Introduction
Arazyme is a novel extracellular metalloprotease
produced by Aranicola proteolyticus (also known as
Serratia proteamaculans, an aerobic Gram negative symbiotic
bacterium isolated from the intestine of the spider, Nephila
clavata) (1,2). A previous report demonstrated that
arazyme has hepatoprotective activity against carbon
tetrachloride-induced hepatic injury and that the mechanism
involves increased expression of SMP30 and antioxidant proteins
(3).
Human umbilical vein endothelial cells (HUVECs) are
used in studies of various diseases, including angiogenesis,
atherosclerosis and the inflammatory process. Notably, HUVECs are
involved in the release of cytokines and chemokines, as well as
cell migration during the inflammatory process (4,5).
Increased levels of vascular cell adhesion molecule-1 (VCAM-1) and
intracellular adhesion molecule-1 (ICAM-1) are early markers of
endothelial activation and dysfunction in inflammatory diseases
(6). Activation of adhesion
molecules on the surface of endothelial cells induces leukocyte
migration and leads to inflammation (7). The HUVEC-associated inflammatory
response is induced by a variety of inflammatory mediators,
including lipopolysaccharide (LPS), tumor necrosis factor-α and
oxidized-low density lipoprotein (6,8,9). LPS
is an integral part of the bacterial outer membrane and is a
significant stimulator of inflammation. LPS induces the generation
of reactive oxygen species (ROS) and upregulates cytokines,
chemokines and adhesion molecules in HUVECs (5). These mechanisms contribute to
endothelial dysfunction and may evoke endothelial cell-associated
diseases. A number of studies have attempted to identify methods to
protect against the inflammatory response and endothelial cell
death (9–11). In the current study, the role of
arazyme in the LPS-mediated inflammatory response in HUVECs was
investigated.
Materials and methods
Reagents
Endothelial cell basal medium-2 (EBM-2), fetal
bovine serum (FBS), recombinant human fibroblast growth factor
(rhFGF), recombinant human epidermal growth factor (rhEGF),
hydrocortisone, gentamicin sulfate, amphotericin-B (GA-1000),
heparin, vascular endothelial growth factor (VEGF), ascorbic acid
and long R insulin-like growth factor-1 (R3-IGF-1) were
purchased from Lonza (Walkersville, MD, USA). Trypsin-EDTA was
purchased from Life Technologies, Inc. (Gaithersburg, MD, USA). LPS
was purchased from Sigma-Aldrich Korea (Seoul, Korea).
2′,7′-dichlorofluorescein diacetate (DCFDA) and Alexa Fluor 488
chicken anti-mouse IgG were purchased from Molecular Probes
(Eugene, OR, USA). Normal rabbit IgG, anti-VCAM-1 and anti-ICAM-1
antibodies were purchased from Santa Cruz Biotechnology (Santa
Cruz, CA, USA).
Enzyme purification
Arazyme was purified as described previously
(2). Briefly, extracellular
fractions were collected by centrifugation of the culture medium or
by filtration using a 0.2-μm membrane filter (Pall Life Sciences,
Port Washington, NY, USA). Chromatography was performed on a
DEAE-cellulose column equilibrated with 50 mM potassium phosphate
buffer (pH 7.6). Bound proteins were eluted with a 0.1–0.5 M sodium
chloride gradient at a flow rate of 400 ml/h and each fraction was
concentrated with a 10 kD cassette membrane (Pall Life Sciences).
The protein solution was then loaded at a flow rate of 20 ml/h onto
a Sephadex G-75 column (GE Healthcare Life Sciences, Pittsburgh,
PA, USA) equilibrated previously with 50 mM potassium phosphate
buffer (pH 7.8). Fractions containing proteolytic activity were
concentrated with the 10 kD cassette membrane and stored at
−20°C.
Cell culture
HUVECs were purchased from the American Type Culture
Collection (Manassas, VA, USA). The cells were cultured on 0.2%
gelatin-coated flasks with EBM-2 medium supplemented with 2% FBS,
rhFGF, rhEGF, hydrocortisone, GA-1000, heparin, VEGF, ascorbic acid
and R3-IGF-1. HUVECs were incubated at 37°C in a 5% CO2
incubator.
MTT assay
The MTT assay was performed with a cell
proliferation kit (Roche Diagnostics, Mannheim, Germany) to
determine cell viability. HUVECs at a concentration of
5×103 cells/100 μl were plated on a 96-well plate.
Following arazyme treatment, the plate was incubated for 24 h at
37°C in a 5% CO2 incubator. Next, 10 μl MTT solution was
added to each well and the plate was incubated at 37°C for 4 h in a
CO2 incubator. A 100 μl aliquot of solubilization
solution was added to each well. Following 24 h incubation, the
absorbance was measured at 550 nm using an enzyme-linked
immunosorbent assay (ELISA) reader (Bio-Tek Instruments Inc.,
Winooski, VT, USA).
Cell apoptosis
An Annexin V-fluorescein isothiocyanate (FITC)
Apoptosis Detection kit (BD Biosciences, San Jose, CA, USA) was
used to detect apoptosis. Following a 24 h treatment with
stimulators, including LPS and arazyme, HUVECs were incubated with
the FITC-labeled Annexin V and propidium iodide (PI) for 15 min at
room temperature. Apoptotic cells were analyzed by flow cytometry
using CellQuest software (BD Biosciences) and were defined as cells
in the right quadrant, which stained positive for Annexin V
with/without PI. A total of 10,000 events were collected for each
sample.
ELISA
HUVECs were treated with LPS for 24 h and the
supernatants were collected. The concentrations of monocyte
chemoattractant protein-1 (MCP-1) and interleukin-6 (IL-6) were
measured in the cell supernatant with a sandwich ELISA (OptEIA™ Set
human IL-6 and MCP-1; BD Biosciences) according to the
manufacturer’s instructions. The concentration of each protein was
calculated from standard curves.
VCAM-1 and ICAM-1 expression
To detect the surface expression of adhesion
molecules, including VCAM-1 and ICAM-1, HUVECs were treated with
LPS for 24 h, incubated with anti-VCAM-1 and anti-ICAM-1 or control
IgG antibodies for 30 min and then with anti-mouse IgG
FITC-conjugated antibodies. The samples were analyzed with
CellQuest software on a FACSCalibur flow cytometer (BD
Biosciences). A total of 10,000 events was collected for each
experiment.
Nuclear factor (NF)-κB p65 transcription
factor assay
Following a 4 h stimulation with LPS, the
DNA-binding activity of NF-κB in HUVECs was assessed using
EZ-Detect™ Transcription Factor kits for NF-κB p65 (Pierce
Biotechnology Inc., Rockford, IL, USA), following the
manufacturer’s instructions. DNA binding specificity was assessed
using wild type or mutant NF-κB oligonucleotides. Chemiluminescent
detection was performed using a luminometer (Thermo Fisher
Scientific, Pittsburgh, PA, USA).
ROS production
Following 24 h LPS stimulation, HUVECs were washed
and resuspended at a concentration of 1×106 cells/ml in
pre-warmed PBS. The cells were exposed to 5 μM DCFDA to label
intracellular ROS and then incubated for 10 min at room
temperature. Labeled cells were immediately observed by flow
cytometry (BD Biosciences).
Statistical analysis
All data are expressed as the mean ± standard error
of the mean. Data were analyzed with Student’s t-test and the SPSS
statistical software package, version 10.0 (SPSS Inc., Chicago, IL,
USA). P<0.05 was considered to indicate a statistically
significant difference.
Results
Arazyme inhibits HUVEC apoptosis induced
by LPS
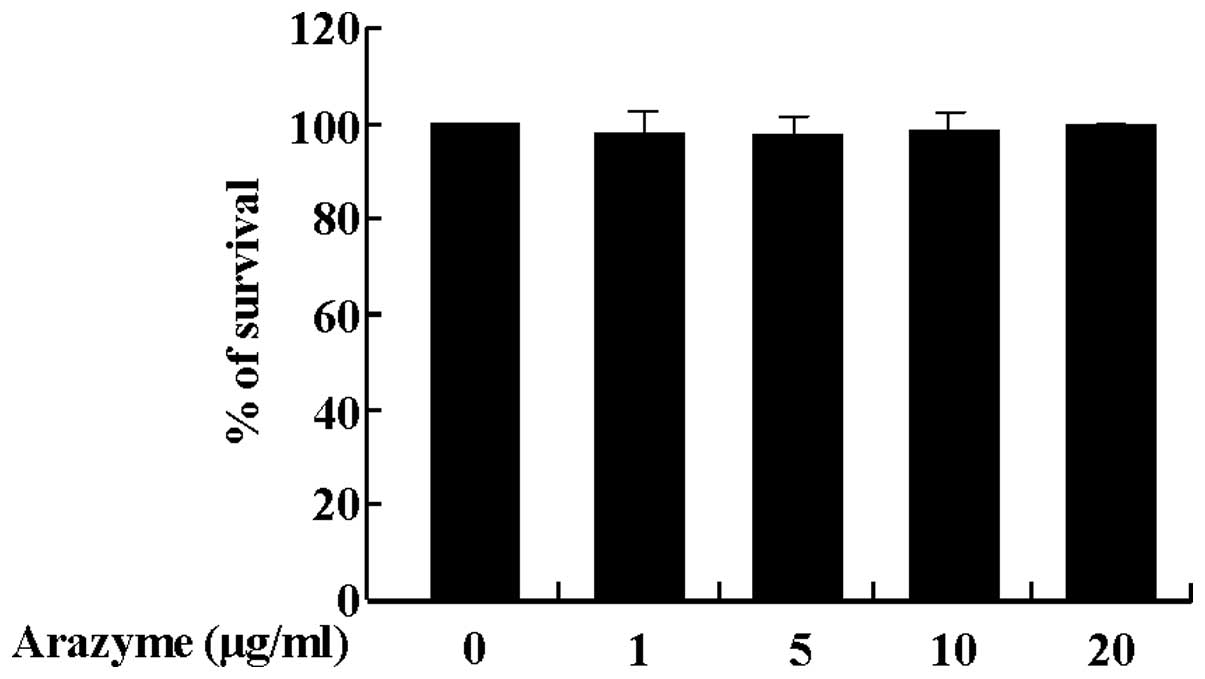
Prior to examining the effect of arazyme on HUVECs,
the effect of arazyme on cell viability was examined. As shown in
Fig. 1, the survival rate of
HUVECs was not altered by arazyme treatment at concentrations of 1,
5, 10 or 20 μg/ml. Thus, 20 μg/ml arazyme was used to determine the
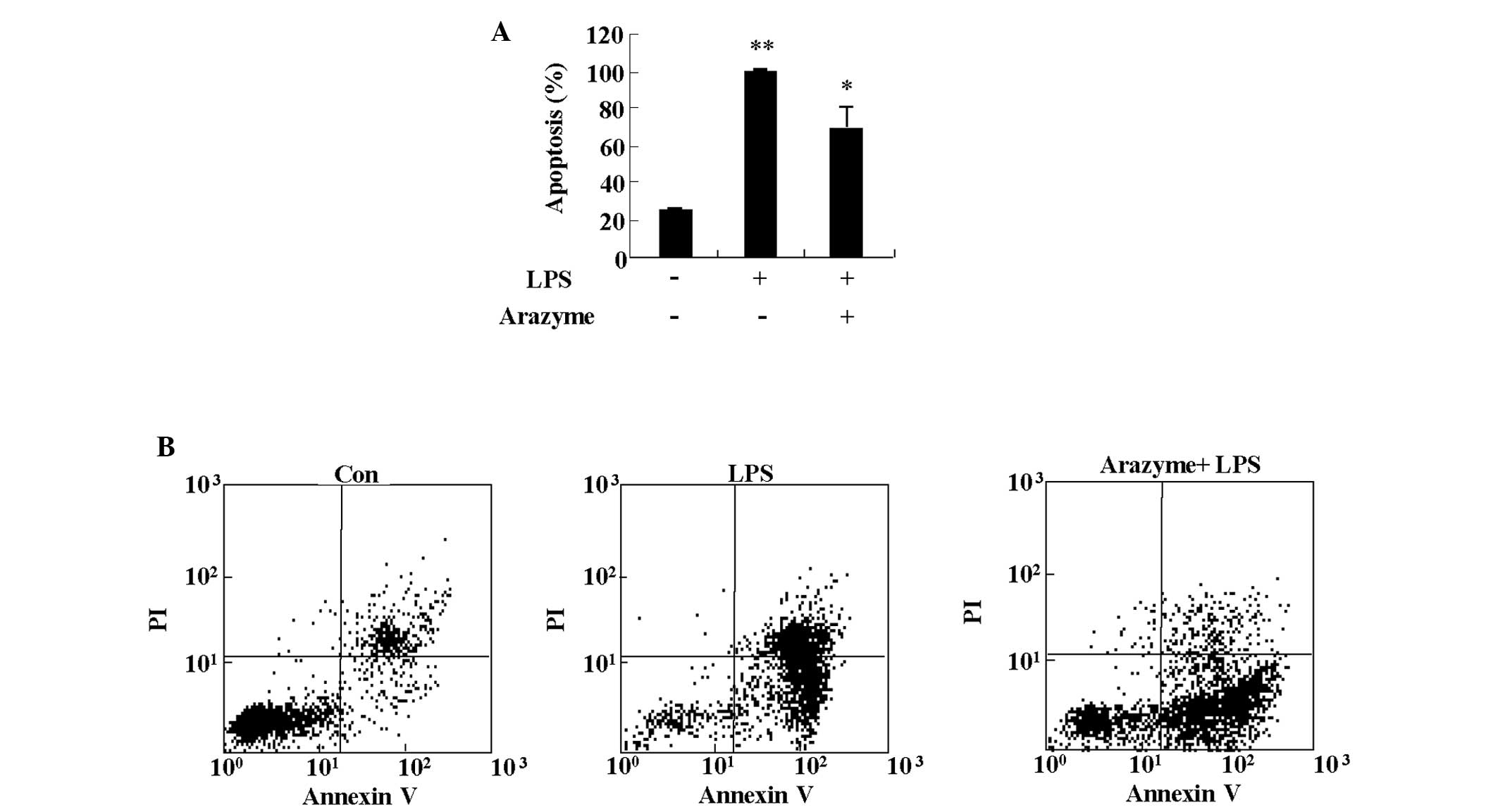
anti-inflammatory effects. Since LPS causes endothelial injury by
inducing apoptosis, the protective effect of arazyme on LPS-induced
apoptosis was determined (12). As
shown in Fig. 2, treatment with 10
μg/ml LPS led to strong induction of apoptosis in HUVECs. Arazyme
significantly blocked LPS-induced apoptosis of HUVECs.
Arazyme decreases secretion of monocyte
chemoattractant protein-1 (MCP-1) and IL-6 in HUVECs
To determine the inhibitory effect of arazyme on
cytokine release from HUVECs, MCP-1 and IL-6 levels were measured
by ELISA in HUVEC supernatants following treatment with 10 μg/ml
LPS in the presence or absence of arazyme. Arazyme decreased MCP-1
secretion considerably in HUVECs stimulated with LPS (Fig. 3A). The release of IL-6 in
LPS-stimulated HUVECs tended to be inhibited by arazyme, but the
difference was not significant (Fig.
3B). These observations indicate that arazyme has
anti-inflammatory effects on LPS-stimulated HUVECs by suppressing
cytokine release.
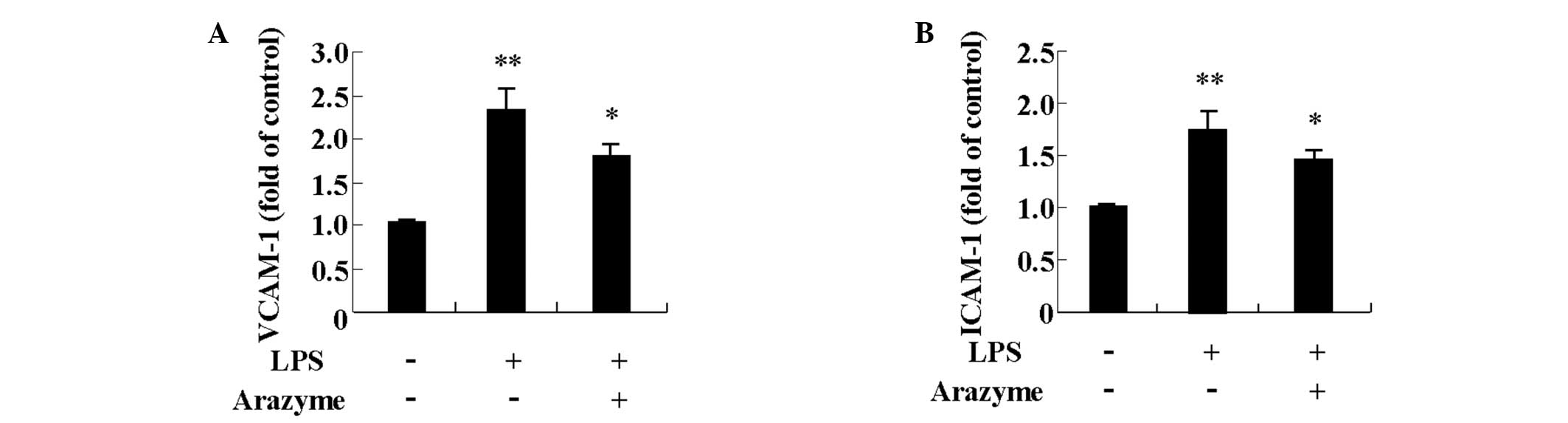
Arazyme inhibits expression of adhesion
molecules in HUVECs
LPS increases cell adhesion molecules, including
VCAM-1 and ICAM-1 in HUVECs (13,14).
To determine other anti-inflammatory effects of arazyme, its effect
on VCAM-1 and ICAM-1 expression induced by LPS was investigated.
HUVECs were pretreated with 10 μg/ml arazyme for 1 h and were then
treated with 10 μg/ml LPS for 24 h. LPS increased surface
expression of VCAM-1 and ICAM-1 and the increased expression was
suppressed by arazyme pretreatment (Fig. 4).
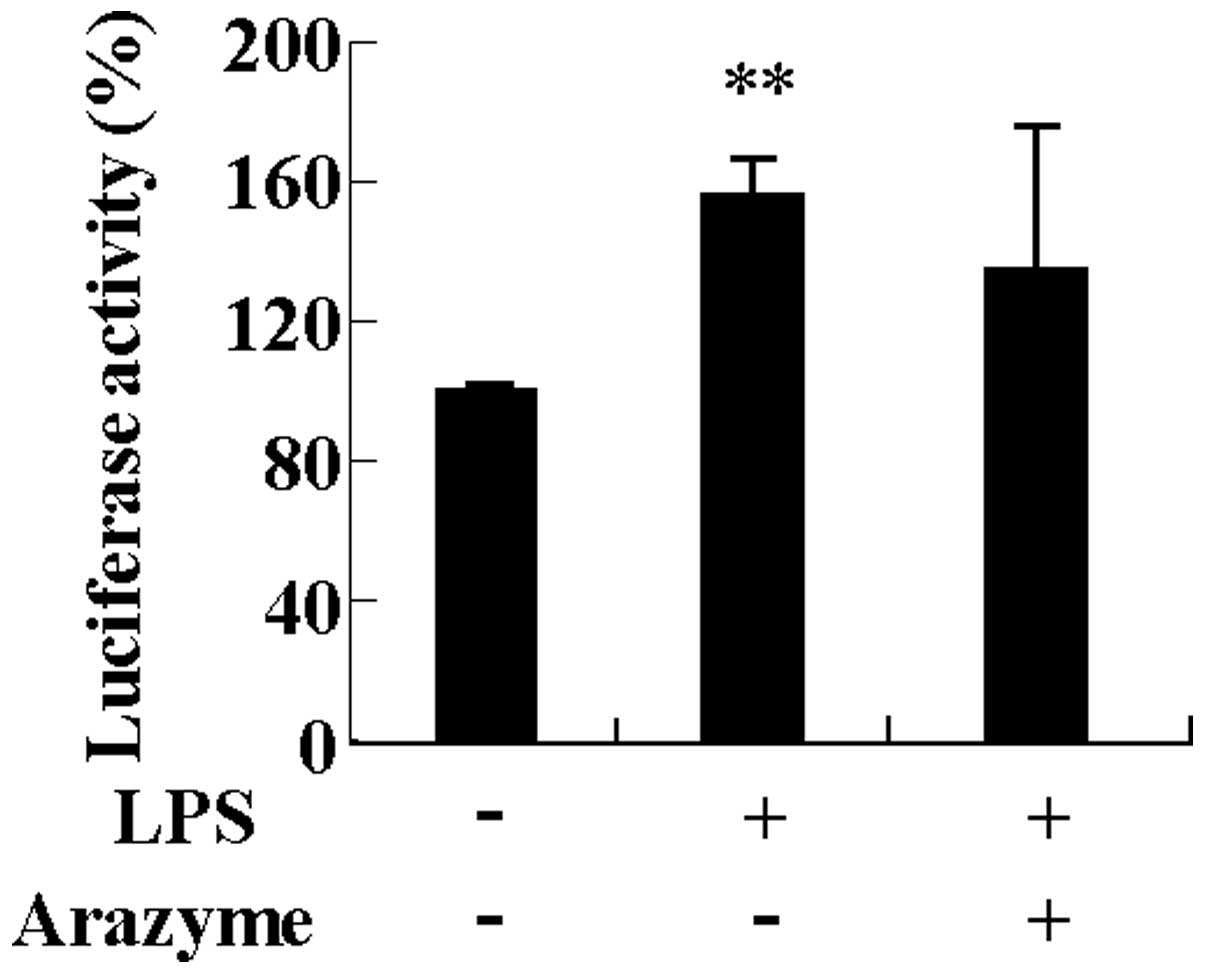
Arazyme is not associated with NF-κB
activation induced by LPS in HUVECs
NF-κB is a pleiotropic regulator of various genes
and is involved in the inflammatory response, including the
expression of chemokines, cytokines and adhesion molecules in
HUVECs (15). Since LPS increases
the expression of inflammatory proteins by activating NF-κB, it was
investigated whether the inhibitory effect of arazyme on cytokine
and adhesion molecule expression was involved in inhibiting NF-κB
activation. As shown in Fig. 5,
arazyme had no effect on NF-κB activation induced by LPS in HUVECs.
This result indicates that arazyme does not modulate the
inflammatory response in HUVECs.
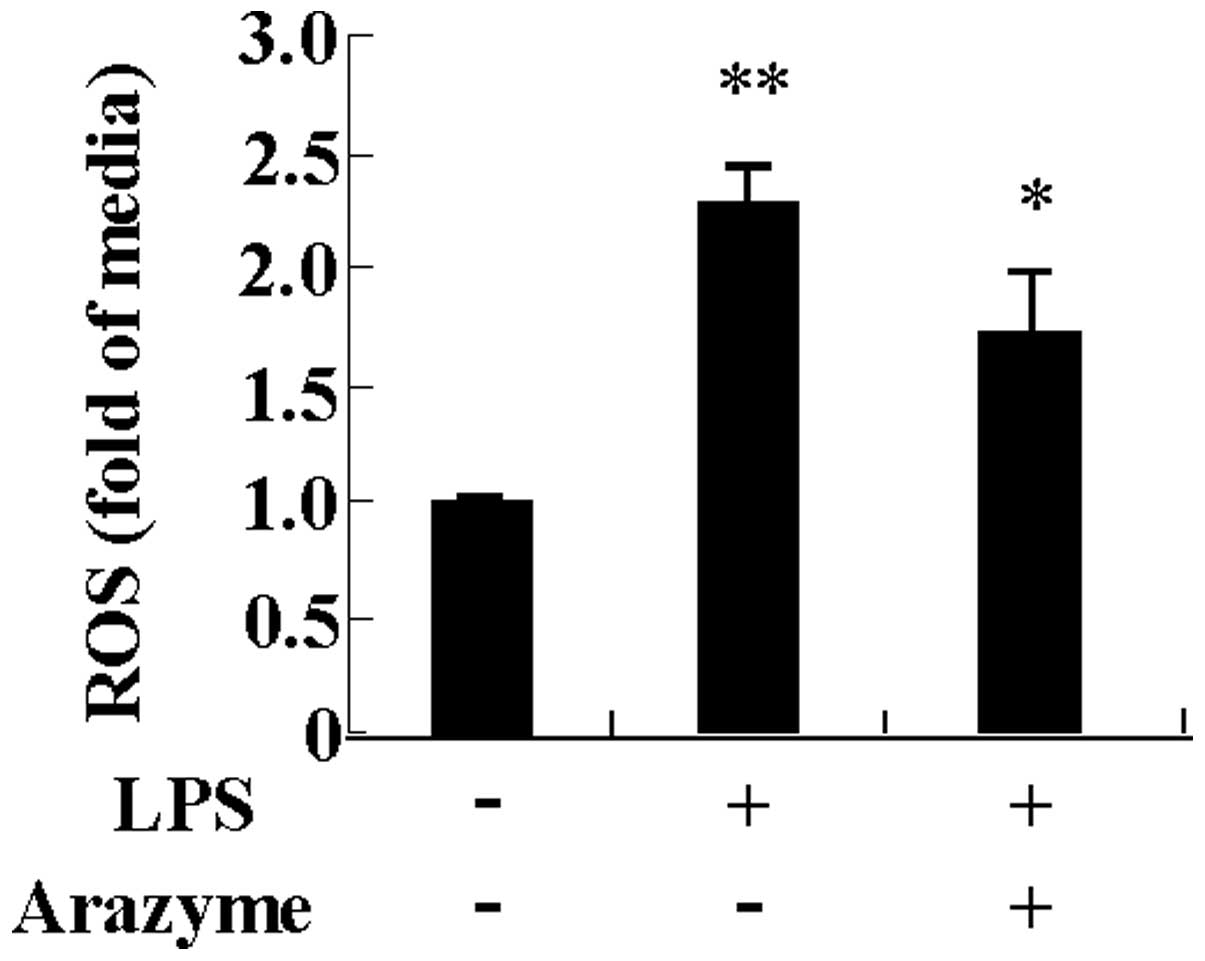
Arazyme inhibits LPS-induced ROS
production in HUVECs
As ROS production is an important mediator of
inflammation, the inhibitory effect of arazyme on ROS production in
HUVECs was examined. As shown in Fig.
6, LPS functioned as a strong inducer of ROS production in
HUVECs. Pre-incubating HUVECs with arazyme suppressed ROS
generation due to LPS. This result indicates that arazyme produces
an anti-inflammatory effect by suppressing oxidative stress.
Discussion
Although arazyme has a protective effect against
hepatic injury, other protective and therapeutic functions,
including inflammation, have not yet been reported (3). Previous studies have identified novel
inhibitory materials against the inflammatory response in
endothelial cells such as Ecklonia cava extracts and
cilostazol (9,11). The current study, focused on
elucidating the effect of arazyme on the inflammatory response in
HUVECs induced by LPS. Cytokine release is an important
inflammatory response. Arazyme inhibited MCP-1 and IL-6 secretion
but to different degrees. MCP-1 is a CCL2 and acts as a chemotactic
factor attracting monocytes (16).
A previous study demonstrated that MCP-1 induces neutrophilic
inflammation by inhibiting spontaneous apoptosis in neutrophils
(17). IL-6 is a multipotent
cytokine and increases the proliferation and differentiation of
numerous cell types, including B cells and skin cells (18). IL-6 induces a shift from acute- to
chronic-phase inflammation, including that observed in allergic
diseases (19,20). Arazyme also inhibited the
expression of VCAM-1 and ICAM-1 increased by LPS. VCAM-1 and ICAM-1
function as adhesion molecules and initiators of the inflammatory
signaling pathway (5). These
results indicate that arazyme acts as an anti-inflammatory agent in
HUVECs. ROS are generated at sites of inflammation and cause
cellular injury and death (4).
HUVECs modulate the movement of macromolecules and circulating
immune cells from the blood into tissue. Increased oxidative stress
in HUVECs induces vascular endothelial permeability and enhances
leukocyte adhesion. Since arazyme suppresses ROS production due to
LPS, arazyme may act as an inhibitor of inflammatory cell migration
into tissue. Since hyperproduction of ROS induces cell death
mediated by cytotoxicity, the anti-apoptotic effect of arazyme was
hypothesized in HUVECs (21). The
experimental results may indicate the validity of this hypothesis,
despite the lack of a direct association between ROS and cell
death.
A variety of inflammatory mediators, including LPS
and ROS activate NF-κB and then induce an increase in adhesion
molecules and the secretion of cytokines and chemokines (21). To examine the arazyme mechanism,
NF-κB activation was evaluated. However, arazyme did not inhibit
NF-κB activation caused by LPS stimulation. Although the mechanism
underlying the effects of arazyme has not been previously
determined, a hypothetical mechanism for the anti-apoptotic and
anti-inflammatory effects may be suggested since arazyme is a
metalloprotease. Arazyme may mediate its anti-apoptotic or
anti-inflammatory functions via the protease-activated receptor
(PAR), which is a G-protein-coupled receptor or through an
unidentified receptor (22). PAR
or an unknown-mediated signal, may be associated with inhibiting
the LPS-induced signal. Secondly, arazyme may directly cleave
cytokines, including MCP-1 and IL-6 and adhesion molecules, since
it may hydrolyze pro-inflammatory molecules, including bradykinin
and histamine (23,24). The exact mechanism of action of
arazyme remains to be determined and further studies are being
conducted to investigate more complex mechanisms.
In conclusion, arazyme has anti-inflammatory effects
in HUVECs that include suppression of cell apoptosis, ROS
production, and expression of IL-6, MCP-1, VCAM-1 and ICAM-1.
Arazyme may be a useful agent to treat endothelial
dysfunction-associated diseases, including atherosclerosis and
cardiovascular diseases.
Acknowledgements
This study was supported by a grant from the Korea
Research Institute of Bioscience and Biotechnology Research
Initiative Program.
References
|
1
|
Bersanetti PA, Park HY, Bae KS, Son KH,
Shin DH, Hirata IY, Juliano MA, Carmona AK and Juliano L:
Characterization of arazyme, an exocellular metalloprotease
isolated from Serratia proteamaculans culture medium. Enzyme
Microb Technol. 37:574–581. 2005. View Article : Google Scholar
|
|
2
|
Kwak J, Lee K, Shin DH, Maeng JS, Park DS,
Oh HW, Son KH, Bae KS and Park HY: Biochemical and genetic
characterization of arazyme, an extracellular metalloprotease
produced from Serratia proteamaculans HY-3. J Microbiol
Biotechnol. 17:761–768. 2007.PubMed/NCBI
|
|
3
|
Park JK, Jeong DH, Park HY, Son KH, Shin
DH, Do SH, Yang HJ, Yuan DW, Hong IH, Goo MJ, et al:
Hepatoprotective effect of arazyme on CCl4-induced acute
hepatic injury in SMP30 knock-out mice. Toxicology. 246:132–142.
2008.PubMed/NCBI
|
|
4
|
Hadi HA, Carr CS and Al Suwaidi J:
Endothelial dysfunction: cardiovascular risk factors, therapy, and
outcome. Vasc Health Risk Manag. 1:183–198. 2005.PubMed/NCBI
|
|
5
|
Liu HT, He JL, Li WM, Yang Z, Wang YZ, Yin
J, Du YG and Yu C: Geniposide inhibits interleukin-6 and
interleukin-8 production in lipopolysaccharide-induced human
umbilical vein endothelial cells by blocking p38 and ERK1/2
signaling pathways. Inflamm Res. 59:451–461. 2010. View Article : Google Scholar
|
|
6
|
Szmitko PE, Wang CH, Weisel RD, Jeffries
GA, Anderson TJ and Verma S: Biomarkers of vascular disease linking
inflammation to endothelial activation: Part II. Circulation.
108:2041–2048. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Sprague AH and Khalil RA: Inflammatory
cytokines in vascular dysfunction and vascular disease. Biochem
Pharmacol. 78:539–552. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
de Vries IJ, Langeveld-Wildschut EG, van
Reijsen FC, Dubois GR, van den Hoek JA, Bihari IC, van Wichen D, de
Weger RA, Knol EF, Thepen T and Bruijnzeel-Koomen CA: Adhesion
molecule expression on skin endothelia in atopic dermatitis:
effects of TNF-alpha and IL-4. J Allergy Clin Immunol. 102:461–468.
1998.PubMed/NCBI
|
|
9
|
Kim TH and Bae JS: Ecklonia cava
extracts inhibit lipopolysaccharide induced inflammatory responses
in human endothelial cells. Food Chem Toxicol. 48:1682–1687. 2010.
View Article : Google Scholar
|
|
10
|
Juzyszyn Z, Czerny B, Pawlik A and
Droździk M: The effect of Artichoke (Cynara scolymus L.)
extract on ROS generatin in HUVEC cells. Phytother Res.
22:1159–1161. 2008.
|
|
11
|
Lim JH, Woo JS and Shin YW: Cilostazol
protects endothelial cells against lipopolysaccharide-induced
apoptosis through ERK1/2- and P38 MAPK-dependent pathways. Korean J
Intern Med. 24:113–122. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Munshi N, Fernandis AZ, Cherla RP, Park IW
and Ganju RK: Lipopolysaccharide-induced apoptosis of endothelial
cells and its inhibition by vascular endothelial growth factor. J
Immunol. 168:5860–5866. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Marui N, Offermann MK, Swerlick R, Kunsch
C, Rosen CA, Ahmad M, Alexander RW and Medford RM: Vascular cell
adhesion molecule-1 (VACM-1) gene transcription and expression are
regulated through an antioxidant-sensitive mechanism in human
vascular endothelial cells. J Clin Invest. 92:1866–1874. 1993.
View Article : Google Scholar
|
|
14
|
Sawa Y, Ueki T, Hata M, Iwasawa K, Tsuruga
E, Kojima H, Ishikawa H and Yoshida S: LPS-induced IL-6, IL-8,
VCAM-1, and ICAM-1 expression in human lymphatic endothelium. J
Histochem Cytochem. 56:97–109. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Joyce DE and Grinnell BW: Recombinant
human activated protein C attenuates the inflammatory response in
endothelium and monocytes by modulating nuclear factor-kappaB. Crit
care med. 20(Suppl 5): S288–S293. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Rossi D and Zlotnik A: The biology of
chemokines and their receptors. Annu Rev Immunol. 18:217–242. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yang EJ, Choi E, Ko J, Kim DH, Lee JS and
Kim IS: Differential effect of CCL2 on constitutive neutrophil
apoptosis between normal and asthmatic subjects. J Cell Physiol.
227:2567–2577. 2012. View Article : Google Scholar
|
|
18
|
Hirano T: Interleukin 6 and its receptor:
ten years later. Int Rev Immunol. 16:249–284. 1998.PubMed/NCBI
|
|
19
|
Gabay C: Interleukin-6 and chronic
inflammation. Arthritis Res Ther. 8(Suppl 2): S32006. View Article : Google Scholar
|
|
20
|
Bellanti JA: Cytokines and allergic
diseases: clinical aspects. Allergy Asthma Proc. 19:337–341. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Viatour P, Merville MP, Bours V and
Chariot A: Phosphorylation of NF-kappaB and IkappaB proteins:
implications in cancer and inflammation. Trends Biochem Sci.
30:43–52. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Arora P, Ricks TK and Trejo J:
Protease-activated receptor signalling, endocytic sorting and
dysregulation in cancer. J Cell Sci. 120:921–928. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Hauck G: Proceedings: Vitalmicroscopic
investigations of the effects of thrombin, a snake venom enzyme and
histamine effect on the mesenteric microvasculature of rabbit and
cat. Arzneimittelforschung. 26:12331976.
|
|
24
|
Wolz RL and Bond JS:
Phe5(4-nitro)-bradykinin: a chromogenic substrate for assay and
kinetics of the metalloendopeptidase meprin. Anal Biochem.
191:314–320. 1990. View Article : Google Scholar : PubMed/NCBI
|