Introduction
Inflammation is a complex process involving various
mediators. In particular, nitric oxide (NO) is considered as an
important inflammatory mediator. NO derived from inducible NO
synthase (iNOS) is crucial in airway inflammation (1). Allergic asthma features airway
inflammation, airway hyperresponsiveness (AHR) and mucus
hypersecretion. The development of allergic asthma results in iNOS
overexpression in the airway, which induces asthmatic responses. A
previous study demonstrated that iNOS expression indirectly induced
the activation of T helper (Th)2 lymphocytes to contribute to the
pathophysiological alterations of asthma. The Th2 lymphocytes
produced various Th2 cytokines, including interleukin (IL)-4, IL-5
and IL-13. Th2 cytokines were closely associated with the
development of allergic asthma via the secretion of
allergen-specific immunoglobulin (Ig)E, chemokines, proinflammatory
mediators and chemoattractants (2).
Picrasma quassioides (D.Don) Benn. (PQ) is a
medicinal herb belonging to the family Simaroubaceae. In China, PQ
is used as a traditional herbal medicine for the treatment of
numerous diseases, including diarrhea, dysentery, inflammation,
microbial infection and fever. Previous studies have shown that PQ
has anti-inflammatory, anticancer and anti-oxidant effects in in
vitro and in vivo experiments (3,4). Liu
et al (5) demonstrated that
PQ attenuated inflammatory bowel disease. Additionally, Fan et
al (6) showed that PQ
inhibited the production of inflammatory mediators in
lipopolysaccharide (LPS)-stimulated macrophage cells and an
adjuvant-induced model of arthritis. However, to the best of our
knowledge, no study has investigated the protective effects of PQ
on airway inflammation in an ovalbumin (OVA)-sensitized/challenged
allergic asthma model.
Therefore, the present study investigated the effect
of PQ on airway inflammation using an OVA-sensitized/challenged
allergic asthma murine model by measuring inflammatory cell counts,
Th2 cytokine and IgE levels, and by performing an histological
analysis of the lung tissue of the mice. To investigate the
anti-inflammatory mechanism of PQ, the levels of NO,
proinflammatory cytokines and iNOS expression were evaluated in the
RAW264.7 murine macrophage cell line.
Materials and methods
Cell culture and cell viability
The RAW264.7 (American Type Culture Collection,
Manassas, VA, USA) murine macrophage cell line was maintained in
Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10%
heat-inactivated fetal bovine serum and antibiotic-antimycotic
(Gibco-BRL, Carlsbad, CA, USA), including penicillin, streptomycin
and Fungizone at 37°C in a 5% CO2 and 95% air incubator.
The cell viability following PQ exposure was measured using a
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT;
Amresco, Solon, OH, USA) assay. The RAW264.7 cells were cultured in
96-well plates at a density of 1×104 cells/well. The PQ
extract was obtained from the Plant Extract Bank at Korea Research
Institute of Bioscience and Biotechnology (KRIBB; PB3612.2;
Ochang-eup, Korea). The PQ extract was added to each individual
well at a concentration of 20, 40, 80 or 100 μg/ml, and then the
plates were incubated for 24 h. MTT solution (10 μl) was added to
each well, and the plates were incubated for 4 h at 37°C. Following
incubation, 100 μl dimethylsulfoxide was added to each well for the
solubilization of formazan. The optical density was measured at 570
nm using a microplate reader (Molecular Devices Co., Sunnyvale, CA,
USA).
Determination of NO production
Cells (5×105 cells/ml) were seeded in
96-well plates with phenol red-free DMEM, treated with different
concentrations of PQ (20, 40, 80 and 100 μg/ml) 1 h prior to LPS
treatment and then incubated in the presence of LPS (0.5 μg/ml) for
24 h. The nitrite accumulation in the culture medium was measured
using the Griess Reagent system (Promega Corporation, Madison, WI,
USA). The absorbance at 540 nm was measured using a microplate
reader (Molecular Devices, LLC).
Immunoblot analysis
The cells were treated as described in the previous
sections and then incubated in the presence of LPS (0.5 μg/ml) for
1 or 24 h. The cells were collected by centrifugation, washed twice
with phosphate-buffered saline (PBS), and suspended using CelLytic
MT Mammalian Tissue Lysis/Extraction Reagent (Sigma-Aldrich, St.
Louis, MO, USA) containing protease inhibitors. The protein
concentration was determined using a protein assay reagent (Bio-Rad
Laboratories, Inc., Hercules, CA, USA), according to the
manufacturer’s instructions. Equal quantities of total cellular
protein (30 g) were resolved by 10% sodium dodecyl
sulfate-polyacrylamide gel electrophoresis and transferred to
nitrocellulose membranes. The membranes were incubated with
blocking solution (5% skimmed milk), followed by an overnight
incubation at 4°C with the appropriate primary antibody. The
following primary antibodies and dilutions were used: Polyclonal
anti-β-actin (anti-rabbit, 1:2,000 dilution; Cell Signaling Inc.,
Danvers, MA, USA) and polyclonal anti-iNOS (anti-rabbit, 1:200
dilution; Enzo Life Sciences Inc., Farmingdale, MA, USA). The blots
were washed three times with Tris-buffered saline containing
Tween-20 (TBST) and then incubated with a 1:10,000 dilution of
horseradish peroxidase-conjugated secondary antibody (Jackson
ImmunoResearch Laboratories, Inc., West Grove, PA, USA) for 30 min
at room temperature. The blots were washed three times with TBST,
and then developed using an enhanced chemiluminescence kit (Thermo
Fisher Scientific Inc., San Jose, CA, USA).
Experimental procedure
Specific pathogen-free female BALB/c mice (weight,
19–21 g; age, 6–8 weeks) were purchased from Koatech Co.
(Pyeongtaek, Korea). The mice were divided into five groups (n=7
per group) and housed under standard conditions (temperature,
22±2°C; humidity, 55±5%; 12-h light/dark cycle) with food and water
ad libitum. All experimental procedures were approved by the
Institutional Animal Care and Use Committee of KRIBB. The mice were
divided into five groups (n=7), and allergic asthma was induced by
OVA in four groups. Each mouse was immunized by intraperitoneal
injection of 20 μg OVA emulsified with 2 mg aluminum hydroxide in
200 μl PBS buffer (pH 7.4) on days 0 and 14. On days 21–23, the
mice were forced to inhale a 1% (w/v) OVA solution aerosolized
using an ultrasonic nebulizer (NE-U12; Omron Corp., Tokyo, Japan)
for 1 h. PQ was administered by oral gavage at dose levels of 15
and 30 mg/kg 1 h prior to the OVA challenge. Dexamethasone was used
as the positive control drug, and was administered by oral gavage
at a dose of 3 mg/kg.
Measurement of AHR
Twenty-four hours after the final OVA challenge, the
AHR was measured by the indirect usage of single-chamber,
whole-body plethysmography (Allmedicus Co., Ltd., Seoul, Korea).
Each mouse was placed in a plastic chamber and exposed to
methacholine aerosols at increasing concentrations (12.5–50 mg/ml
in PBS) for 3 min. Following each methacholine challenge, the Penh
values were measured for 3 min.
Measurement of inflammatory cell counts
in bronchoalveolar lavage (BAL) fluid
To obtain BAL fluid, mice were sacrificed 48 h after
the final OVA challenge with an intraperitoneal injection of
phenobarbital (50 mg/kg; Hanlim Pharm., Co., Seoul, Republic of
Korea) and tracheotomy was performed. The trachea was cannulated
and the left bronchi were tied for histopathological examination.
Following instilling ice-cold PBS (0.4 ml) into the lung, BALF was
obtained by three successive aspirations (total volume 1.2 ml) via
tracheal cannulation. The total numbers of inflammatory cells were
determined by counting cells in at least five squares of a
hemocytometer (Reichert, Buffalo, NY, USA) following the exclusion
of dead cells by Trypan blue staining. The differential cell count
of the BAL fluid was performed using Diff-Quik® staining
reagent (B4132-1A; IMEB Inc., San Marcos, CA, USA), according to
the manufacturer’s instructions. Blood was collected from the
inferior vena cava and centrifuged (200 g, 10 min, 4°C). The
supernatant was stored at −70°C.
Measurement of the levels of cytokines in
BAL fluid and IgE in serum
The levels of certain cytokines (IL-4, IL-5 and
IL-13) in the BAL fluid were measured using ELISA kits (R&D
Systems, Minneapolis, MN, USA), according to the manufacturer’s
instructions. The levels of total IgE and OVA-specific IgE in the
serum were measured by ELISAs. Microtiter plates were coated with
anti-IgE antibodies (anti-mouse IgE; 10 g/ml; AbD Serotec, Oxford,
UK) or OVA (Sigma-Aldrich) in PBS-Tween-20, and incubated with the
serum samples. The plates were then washed four times, and 200 μl
o-phenylenediamine dihydrochloride (Sigma-Aldrich) was added to
each well. The plates were incubated for 10 min in the dark and the
absorbance was measured at 450 nm using a microplate reader
(Molecular Devices Co.).
Histopathological analysis of the lung
tissue
The left lung tissue was fixed in 4% (v/v)
paraformaldehyde, embedded in paraffin, sectioned at a thickness of
4 μm, and stained with hematoxylin and eosin solution
(Sigma-Aldrich) or periodic acid-Schiff (PAS) (IMEB Inc.) to
estimate the levels of inflammation and mucus production,
respectively.
Statistical analysis
Data are expressed as the mean ± standard deviation.
The statistical significance was determined using analysis of
variance followed by a multiple comparison test with Dunnet’s
adjustment. P<0.05 was considered to indicate a statistically
significant difference.
Results
PQ reduces the AHR induced by OVA
challenge
The OVA-sensitized/challenged mice exhibited a
significant increase in AHR with the exposure to increasing
concentrations of methacholine compared with that of the normal
controls. The dexamethasone-treated mice showed a significant
reduction in the AHR compared with that of the
OVA-sensitized/challenged mice. The PQ-treated mice also exhibited
a reduced AHR at the 25 and 50 mg/ml methacholine concentrations
compared with that of the OVA-sensitized/challenged mice. These
reductions were similar to the results of the dexamethasone-treated
mice (Fig. 1).
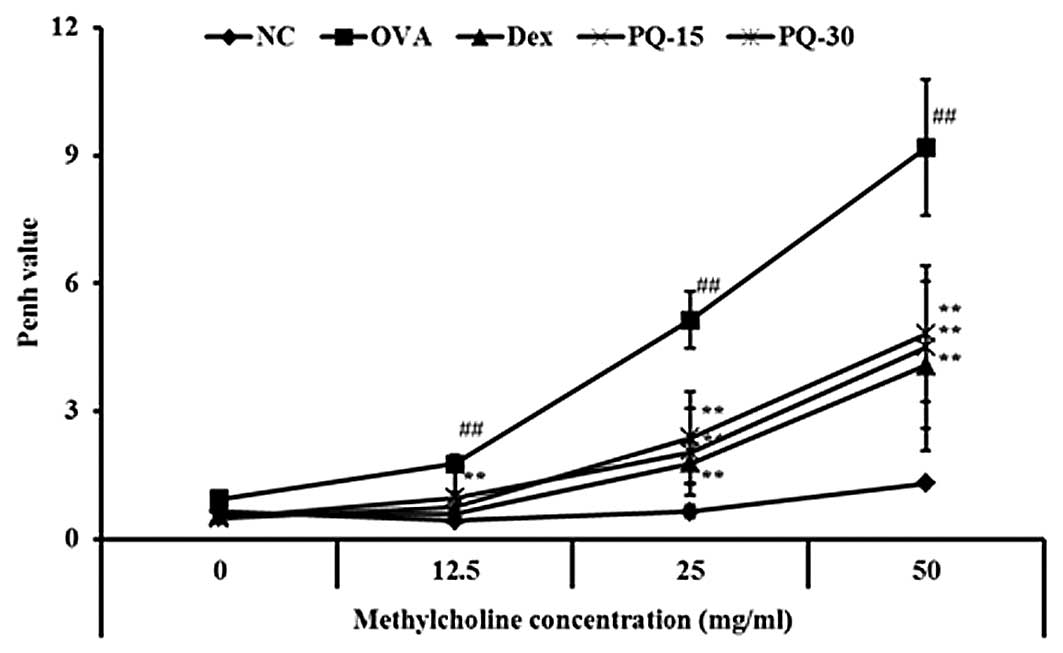 | Figure 1PQ reduced AHR in
OVA-sensitized/challenged mice. AHR was measured 24 h after the
last challenge in mice administered with various concentrations of
methacholine (12.5–50 mg/ml) using single-chamber, whole-body
plethysmography. NC, normal control mice; OVA,
OVA-sensitized/challenged mice; Dex, dexamethasone (3 mg/kg) +
OVA-sensitized/challenged mice; PQ-15, PQ (15 mg/kg) +
OVA-sensitized/challenged mice; PQ-30, PQ (30 mg/kg) +
OVA-sensitized/challenged mice; OVA, ovalbumin; PQ, Picrasma
quassioides; AHR, airway hyperresponsiveness. Values are
expressed as the mean ± standard deviation (n=7 per group).
##P<0.01, compared with NC and
**P<0.01, compared with OVA. |
PQ reduces the number of inflammatory
cells in BAL fluid
The OVA-sensitized/challenged mice exhibited a
significant increase in the number of inflammatory cells, in
particular eosinophils, in the BAL fluid compared with that of the
normal controls. However, the PQ-treated mice showed a markedly
reduced number of inflammatory cells, including eosinophils,
macrophages, lymphocytes and neutrophils, in the BAL fluid compared
with that of the OVA-sensitized/challenged mice (Fig. 2).
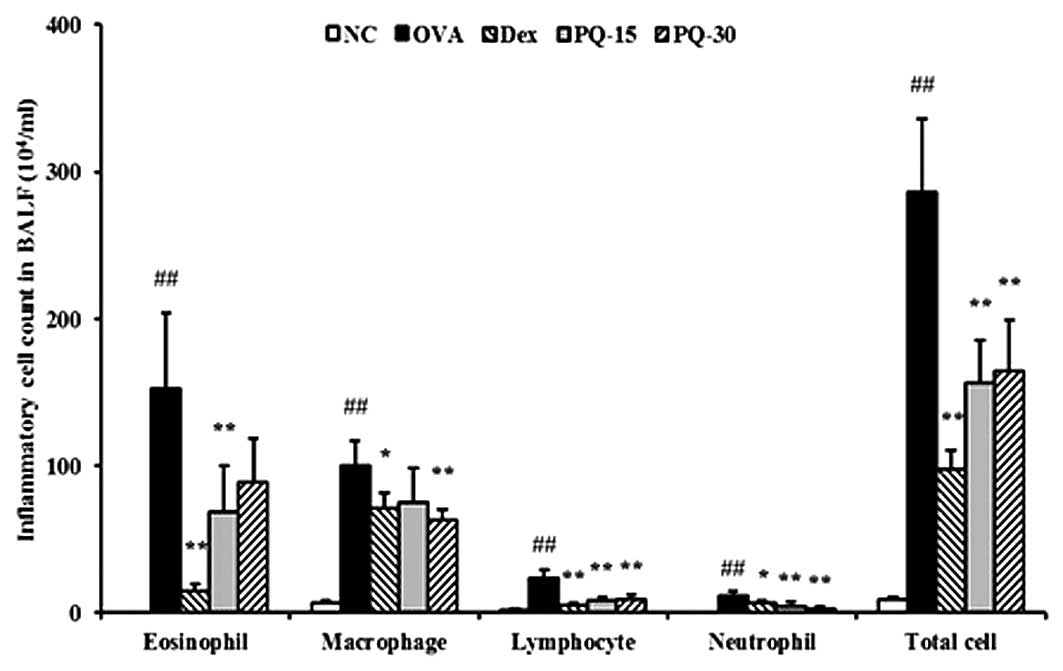 | Figure 2PQ inhibited inflammatory cells in the
BAL fluid of the mice. Cells were isolated by centrifugation and
stained with Diff-Quik staining reagent. NC, normal control mice;
OVA, OVA-sensitized/challenged mice; Dex, dexamethasone (3 mg/kg) +
OVA-sensitized/challenged mice; PQ-15, PQ (15 mg/kg) +
OVA-sensitized/challenged mice; PQ-30, PQ (30 mg/kg) +
OVA-sensitized/challenged mice; BAL, bronchoalveolar lavage; OVA,
ovalbumin; PQ, Picrasma quassioides. Values are expressed as
the mean ± standard deviation (n=7/group). ##P<0.01,
compared with NC; *P<0.05 and **P<0.01,
compared with OVA. |
PQ reduces the levels of proinflammatory
cytokines in BAL fluid and IgE in serum
The levels of IL-4, IL-5 and IL-13 in the BAL fluid
were significantly increased in the OVA-sensitized/challenged mice
compared with those in the normal controls. By contrast, the
administration of PQ treatment induced a significant reduction in
these levels in the BAL fluid compared with those of the OVA
sensitized/challenged mice (Fig.
3A). In addition, the levels of total IgE and OVA-specific IgE
in the serum of the OVA-sensitized/challenged mice were
significantly elevated compared with those in the normal controls.
However, the PQ-treated mice exhibited a significant reduction in
the levels of total IgE and OVA-specific IgE in the serum compared
with those in the OVA-sensitized/challenged mice (Fig. 3B and C).
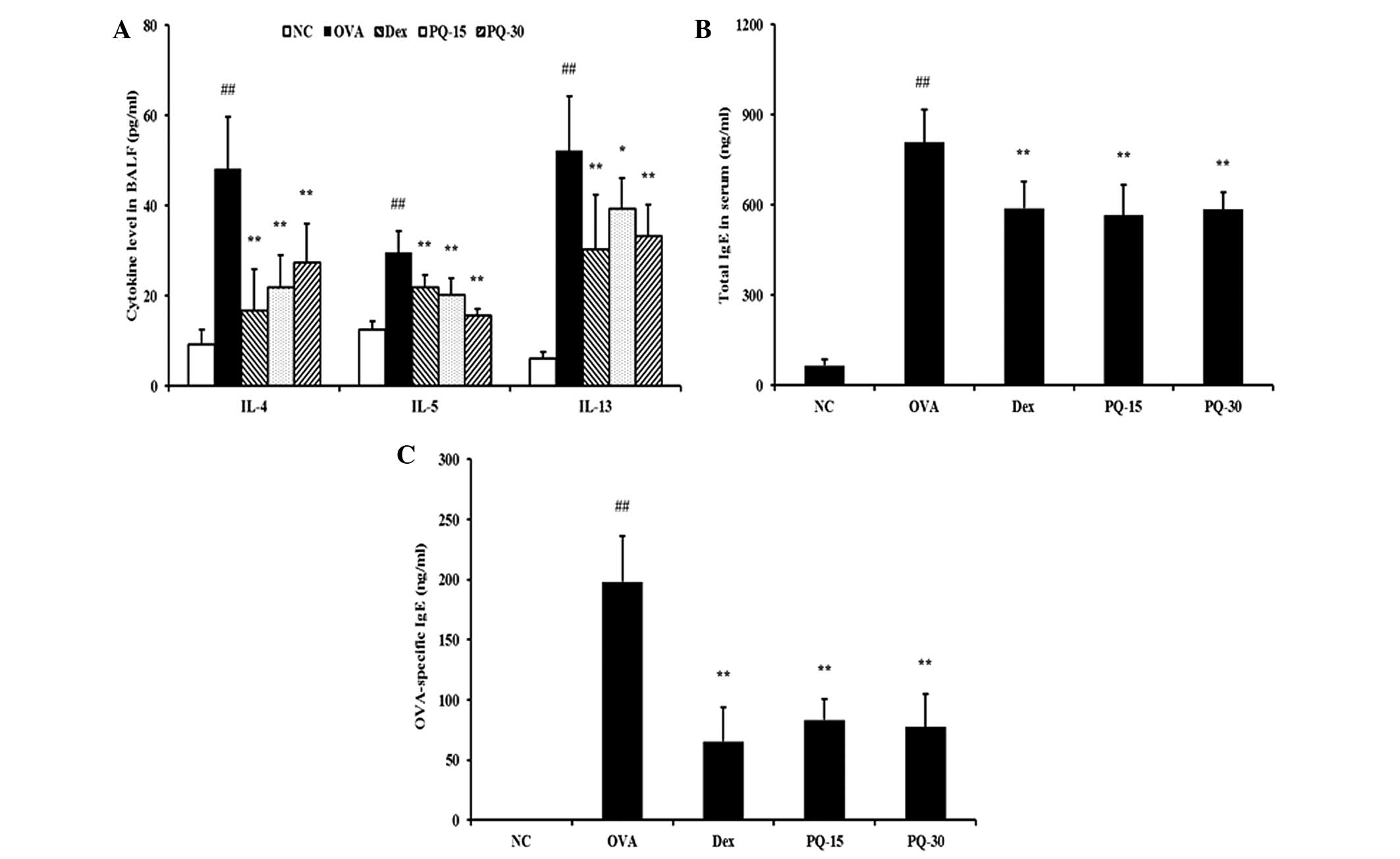 | Figure 3PQ extract inhibited proinflammatory
cytokine and IgE levels. (A) Levels of pro-inflammatory cytokines
in the BAL fluid, (B) total IgE levels in the serum, (C)
OVA-specific IgE levels in the serum determined by ELISA. NC,
normal control mice; OVA, OVA-sensitized/challenged mice; Dex,
dexamethasone (3 mg/kg) + OVA-sensitized/challenged mice; PQ-15, PQ
(15 mg/kg) + OVA-sensitized/challenged mice; PQ-30, PQ (30 mg/kg) +
OVA-sensitized/challenged mice; BAL, bronchoalveolar lavage; OVA,
ovalbumin; PQ, Picrasma quassioides; Ig, immunoglobulin.
Values are expressed as the mean ± standard deviation (n=7/group).
##P<0.01, compared with NC; *P<0.05 and
**P<0.01, compared with OVA. |
PQ decreases the levels of inflammatory
cell infiltration and mucus production
Lung tissue from the OVA-sensitized/challenged mice
exhibited infiltration of inflammatory cells into peribronchial and
perivascular lesions, and mucus hypersecretion from the airway
epithelial cells (Fig. 4). By
contrast, the lung tissue from the PQ-treated mice showed markedly
lower levels of inflammatory cell infiltration and mucus secretion
compared with that from the OVA-sensitized/challenged mice.
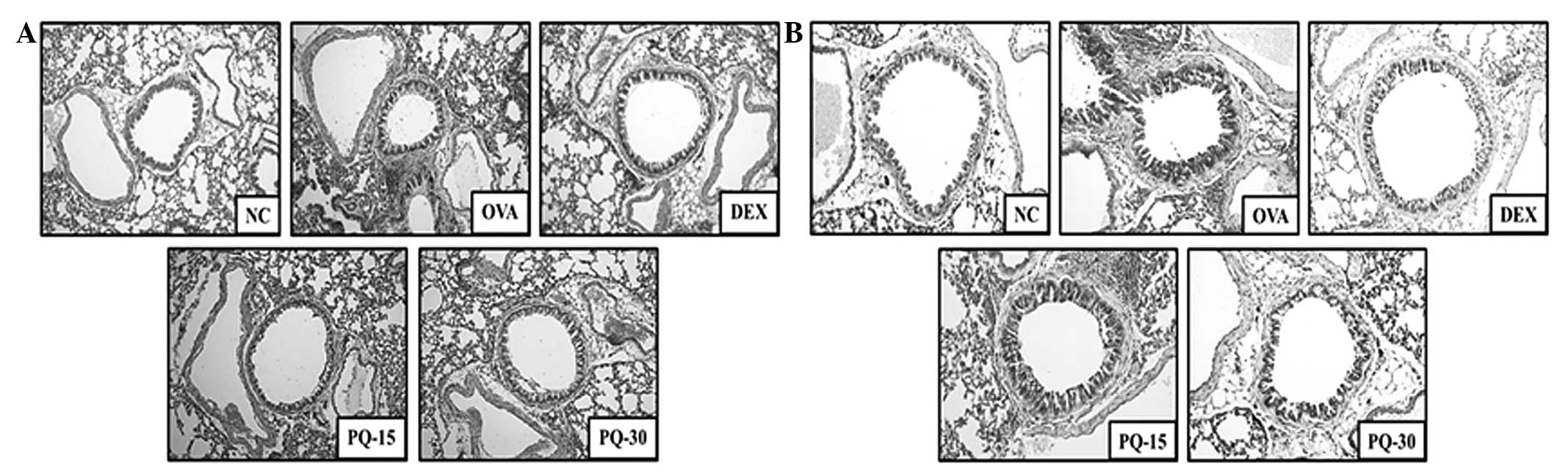 | Figure 4PQ inhibited airway inflammation and
mucus production. (A) Histological examination of airway
inflammation in the lung tissue by H&E staining (magnification,
×200). (B) Histological examination of mucus production in the lung
tissue by PAS staining (magnification, ×200). NC, normal control
mice; OVA, OVA-sensitized/challenged mice; Dex, dexamethasone (3
mg/kg) + OVA-sensitized/challenged mice; PQ-15, PQ (15 mg/kg) +
OVA-sensitized/challenged mice; PQ-30, PQ (30 mg/kg) +
OVA-sensitized/challenged mice; OVA, ovalbumin; PQ, Picrasma
quassioides; H&E, hematoxylin and eosin; PAS, periodic
acid-Schiff. |
PQ inhibits the production of NO in
LPS-stimulated RAW264.7 cells
In this study, nontoxic concentrations of PQ (20,
40, 80 and 100 μg/ml; Fig. 5A)
were tested for their ability to inhibit LPS-stimulated NO
production. The LPS-stimulated cells exhibited an increase in the
cellular NO levels compared with those in the non-stimulated cells.
However, the cells pretreated with PQ showed inhibition of the
production of NO in a concentration-dependent manner compared with
that in the LPS-stimulated cells (Fig.
5B).
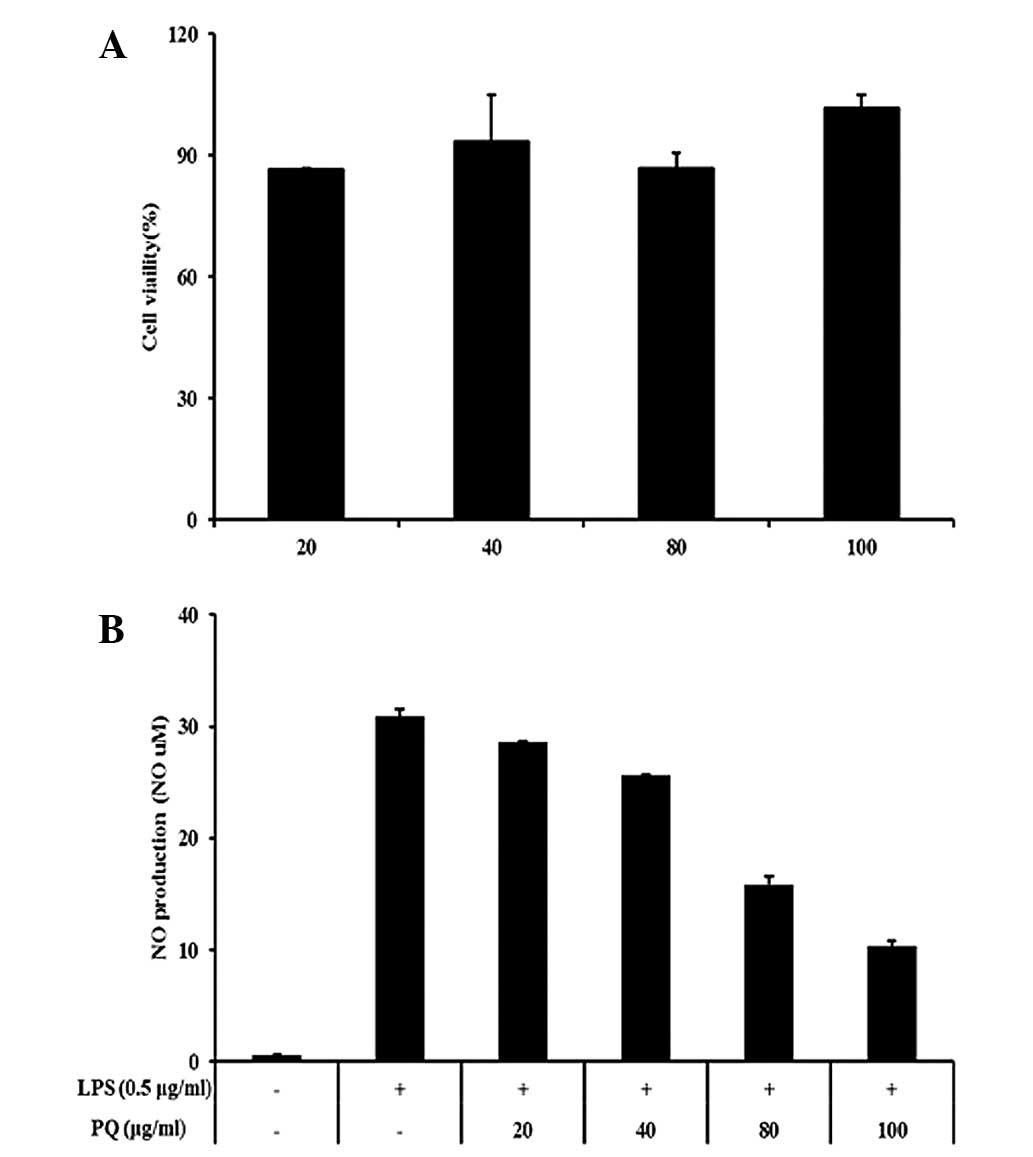 | Figure 5RAW 264.7 cells were treated with 20,
40, 80 or 100 μg/ml PQ for 24 h. (A) Survival rates were tested by
MTT assay. (B) PQ inhibited NO production in the LPS-stimulated RAW
264.7 cells. The cells were induced by PQ (20, 40, 80 or 100 μg/ml)
for 1 h and treated with LPS (0.5 μg/ml), incubation for 24 h.
Values are expressed as the mean ± standard deviation.
##P<0.01, compared with NC; *P<0.05 and
**P<0.01, compared with OVA. NO, nitric oxide; LPS,
lipopolysaccharide; PQ, Picrasma quassioides; MTT,
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide. |
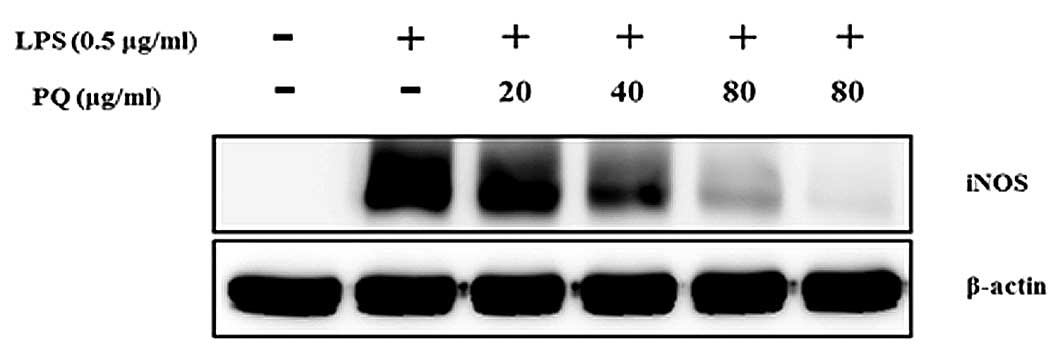
PQ suppresses iNOS expression in
LPS-stimulated RAW264.7 cells
As shown in Fig. 6,
the levels of iNOS protein expression were almost undetectable in
the non-stimulated cells. The LPS-stimulated cells exhibited
increased iNOS protein expression levels compared with those of the
non-stimulated cells. However, the cells pre-treated with PQ showed
an inhibition of the iNOS expression in a concentration-dependent
manner.
Discussion
The present study evaluated the effects of PQ on
inflammatory responses using an OVA-sensitized/challenged allergic
asthma murine model and LPS-stimulated RAW264.7 cells.
Administration of PQ resulted in a significant reduction in the
elevated inflammatory cell counts, AHR, and levels of Th2 cytokines
and IgE induced by the OVA challenge. PQ attenuated the
infiltration of inflammatory cells into the airway and mucus
hypersecretion. In addition, the LPS-stimulated RAW264.7 cells
exhibited significantly reduced levels of NO production when
treated with PQ. Furthermore, PQ inhibited the overexpression of
iNOS caused by LPS treatment.
Airway inflammation is an important feature of
allergic asthma. In the initiation or development of airway
inflammation, Th2 cytokines are central in causing the release of
various inflammatory mediators, including IL-4, IL-5 and IL-13
(7–10). These cytokines are involved in the
maturation and activation of eosinophils, B-cell isotype switching
and the release of chemokines and chemoattractants, which result in
the phenotypes of allergic asthma, including airway inflammation,
AHR and airway remodeling (11–14).
In the present study, PQ reduced the number of inflammatory cells,
AHR and the levels of IgE as a result of the reduction in the
levels of IL-4, IL-5 and IL-13. These finding indicate that PQ
effectively suppressed airway inflammation via the downregulation
of Th2 cytokines. These results were consistent with the
histological analysis of the lung tissue in the present study. The
lung tissue from the PQ-treated mice exhibited a reduction in the
recruitment of inflammatory cells into the airway and mucus
production in the airway compared with those in the
OVA-sensitized/challenged mice.
NO, an important inflammatory mediator, is a gaseous
molecule synthesized by NOS that has numerous detrimental
functions. NOS has three isoforms, constitutive NOS, endothelial
NOS and iNOS. Of the NOS isoforms, iNOS is closely associated with
the production of NO (15). A
previous study has demonstrated that overproduction of NO acts as a
crucial mediator of airway inflammation in allergic asthma
(16). NO contributes to the
infiltration of inflammatory cells, including neutrophils,
macrophages and eosinophils (8).
In addition, NO is a potent stimulator of the Th2 responses of
allergic asthma. According to a previous study, increased NO
production induced by iNOS aggravated the allergic asthmatic
responses, including airway inflammation and AHR, via the elevation
of the levels of Th2 cytokine release. In a previous study, iNOS
knockout mice exhibited a reduction in NO production, resulting in
a decrease in the Th2 cytokine levels in an allergic asthma model
(1). Therefore, numerous
anti-inflammatory materials have been developed that focus on the
suppression of iNOS expression. A number of natural products have
been investigated for their anti-inflammatory effects using
numerous experimental models (3–6). In
the present study, PQ inhibited the elevated production of NO in
LPS-stimulated RAW264.7 cells in a concentration-dependent manner.
PQ also suppressed the overexpression of iNOS induced by LPS
treatment. These results indicate that PQ effectively inhibits
inflammatory responses via the suppression of iNOS expression.
Numerous oriental medicine practitioners have
administered herbal treatments to patients to cure various diseases
and PQ has been used as a traditional herbal remedy for several
disorders, particularly inflammatory diseases (5). The present study provided evidence of
the anti-inflammatory effects of PQ using an
OVA-sensitized/challenged allergic asthma murine model and
LPS-stimulated RAW264.7 cells. These effects were consistent with
the results of a previous study (6). Fan et al (6) demonstrated that PQ possesses
protective effects against carrageenan-induced paw edema,
xylene-induced ear edema and adjuvant-induced arthritis.
In conclusion, PQ effectively suppressed airway
inflammation induced by OVA challenge in the present study. The
anti-inflammatory effects of PQ were considered to be associated
with the suppression of iNOS expression based on the results of the
in vitro experiments. Therefore, this study suggests that PQ
may be a potent therapeutic agent for airway inflammatory diseases,
including allergic asthma.
Acknowledgements
This study was supported by a grant from the Korea
Research Institute of Bioscience and Biotechnology Research
Initiative program (grant no. KGM1221312) of the Republic of
Korea.
References
|
1
|
Prado CM, Martins MA and Tibério IF:
Nitric oxide in asthma physiopathology. ISRN Allergy.
2011:8325602011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Schuijs MJ, Willart MA, Hammad H and
Lambrecht BN: Cytokine targets in airway inflammation. Curr Opin
Pharmacol. 13:351–361. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Jiao WH, Gao H, Zhao F, Lin HW, Pan YM,
Zhou GX and Yao XS: Anti-inflammatory alkaloids form the stems of
Picrasma qussioides BENNET. Chem Pharm Bull (Tokyo).
59:359–364. 2011. View Article : Google Scholar
|
|
4
|
Yin Y, Lee SK and Wang MH: Isolation and
biological activities of an alkaloid compound
(3-methylcanthin-5,6-dione) from Picrasma quassiodes (D.Don)
Benn. Nat Prod Sci. 17:5–9. 2011.
|
|
5
|
Liu JF, Shao M, Zhai DW, Liu K and Wu LJ:
Protective effect of 4-methoxy-5-hyfuoxycanthin-6-one, a natural
alkaloid, on dextran sulfate sodium-induced rat colitis. Planta
Med. 75:142–145. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Fan H, Qi D, Yang M, Fang H, Liu K and
Zhao F: In vitro and in vivo anti-inflammatory effects of
4-methoxy-5-hydroxycanthin-6-one, natural alkaloid from Picrasma
quassioides. Phytomedicine. 20:319–323. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ra J, Lee S, Kim HJ, Jang YP, Ahn H and
Kim J: Bambusae Caulis in Taeniam extract reduces ovalbumin-induced
airway inflammation and T helper 2 responses in mice. J
Ethnopharmacol. 128:241–247. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Nader MA, El-Awady MS, Shalaby AA and
El-Aqamy DS: Sitagliptin exerts anti-inflammatory and anti-allergic
effects in ovalbumin-induced murine model of allergic airway
disease. Naunyn Schmidebergs Arch Pharmacol. 385:909–919. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Lee MY, Shin IS, Jeon WY, Lim HS, Kim JH
and Ha H: Pinellia ternate Breitenbach attenuates
ovalbumin-induced allergic airway inflammation and mucus secretion
in a murine model of asthma. Immunopharmacol Immunotoxicol.
35:410–418. 2013. View Article : Google Scholar
|
|
10
|
Schmudde I, Laumonnier Y and Köhl J:
Anaphylatoxins coordinate innate and adaptive immune responses in
allergic asthma. Semin Immunol. 25:2–11. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Shin IS, Lee MY, Lim HS, Ha H, Seo CS, Kim
JC and Shin HK: An extract of Crataegus pinnatifida fruit
attenuates airway inflammation by modulation of matrix
metalloproteinase-9 in ovalbumin induced asthma. PLoS One.
7:e457342012.PubMed/NCBI
|
|
12
|
Lee YT, Lee SS, Sun HL, Lu KH, Ku MS, Sheu
JN, Ko JL and Lue KH: Effect of the fungal immunomodulatory protein
FIP-fve on airway inflammation and cytokine production in
mouse asthma model. Cytokine. 61:237–244. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Simoes DC, Xanthou G, Petrochilou K,
Panoutsakopoulou V, Roussos C and Gratziou C: Osteopontin
deficiency protects against airway remodeling and
hyperresponsiveness in chronic asthma. Am J Respir Crit Care Med.
179:894–902. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Shen JJ, Chiang MS, Kuo ML, Leu YL, Hwang
TL, Liou CJ and Huang WC: Partially purified extract and viscolin
from Viscum coloratum attenuate airway inflammation and
eosinophil infiltration in ovalbumin-sensitized mice. J
Ethnopharmacol. 135:646–653. 2011.PubMed/NCBI
|
|
15
|
Koarai A, Ichinose M, Sugiura H, Tomaki M,
Watanabe M, Yamagata S, Komaki Y, Shirato K and Hattori T: iNOS
depletion completely diminishes reactive nitrogen-species formation
after an allergic response. Eur Respir J. 20:609–616. 2002.
View Article : Google Scholar
|
|
16
|
Deshane J, Zmijewski JW, Luther R, Gaggar
A, Deshane R, Lai JF, Xu X, Spell M, Estell K, Weaver CT, Abraham
E, Schwiebert LM and Chaplin DD: Free radical-producing
myeloid-derived regulatory cells: potent activators and suppressors
of lung inflammation and airway hyperresponsiveness. Mucosal
Immunol. 4:503–518. 2011. View Article : Google Scholar
|