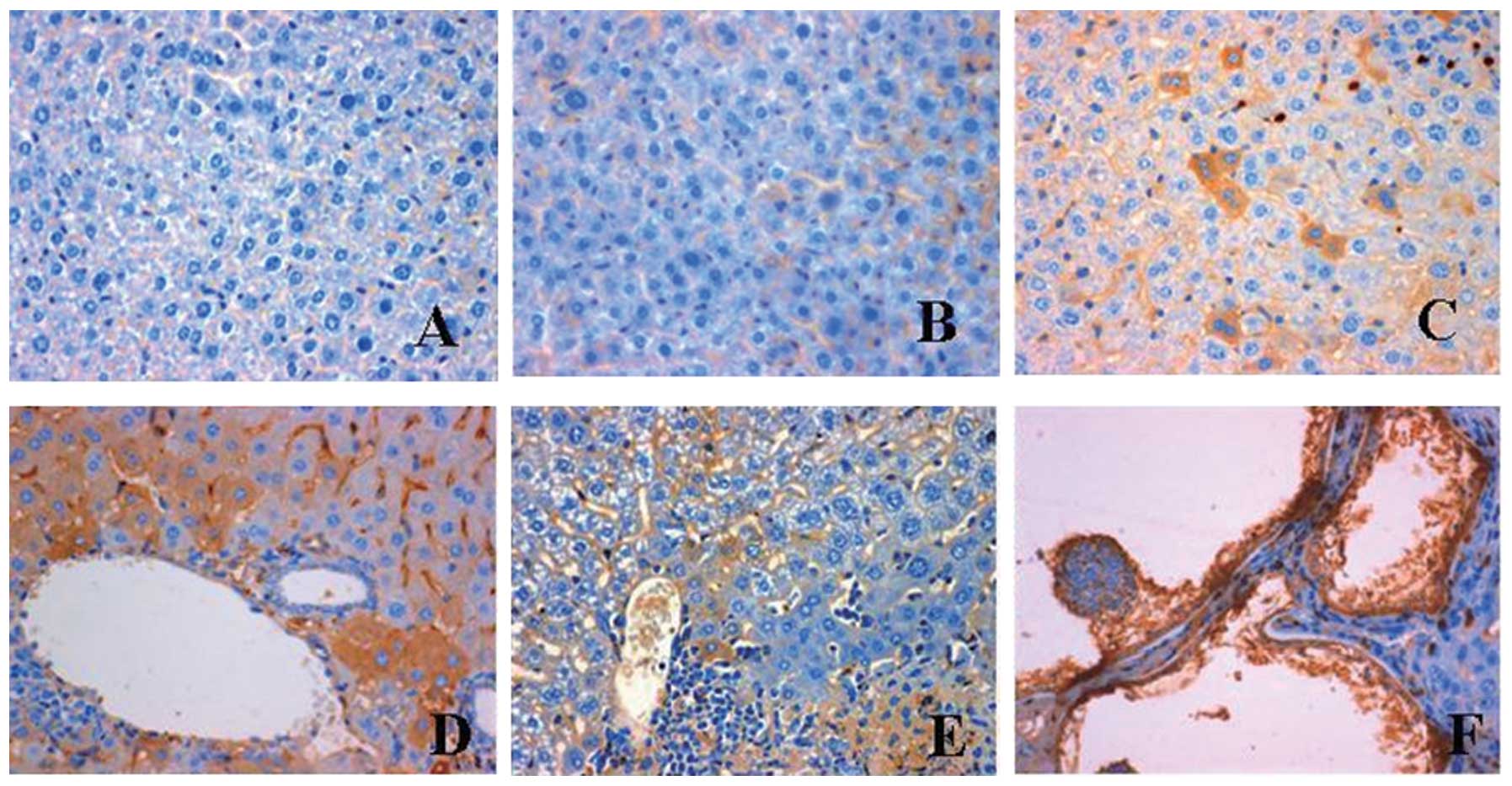
tissue and organs. Staining for IL-17 expression was negative in
the uninfected control sections (Fig.
1A). No expression of IL-17 was observed in the cytoplasm of
hepatic cells at 2 days post-infection (Fig. 1B), while IL-17 was only expressed
in a few hepatic sinusoid endothelial and hepatic cells at 1 month
post-infection (Fig. 1C). Notably,
at 3 months post-infection, a strong positive staining for IL-17
was found in hepatic and Kupffer cells, particularly in those
around the central veins of the livers (Fig. 1D). At 4 months post-infection,
parasitic germinal and laminated layers were formed in the liver,
and the perisinusoidal spaces were enlarged. A few stained hepatic
cells were observed at 6 months post-infection (Fig. 1E). At 10 months post-infection, the
parasitic vesicles were surrounded by a necrotic zone and a large
number of fibroblasts. Both the parasitic germinal layer and the
hepatic cells surrounding the parasitic vesicles were intensively
stained for IL-17 (Fig. 1F). In
summary, IL-17 expression occurred in hepatic cells at 1 month
post-infection, reached a maximum at 3 months post-infection and
then decreased gradually.
Dynamic changes in cytokine levels in
mice infected with Em
To assess the dynamic change in cytokine levels in
mice infected with Em, ELISA was performed. The concentration of
IL-17 remained low in uninfected controls. During the infection, a
trend of constantly increasing IL-17 levels was observed, with
levels at 6 and 10 months post-infection significantly higher than
those in the control. Levels at 6 and 10 months post-infection were
also significantly higher than those in the groups at 2 and 8 days
post-infection, respectively (Fig.
2, Table I).
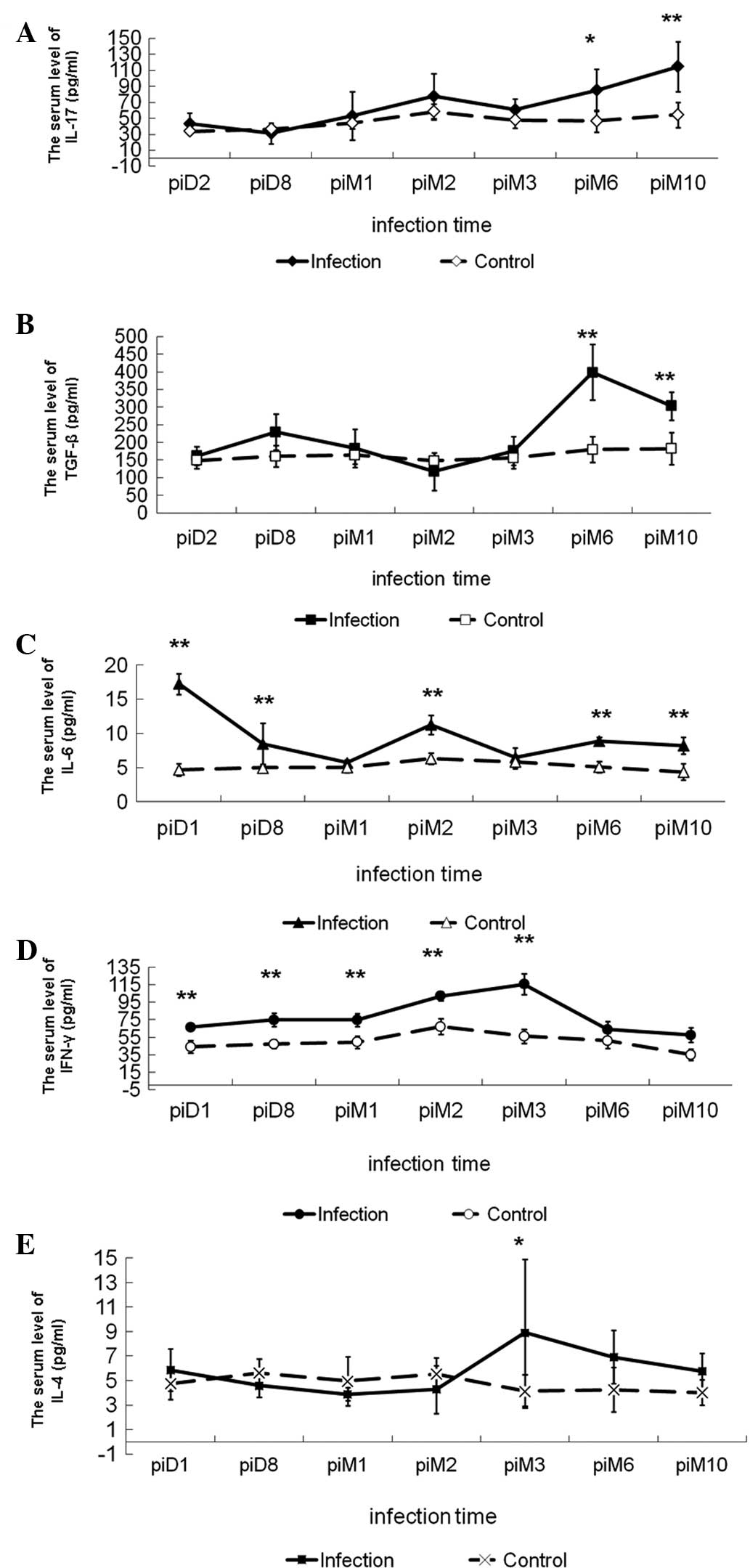 | Figure 2Changes in cytokine levels in serum of
BALB/c mice infected with Echinococcus multilocularis.
Levels of (A) IL-17, (B) TGF-β1, (C) IL-6, (D) IFN-γ and (E) IL-4.
*P<0.05, **P<0.01, significant
difference compared with group control. IL, interleukin; TGF-β,
transforming growth factor-β; IFN-γ, interferon-γ; pi,
post-infection; D, day; M, month. |
 | Table ILevels of IL-17, TGF-β1, IL-6, INF-γ
and IL-4 in sera of mice. |
Table I
Levels of IL-17, TGF-β1, IL-6, INF-γ
and IL-4 in sera of mice.
| Cytokine levels
(pg/ml) | Controls | piD2 | piD8 | piM1 | piM2 | piM3 | piM6 | piM10 |
|---|
| IL-17 | 33.76±3.37f,G | 43.44±13.64G | 31.22±13.12d,f | 53.28±29.95G | 77.86±28.33 | 60.65±13.84G | 85.24±26.13 | 114.74±31.34 |
| TGF-β1 | 150.54±22.37F,G | 161.34±26.13F,G | 229.51±50.68d,F | 183.41±54.60F,g | 117.84±53.48F,G | 176±40.64F,G | 398.54±78.78 | 303.27±39.56 |
| IL-6 | 4.69±0.89A,B,D,F,G | 17.25±1.54B,C,D,E,F | 8.50±2.97 | 5.71±0.42D,f | 11.26±1.41 | 6.46±1.42 | 8.89±0.59 | 8.24±1.23 |
| IFN-γ | 44.21±7.13A,B,C,D,E | 66.53±3.09D,E | 75.09±7.63D,E | 74.67±7.54D,E | 102.07±5.04F,G | 115.73± 11.69F,G | 64.10±9.08 | 57.44±8.25 |
| IL-4 | 4.75±1.27e | 5.87±1.72 | 4.59±0.93e | 3.89±0.52E | 4.28±1.96 | 8.91±5.99 | 6.93±2.19f | 5.76±1.45 |
Expression of TGF-β1 showed a similar trend to
IL-17. Compared with the control and the groups at 2 days, 8 days
and 1, 2 and 3 months post-infection, the TGF-β1 concentration was
significantly increased at 6 and 10 months post-infection (Fig. 2, Table
I). Levels of IL-6 reached a maximum at 2 days post-infection
and then decreased to a minimum at 1 month post-infection, prior to
steadily increasing until 2 months post-infection. IL-6 production
in all infected groups was significantly higher than that in the
uninfected control (Fig. 2,
Table I). IFN-γ levels increased
following Em infection, reaching a maximum at 3 months
post-infection and decreasing thereafter, while the IL-4 levels
remained low until 2 months post-infection and increased at 3
months post-infection (Fig. 2,
Table I). These results suggest
that Th17 cells may be involved in the Th1/Th2-cell balance during
the immune response.
Discussion
A previous study suggested that IFN-γ (Th1) activity
was associated with the killing of both the protosceles and
established cysts of E. granulosus (10). However, Wei et al (11) suggested that the induction of
Th2-antibody-mediated immunity (AMI), with a parallel expansion of
Th1-cell-mediated inflammatory (CMI) responses, was an important
mechanism for host defence against metacestodes. Th1 CMI has an
important role at the early stages of infection, while Th2 AMI is
crucial for the later stages of infection.
A variety of immune mechanisms exist at an early
stage of secondary E. granulosus infection, and Th cells
show no dominant imbalance (12).
It has been shown that during the intermediate stage of infection,
when hydrated antigen levels in vivo are comparatively low,
the immune response becomes Th2-skewed. On entering the chronic
infection stage, the number of antigens gradually increases and Th1
cells become increasingly activated. This suggests that the early
induction of Th2, with a parallel expansion of Th1, cells
represents an important mechanism involved in the host defence
against E. granulosus.
IFN-γ, induced by Th1-type antigens, can inhibit the
secretion of Th2 cytokines in the anti-parasitic response primarily
by enhancing macrophage functions and limiting the proliferation of
intracellular parasitic protozoa in order to control the infection
(13). By contrast, IL-4, produced
by activated Th2 cells, stimulates the proliferation and
differentiation of B cells to inhibit cell proliferation and the
Th1 response. The present study showed that levels of IFN-γ
increased gradually following Em infection, reached a maximum at 3
months post-infection and then decreased. Levels of Th2-type
cytokines were also high at 3 months post-infection. This suggests
that the dominant Th1 immune response is shifted to a Th2 response
in Em infection. In addition, IFN-γ can promote Th1-cell maturation
and inhibit Th2-cell function by reducing the production of
Th2-type cytokines. Furthermore, macrophages activated by IFN-γ can
kill parasites and inhibit the growth of hydatid tissue. By
contrast, high IL-4 levels delay the secretion of Th1-type
cytokines and cell proliferation. In the present study, the levels
of IL-4 remained low until 2 months post-infection, and increased
after 3 months. As a result of the high secretion of IL-4,
decreased levels of Th1-type cytokines led to rapid growth of Em
tissue in the body. Considering that Th2-type cytokines are
generally associated with parasite persistence and growth (14,15),
a Th2-type immune response may facilitate the evasion of the host
immune response by the parasite. High levels of cytokines,
including IL-4, IL-5, IL-9 and IL-13, secreted by different types
of immune cells in response to parasite antigens, not only reduce
the Th1 response, but also promote parasite expulsion and tissue
renewal and repair (16).
IL-17, induced by IL-23 and modulated by IL-21,
IL-22 and TGF-β1, is mainly involved in T-cell-mediated activation
of innate immunity/inflammation, as well as immune tolerance
(17). BALB/c mice infected with
Schistosoma japonicum have been shown to exhibit significant
changes in cytology, particularly the decrease in Th1 and plasma
cells and increase in Th2 and Th17 cells (18). In the present study, levels of
IL-17 were relatively low in the uninfected control; however,
levels increased gradually following Em infection, particularly
during the middle-late stage of infection. In the early stage of
infection, Th1 and Th17 cells may have roles in clearing the
parasites; however, with the extension of infection time,
particularly after 3 months, Th2-type cytokines may begin to
inhibit Th1-cell proliferation and immune response. Thus, Th1 cells
were downregulated, and the high levels of IL-17 secreted by Th17
cells resulted in a strong immunopathological injury of the host
liver. The Th2-type immune response can protect alveolar hydatids
from the host immune attack. This may explain the slow growth of
alveolar hydatids during the first 3 months post-infection and the
rapid growth in the abdominal cavity and liver after 3 months,
breaking through the diaphragmatic muscle and reaching the chest
area.
Compared with the uninfected control, IL-6 levels in
the infected groups were increased and reached a maximum at 2 days
post-infection. These data show that when the systemic immune
response occurred following invasion of the body by alveolar
hydatids, IL-6 participated in an acute inflammation process and
levels of IL-6 increased rapidly at the early stage of infection.
When Em parasites were unable to be cleared from the body, the
inflammation continually persisted. The production of TGF-β1 showed
a similar trend to IL-17 and rapidly increased at the middle-late
stage of infection. TGF-β1 was highly expressed at the late stage
of infection and exhibited similar variations in expression to
IL-17, indicating that TGF-β1 may have an important role in
promoting Th17-cell differentiation. Hepatic stellate cells
stimulated by TGF-β1 can promote liver fibrosis (19). With the growth of hydatid cysts,
inflammatory reaction infiltrates formed a fibrous layer and
separated the hydatid cysts from the host tissue.
In conclusion, the present study may explain the
cellular immunity observed in the Th1/Th2 axis and broaden the
understanding of the immunopathological effects of Th17 cells in
the development of AE.
Acknowledgements
The present study was supported by the Scientific
Research Project of Science Department of Xinjiang Autonomous
Region (no. 2012211A034) and the National Natural Science
Foundation (nos. 31160194, 81260253, 30960358, 31000411, 30901374,
81160378, 81060135 and 30860263) and the Scientific Research
Project of the Science Department of Xinjiang Autonomous Region
(no. 2012211A034).
References
|
1
|
Wang X, Lu R, Liu QF, et al: Production
and immunoanalytical application of 32 monoclonal antibodies
against metacestode somatic antigens of Echinococcus
multilocularis. Parasitol Res. 107:177–185. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Barthelmann J, Nietsch J, Blessenohl M, et
al: The protective Th1 response in mice is induced in the T-cell
zone only three weeks after infection with Leishmania major
and not during early T-cell activation. Med Microbiol Immunol.
201:25–35. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Pang NN, Ding JB, Zhao H, et al: The
dynamic observation of liver T-bet, GATA-3, ROR-γt and IL-17 mRNA
expression in BALB/c mice infected with Echinococcus
multilocularis. Chin J Immunol. 27:395–399. 2011.(In
Chinese).
|
|
4
|
Ma XM, Xu Q, Hou M, et al: Effects of
Echinococcus granulosus infection on IL-17 level and Th1/Th2
balance in serum of asthmatic rats. Immunol J. 27:110–113. 2011.(In
Chinese).
|
|
5
|
Mendes EA, Mendes TA, dos Santos SL, et
al: Expression of IL-4, IL-10 and IFN-γ in the liver tissue of
cattle that are naturally infected with Fasciola hepatica.
Vet Parasitol. 195:177–182. 2013.
|
|
6
|
Infante-Duarte C, Horton HF, Byrne MC and
Kamradt T: Microbial lipopeptides induce the production of IL-17 in
Th cells. J Immunol. 165:6107–6115. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Mbow M, Larkin BM, Meurs L, et al:
T-helper 17 cells are associated with pathology in human
schistosomiasis. J Infect Dis. 207:186–195. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zhang Y, Chen L, Gao W, et al: IL-17
neutralization significantly ameliorates hepatic granulomatous
inflammation and liver damage in Schistosoma japonicum
infected mice. Eur J Immunol. 42:1523–1535. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhang H, Hu X, Liu X, et al: The Treg/Th17
imbalance in Toxoplasma gondii-infected pregnant mice. Am J
Reprod Immunol. 67:112–121. 2012.
|
|
10
|
Wang S, Xu Y, Zhu M, et al: Cytokine
development in mice with secondary Echinococcus granulosus
infection. Endemic Diseases Bulletin. 17:8–11. 2002.(In
Chinese).
|
|
11
|
Wei XL, Ding JB, Xu Z, et al: Change of
cytokines in mice with Echinococcus multilocularis
infection. Chin J Parasitol Parasit Dis. 22:361–364. 2004.(In
Chinese).
|
|
12
|
Rogan MT: T-cell activity associated with
secondary infections and implanted cysts of Echinococcus
granulosus in BALB/c mice. Parasite Immunol. 20:527–533. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Li Y, Terkawi MA, Nishikawa Y, et al:
Macrophages are critical for cross-protective immunity conferred by
Babesia microti against Babesia rodhaini infection in
mice. Infect Immun. 80:311–320. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Xu X, Wen X, Chi Y, et al:
Activation-induced T helper cell death contributes to Th1/Th2
polarization following murine Schistosoma japonicum
infection. J Biomed Biotechnol. 2010:2023972010.PubMed/NCBI
|
|
15
|
Vuitton DA: The ambiguous role of immunity
in echinococcosis: protection of the host or of the parasite? Acta
Trop. 85:119–132. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Pennock JL and Grencis RK: The mast cell
and gut nematodes: damage and defence. Chem Immunol Allergy.
90:128–140. 2006.PubMed/NCBI
|
|
17
|
Wilson NJ, Boniface K, Chan JR, et al:
Development, cytokine profile and function of human interleukin
17-producing helper T cells. Nat Immunol. 8:950–957. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhao JJ, Huang J, Mai JY, et al: Changes
of cytology of spleen in BALB/c infected with Schistosoma
japonicum. J Trop Med. 9:140–143. 2009.(In Chinese).
|
|
19
|
Jia D, Duan F, Peng P, et al:
Up-regulation of RACK1 by TGF-β1 promotes hepatic fibrosis in mice.
PLoS One. 8:e601152013.
|