Introduction
Pelvic organ prolapse (POP) is the displacement of
pelvic organs caused by various types of damage to the pelvic floor
fascia and ligaments, which have failed to fully recover, or by
weakened supporting structures, due to hypotonia. The predominant
clinical manifestations are anterior and/or posterior vaginal wall
prolapse as well as uterine prolapse (1). POP, a pelvic floor dysfunction (PFD)
disorder, is a global health problem that affects 50% of parous
females. It contributes to reduced quality of life and is a major
reason for gynecological surgery in aging females (2). The pelvic organs are supported by
pelvic floor muscles, the bony pelvis, ligaments and fascial
supports. Abnormalities in the connective tissues of the pelvic
support system have been suggested to contribute to the genesis of
POP (3,4). The exact pathophysiology and natural
history of POP, however, are not fully understood.
The fascia and ligaments in the bladder and urethra
are mainly composed of connective tissue, and the main structural
protein is type I collagen-a heterotrimer, comprising two α-1 and
single α-2 chains encoded by the genes COL1A1 and COL1A2,
respectively (5). Collagen is also
a structural protein in the extracellular matrix (ECM) and is
responsible for the integrity of the pelvic floor structure
(6). Recent studies have
demonstrated that the content, components and ultrastructure of
collagen are modified in the pelvic floor structure of POP patients
(7). Abnormalities in the
ultrastructure and biochemical properties of collagenous fibers
have also been indicated in the occurrence of PFD. A reduction in
the collagen content has been shown to be due to an increase in
collagen decomposition but not to a reduction in collagen
synthesis. Therefore, the accelerating degradation of collagen
accounts for the reduction of total collagen content in PFD
patients (8).
Matrix metalloproteinases (MMPs) and tissue
inhibitors of metalloproteinase (TIMPs) are two important enzymes
capable of effectively regulating collagen metabolism and other ECM
degradation. MMPs are secreted as inactive pre-enzymes and are
transformed into active forms following cleavage of a propeptide
domain in the molecule (9). Among
>20 human MMPs, MMP-1 – 3 and -9 are the key enzymes resulting
in weak ductility and lack of integrity in the collagen tissues
(10). TIMPs combine with the
relevant MMP pre-enzymes and enzymes in active form to inhibit the
production and activation of MMPs, as well as the degradation of
collagen. Furthermore, an imbalance between MMPs and TIMPs in ECM
has been reported to be closely associated with collagen metabolism
(11).
The expression and involvement of several MMPs and
TIMPs in POP patients have been determined in several studies
(12–14). However, the studies have produced
conflicting results regarding the contribution of MMPs and TIMPs to
the clinicopathological findings and prognosis of POP patients. In
the present study, immunohistochemistry and quantitative polymerase
chain reaction (qPCR) were employed to detect the expression levels
of MMP-1–3 and -9 as well as TIMP protein and mRNA in vaginal wall
tissues from POP patients and control subjects, and the
correlations among these expression levels were examined. This may
aid in the elucidation of the molecular mechanisms of the
involvement of MMPs and TIMP in POP, and also provide an underlying
theoretical basis for the etiology, and the prevention and
treatment of POP.
Materials and methods
Sample collection
The present study was reviewed and approved by the
Human Ethics Committees Review Board at Fujian University of
Traditional Chinese Medicine (Fuzhou, China) with written informed
consent obtained from each respondent.
The subjects were 72 POP patients who received
surgical treatment at the People’s Affiliated Hospital of Fujian
University of Traditional Chinese Medicine between September 2011
and September 2012. All prolapse cases were diagnosed and recorded
according to the pelvic organ prolapse quantification score issued
by the International Temperance Association in 1996. A total of 72
non-POP cases that underwent hysterectomy during the same period
due to benign tumors served as a control group. All respondents
were from the Fuzhou district, but of different socioeconomic
backgrounds. All respondents met the following criteria: The
respondents agreed to be involved in this study; the respondents
were able to understand and complete the assessment scales
correctly, and fill out questionnaires; and the respondents were
neither taking hormonal drugs nor suffering from a functional
ovarian tumor within the last three months. The patients had not
undergone prior vaginal surgery. Patients with cancer,
endometriosis, gynecological inflammation, connective tissue
disease or other diseases associated with MMPs, in addition to
those who had previously given birth by caesarian, were excluded
from the present study.
Immunohistochemistry
The anterior vaginal wall tissues from the POP
patients and the control group were cut into 5-μm sections, placed
on glass slides and fixed with 4% paraformaldehyde. The slides were
then stained using an avidin-biotin complex (Thermo Fisher
Scientific, Waltham, MA, USA)method. The sections were incubated
with primary antibodies as follows: MMP-1, 1:50, overnight at room
temperature; MMP-2, 1:50, 60 min at room temperature; MMP-3, 1:50,
60 min at room temperature; MMP-9, 1:100, 60 min at room
temperature; and TIMP-1, 1:100, 60 min at room temperature (all
antibodies were purchased from Thermo Fisher Scientific).
Subsequent to washing with phosphate-buffered saline,
biotin-labeled link secondary antibodies (1:1,000; Thermo Fisher
Scientific) and streptavidin-biotin peroxidase were applied using a
labeled streptavidin-biotin (LSAB) kit (Dako, Carpinteria, CA,
USA). Bound peroxidase was detected by diaminobenzidine. A brown
reaction product indicated a positive reaction for antibody
staining.
RNA isolation and quality inspection
The anterior vaginal wall tissues from POP patients
and the control group were collected during surgery. Subsequent to
removal from the body, the tissue samples were immediately
dissolved with RNAlater Stabilization Reagent (Qiagen, Valencia,
CA, USA) and then placed in a −80°C refrigerator. Total RNA from
each sample was individually isolated using TRIzol (Invitrogen Life
Technologies, Carlsbad, CA, USA) and a miRNeasy mini kit (Qiagen)
according to the manufacturer’s instructions. RNA quality and
quantity were measured with a NanoDrop spectrophotometer (ND-1000;
NanoDrop Technologies, Wilmington, DE, USA), and RNA integrity was
determined by gel electrophoresis imaged by Chemi Doc XRS + gel
imaging system (Bio-Rad, Hercules, CA, USA).
qPCR analysis
The expression levels of MMP and TIMP mRNA were
analyzed using a SYBR-based qPCR method. Briefly, 100 ng total RNA
was reverse-transcribed to cDNA with gene-specific stem-loop RT
primers in a Veriti Thermal Cycler Detector (Applied Biosystems,
Foster City, CA, USA). qPCR was performed using Thunderbird SYBR
qPCR mix (Toyobo Corporation, Osaka, Japan), according to the
manufacturer’s instructions, in a Mastercycler® ep
Realplex PCR instrument (Eppendorf, Hamburg, Germany). The MMP and
TIMP mRNA levels were normalized to those of U6, which served as an
internal control. The relative abundance of each mRNA was
calculated using the comparative Ct (2−ΔΔCt) method and
the results were assessed by t-test. The primers are listed in
Table I.
 | Table IPrimer sequences for polymerase chain
reaction. |
Table I
Primer sequences for polymerase chain
reaction.
| Primer | Sequence | Product Length
(bp) |
|---|
| MMP-1-F |
GGTGATGAAGCAGCCCAGATG | 187 |
| MMP-1-R |
CAGAGGTGTGACATTACTCCAGAG | |
| MMP-2-F |
CGCAGTGACGGAAAGATGTGG | 198 |
| MMP-2-R |
CAGACGGAAGTTCTTGGTGTAGG | |
| MMP-3-F |
ATGAACAATGGACAAAGGATACAACAG | 165 |
| MMP-3-R |
CATCTTGAGACAGGCGGAACC | |
| MMP-9-F |
ACGCCGCTCACCTTCACTC | 182 |
| MMP-9-R |
AGGGACCACAACTCGTCATCG | |
| TIMP-1-F |
CTGGCTTCTGGCATCCTGTTG | 162 |
| TIMP-1-R |
ACGCTGGTATAAGGTGGTCTGG | |
Statistical analysis
Comparisons of two-group parameters were performed
using Student’s t-test. Comparisons of multiple group data were
performed using one-way analysis of variance followed by Turkey’s
post-hoc test. P<0.05 was considered to indicate a statistically
significant difference. Statistical analysis was performed using
the SPSS statistical package, version 19.0 (IBM SPSS, Armonk, NY,
USA).
Results
Clinical characteristics of patients
The vaginal wall tissues from the 72 female POP
patients and 72 female control subjects were collected and
analyzed. The median ages of the POP patients and the control group
were both 59 years. The average number of deliveries of POP
patients was 2.24±0.617, whereas that of the control group was
2.29±0.615. In the POP group, 12 of 72 patients had entered
menopause and in the control group, 11 of 72 patients were
menopausal. No statistical differences were identified in age,
numbers of deliveries or menopausal status between the two groups
(P>0.05, Table II).
 | Table IIClinical data of the POP group and
control groups. |
Table II
Clinical data of the POP group and
control groups.
| POP group (n=72) | Control group
(n=72) | Mann-Whitney test
P-value |
|---|
| Age, years | 59.01±7.657 | 59.53±8.962 | 0.540 |
| Deliveries, n | 2.24±0.617 | 2.29±0.615 | 0.651 |
| Menopausal status, n
(%) | 12 (16.67%) | 11 (15.28%) | 0.820 |
Expression levels of MMPs and TIMP-1
proteins evaluated by immunohistochemistry
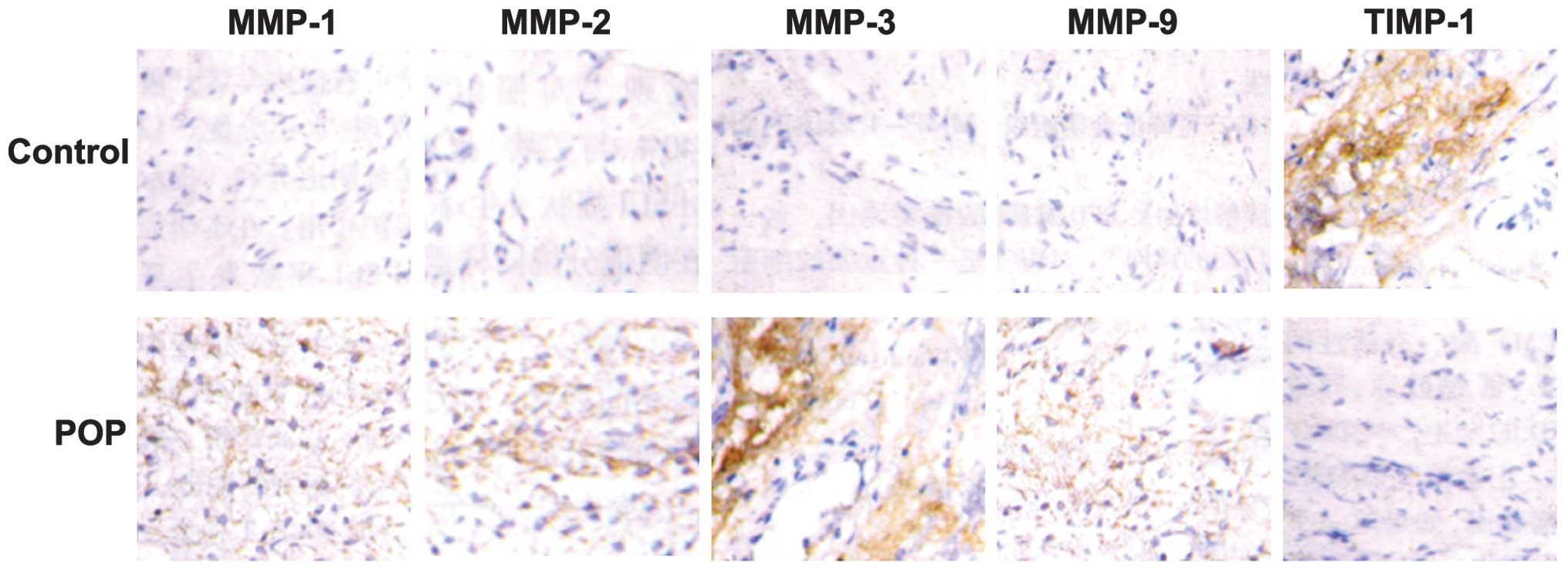
Fig. 1 shows the
intensity and the extent of MMP and TIMP-1 expression in the
vaginal tissues from POP and control patients. MMPs and TIMPs
exhibited cytoplasmic immunoreactivity in all tissue samples, but
the staining intensities of the MMPs (MMP-1, 2, 3 and 9) in the POP
group were significantly higher than those in the control group
(P<0.05). By contrast, the staining intensity of TIMP-1 in the
POP group was significantly lower than that in the control group
(P<0.05).
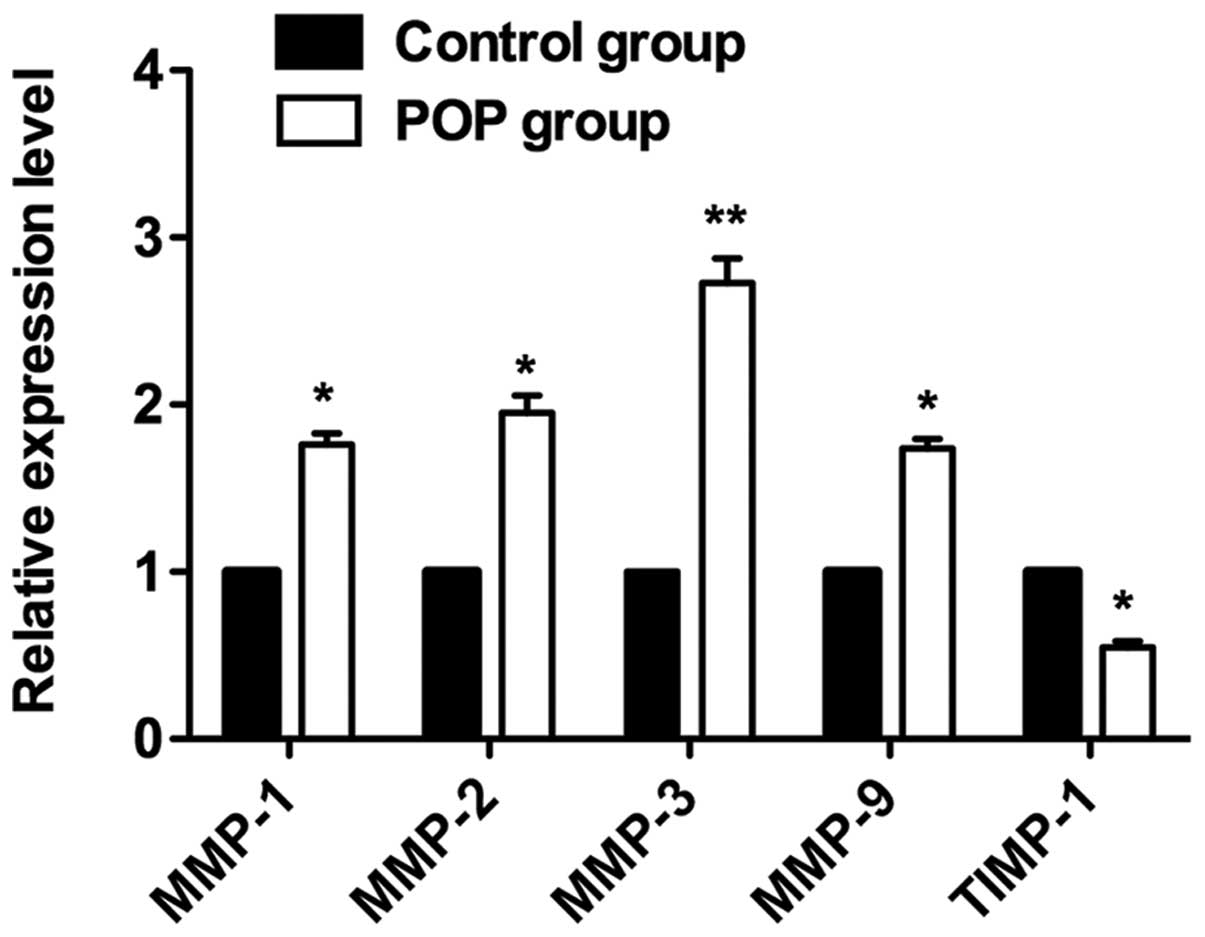
Expression levels of MMP and TIMP-1 mRNA
analyzed by qPCR
To validate the immunohistochemistry results, a qPCR
assay was performed to analyze mRNA expression levels in the tissue
samples. MMP and TIMP mRNA expression was detected in all vaginal
tissue samples, although the expression levels varied among cases.
The relative expression levels of MMP-1, -2, -3 and -9 mRNA in the
vaginal tissues of the POP group were significantly higher than
those of the control group (Fig.
2, P<0.05 and P<0.005). By contrast, the relative
expression levels of TIPM-1 mRNA in the POP group were
significantly lower than those in the control group
(P<0.05).
Correlations among gene expression
levels
The results that followed the normal distribution
were then investigated by linear correlational analysis. As shown
in Table III, significant
positive correlations were identified among the expression levels
of MMP-2, -3 and-9. The r correlation coefficients were: 0.865
between MMP-2 and MMP-3, 0.722 between MMP-2 and MMP-9, and 0.934
between MMP-3 and MMP-9. Conversely, significant negative
correlations were detected between the expression levels of TIMP-1
and those of MMP-3 and MMP-9 (r = −0.944 and −0.891, respectively).
However, no significant correlation was identified between MMP-2
and TIMP-1 expression levels (P>0.05). The results that did not
follow the normal distribution were investigated by linear
regression analysis. As shown in Table IV, positive linear regression
correlation was detected between the expression levels of MMP-1 and
those of other MMPs (P<0.05), and negative linear regression
correlation between the expression levels of MMP-1 and those of
TIMP-1 (P<0.05).
 | Table IIILinear correlational analysis among
gene expression levels. |
Table III
Linear correlational analysis among
gene expression levels.
| Gene | MMP-2 (P-value/r
value) | MMP-3 (P-value/r
value) | MMP-9 (P-value/r
value) | TIMP-1 (P-value/r
value) |
|---|
| MMP-2 | 1.000 | 0.006/0.865 | 0.043/0.722 | 0.065/−0.678 |
| MMP-3 | 0.006/0.865 | 1.000 | 0.001/0.934 | 0.000/−0.944 |
| MMP-9 | 0.043/0.722 | 0.001/0.934 | 1.000 | 0.003/−0.891 |
| TIMP-1 | 0.065/−0.678 | 0.000/−0.944 | 0.003/−0.891 | 1.000 |
 | Table IVLinear regression analysis among gene
expression levels. |
Table IV
Linear regression analysis among gene
expression levels.
| MMP-1 | R Square | 95% confidence
interval | Intercept a | Regression
coefficient b |
|---|
| MMP-2 | 0.502 | (−36.282, 4.051) | −16.115 | 1.468 |
| MMP-3 | 0.703 | (−67.843,
−3.047) | −35.445 | 3.613 |
| MMP-9 | 0.504 | (−20.720, 8.820) | −5.950 | 1.219 |
| TIMP-1 | 0.614 | (11.627, 75.209) | 43.418 | −2.907 |
Discussion
POP is a common disorder that affects the health and
the life quality of elderly female patients. The understanding of
pelvic floor disorders has advanced over the last few years. The
etiology of POP is likely to be multifactorial; however, the
biochemical features of the pelvic floor and the possible
connective tissue alterations in patients with POP are not fully
understood.
Collagen fiber is the main constituent of the pelvic
floor fascia and ligaments. Collagen is also the main
organizational structural component connecting the pelvic organs
(6). A number of studies have
reported that collagen is involved in the pathogenesis of POP, and
these studies have mainly focused on the collagen content,
morphology and metabolism (7,8). The
collagen content was considered to be reduced in the pelvic floor
connective tissue of POP patients, with a reduction in the number
of collagen fibers leading to the relaxation of the ligaments,
fascia and other supportive structures, eventually resulting in the
occurrence of POP. Further studies revealed that the collagen
content in POP patients was significantly reduced, while the
procollagen gene expression levels were not affected (15). The reduction in the collagen
content in the pelvic floor tissue is thus considered to be due to
increases in collagenase degradation. Changes in the ultrastructure
and biochemical properties of collagen fibers have been
demonstrated to be involved in the occurrence of POP (16).
MMPs are important enzymes that degrade collagen in
the ECM. TIMPs inhibit the production and activation of MMPs, and
thus inhibit the degradation of collagen. The imbalance between
MMPs and TIMPs in ECM is closely associated with collagen
metabolism (17). MMPs and TIMPs
work together to increase the degradation of collagen in the pelvic
floor connective tissue, leading to a reduction in collagen levels
and the relaxation of the pelvic floor, eventually resulting in the
occurrence of POP. Interstitial collagenase (MMP-1), gelatinase
(MMP-2, MMP-9) and stromelysin (MMP-3) in pelvic tissue have also
been found to be associated with the occurrence of POP. The
expression levels of MMPs were observed to be increased, but the
expression levels of TIMPs were demonstrated to be reduced in
prolapse patients (18). Wu et
al (19) found that MMP-9
expression levels in patients with POP were significantly higher
than those in a control group, indicating that MMP-9 overexpression
may be involved in the occurrence of POP. Strinic et al
(20) have shown that the
expression levels of MMP-1 and MMP-2 in POP patients were also
significantly higher that those in a control group. Skorupski et
al (21) reported that MMP-1
and MMP-3 expression levels in POP patients were markedly higher
than those in healthy controls, suggesting that these levels were
correlated with the incidence of POP. However, Gabriel et al
(12) compared the expression
levels of MMP-1 and MMP-2 in female patients with and without POP
by immunohistochemistry and found that increased MMP-2 expression
levels in the uterosacral ligament were associated with POP, while
no significant difference in MMP-1 expression levels was identified
between females with POP and healthy controls. As collagen
regulatory factors, MMPs have attracted extensive attention.
However, these previous studies observed somewhat conflicting
results with regard to the expression levels of MMPs in POP, and
accordingly, the understanding of the underlying mechanism of POP
occurrence and development is limited.
In analogy to previous studies, in the present
study, the differential expression of MMPs and TIMP-1 protein and
mRNA in the anterior vaginal wall tissues from POP patients and
control subjects was detected using immunohistochemistry and qPCR.
The expression levels of MMP-1, -2, -3 and -9 mRNA and protein in
the POP group were found to be markedly higher than those in the
control group. By contrast, the expression levels of TIMP-1 mRNA
and protein in the POP group were significantly lower than those in
the control group. Correlational analysis revealed a positive
correlation among MMP-2, -3 and -9 expression levels. TIMP-1
expression levels were negatively correlated with those of MMP-3
and -9 (Table III). Conversely,
MMP-1 expression levels exhibited a positive correlation with those
of the other MMPs (MMP-2, -3 and -9), but a negative correlation
with TIMP-1 expression levels (Table
IV). These findings demonstrated that the increased expression
levels of MMPs and the reduced expression levels of TIMPs were
directly associated with the presence of uterine prolapse,
indicating that the differential expression of MMPs and TIMPs is
associated with the occurrence and development of POP. Increased
MMP expression levels and/or reduced TIMP-1 expression levels may
result in a reduction in the collagen content in the vaginal wall
connective tissue, resulting in damage to the pelvic floor
structure, subsequently losing integrity and stability (22).
In conclusion, the expression levels of MMP-1, -2,
-3 and -9 were demonstrated to be markedly increased, while TIMP-1
expression levels were decreased in the vaginal wall tissues from a
POP group as compared with those from a control group. These
findings suggested that the differential expression of MMPs and
TIMPs was involved in the onset and development of POP. An
effective way to treat POP may therefore be through the inhibition
of MMP expression and increasing TIMP expression levels, which may
inhibit the degradation of collagen and increase the local collagen
content, thus enhancing tissue elasticity.
Acknowledgements
This study was supported by the National Natural
Science Foundation of China (grant no. 81102713), the Key Program
of Health Committee of Fujian Province of China (grant no.
wzzz0911) and the Chen Keji Development Fund (grant no.
CKJ2010006).
References
|
1
|
Weber AM and Richter HE: Pelvic organ
prolapse. Obstet Gynecol. 106:615–634. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Goh JT: Biomechanical and biochemical
assessments for pelvic organ prolapse. Curr Opin Obstet Gynecol.
15:391–394. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Suzme R, Yalcin O, Gurdol F, Gungor F and
Bilir A: Connective tissue alterations in women with pelvic organ
prolapse and urinary incontinence. Acta Obstet Gynecol Scand.
86:882–888. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Alperin M and Moalli PA: Remodeling of
vaginal connective tissue in patients with prolapse. Curr Opin
Obstet Gynecol. 18:544–550. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Gelse K, Pöschl E and Aigner T: Collagens
- structure, function, and biosynthesis. Adv Drug Deliv Rev.
55:1531–1546. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ewies AA, Al-Azzawi F and Thompson J:
Changes in extracellular matrix proteins in the cardinal ligaments
of post-menopausal women with or without prolapse: a computerized
immunohistomorphometric analysis. Hum Reprod. 18:2189–2195. 2003.
View Article : Google Scholar
|
|
7
|
Kerkhof MH, Hendriks L and Brölmann HA:
Changes in connective tissue in patients with pelvic organ prolapse
- a review of the current literature. Int Urogynecol J Pelvic Floor
Dysfunct. 20:461–474. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Campeau L, Gorbachinsky I, Badlani GH and
Andersson KE: Pelvic floor disorders: linking genetic risk factors
to biochemical changes. BJU Int. 108:1240–1247. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Carmeliet P, Moons L, Lijnen R, et al:
Urokinase-generated plasmin activates matrix metalloproteinases
during aneurysm formation. Nat Genet. 17:439–444. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Amălinei C, Căruntu ID, Giuşcă SE and
Bălan RA: Matrix metalloproteinases involvement in pathologic
conditions. Rom J Morphol Embryol. 51:215–228. 2010.
|
|
11
|
Bourboulia D and Stetler-Stevenson WG:
Matrix metalloproteinases (MMPs) and tissue inhibitors of
metalloproteinases (TIMPs): Positive and negative regulators in
tumor cell adhesion. Semin Cancer Biol. 20:161–168. 2010.
View Article : Google Scholar
|
|
12
|
Gabriel B, Watermann D, Hancke K, et al:
Increased expression of matrix metalloproteinase 2 in uterosacral
ligaments is associated with pelvic organ prolapse. Int Urogynecol
J Pelvic Floor Dysfunct. 17:478–482. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Jackson S, James M and Abrams P: The
effect of oestradiol on vaginal collagen metabolism in
postmenopausal women with genuine stress incontinence. BJOG.
109:339–344. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Phillips CH, Anthony F, Benyon C and Monga
AK: Collagen metabolism in the uterosacral ligaments and vaginal
skin of women with uterine prolapse. BJOG. 113:39–46. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lammers K, Lince SL, Spath MA, et al:
Pelvic organ prolapse and collagen-associated disorders. Int
Urogynecol J. 23:313–319. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Johanes I, Mihelc E, Sivasankar M and
Ivanisevic A: Morphological properties of collagen fibers in
porcine lamina propria. J Voice. 25:254–257. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Gueders MM, Foidart JM, Noel A and Cataldo
DD: Matrix metalloproteinases (MMPs) and tissue inhibitors of MMPs
in the respiratory tract: potential implications in asthma and
other lung diseases. Eur J Pharmacol. 533:133–144. 2006. View Article : Google Scholar
|
|
18
|
Chen BH, Wen Y, Li H and Polan ML:
Collagen metabolism and turnover in women with stress urinary
incontinence and pelvic prolapse. Int Urogynecol J Pelvic Floor
Dysfunct. 13:80–87. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wu JM, Visco AG, Grass EA, et al: Matrix
metalloproteinase-9 genetic polymorphisms and the risk for advanced
pelvic organ prolapse. Obstet Gynecol. 120:587–593. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Strinic T, Vulic M, Tomic S, et al: Matrix
metalloproteinases-1, -2 expression in uterosacral ligaments from
women with pelvic organ prolapse. Maturitas. 64:132–135. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Skorupski P, Jankiewicz K, Miotła P, et
al: The polymorphisms of the MMP-1 and the MMP-3 genes and the risk
of pelvic organ prolapse. Int Urogynecol J. 24:1033–1038. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Moore CS and Crocker SJ: An alternate
perspective on the roles of TIMPs and MMPs in pathology. Am J
Pathol. 180:12–16. 2012. View Article : Google Scholar : PubMed/NCBI
|