Introduction
Gastric cancer is the fourth most common type of
cancer and the second leading cause of cancer-associated mortality
worldwide (1). Cancer metastasis
is the leading cause of cancer treatment failure and is chiefly
responsible for the poor prognosis of patients with gastric cancer
(2). The biological mechanisms of
metastasis appear to involve a complex array of genetic
alterations, including changes in the expression of adhesion
molecules (such as, integrins and cadherins), proteolytic enzymes
(such as, matrix metalloproteinases) and signaling pathway
components [such as, mitogen-activated protein kinase (MAPK) and
Akt] (3). However, the molecular
mechanisms involved in gastric cancer remain unclear. Therefore,
examining the biological mechanisms underlying gastric cancer
metastasis and developing a novel effective therapeutic target for
gastric cancer treatment remains critical.
Calcium/calmodulin-dependent protein kinase II
(CaMKII) is a multifunctional serine/threonine protein kinase with
wide distribution and multiple substrates in mammalian cells
(4,5). CaMKII is composed of a combination of
four enzyme isoforms (α, β, γ or δ), and each subunit contains a
catalytic domain, a regulatory domain (including an auto-inhibitory
region) and a calmodulin-binding region (6). In resting cells, CaMKII is a
dodecameric holoenzyme that auto-inhibits catalytic activity via
the auto-inhibitory domain. Binding of calcium/calmodulin to CaMKII
relieves its auto-inhibition, resulting in
Ca2+-independent CaMKII activation and phosphorylation
of the Thr 286 site to sustain CaMKII activity (7). CaMKII inhibitors have been used to
investigate CaMKII function, specifically several CaMKII inhibitors
(including chemically synthesized KN-62 and KN-93) and endogenous
inhibitory proteins (e.g. CaMKIINα and CaMKIINβ derived from humans
and mice). KN-62 and KN-93 inhibit CaMKII phosphorylation by
interfering with calcium/calmodulin binding (8,9),
thereby suppressing CaMKII activity. Endogenous inhibitory proteins
CaMKIINα and CaMKIINβ interact with activated CaMKII and directly
inhibit CaMKII activity (10,11).
CaMKII is involved in numerous different
physiological and pathological cellular processes (12,13).
Multiple studies have identified CaMKII to be important for cancer
cell control by regulating cell cycle progression, cellular
apoptosis and proliferation (8–11).
For example, KN-62 and KN-93 induce cancer cell cycle arrest
(8,9). The endogenous inhibitors hCaMKIINα
and hCaMKIINβ suppress tumor cell growth by inducing cell cycle
arrest and apoptosis (10,11). In addition, CaMKII has been
reported to be required for prostate cancer cell survival (14). Accumulating evidence suggests that
CaMKII is involved in the regulation of cell migration (15–19);
specifically, it enhances vascular smooth muscle migration after
injury and stress (15–17). CaMKII is also required for
ghrelin-induced glioma cell migration (18) and ClC-3-induced glioma invasion
(19), suggesting that CaMKII is
associated with cancer cell migration and invasion. However, the
function of CaMKII in gastric cancer cell metastasis remains to be
elucidated.
The present study investigated the function of
CaMKII in gastric cancer cell metastasis and the underlying
molecular mechanisms.
Materials and methods
Reagents and cell culture
KN-62 and PDTC were purchased from Calbiochem (San
Diego, CA, USA). Dimethylsulfoxide was purchased from Sigma-Aldrich
(St. Louis, MO, USA). The primary antibodies against β-actin
(sc-8432; mouse monoclonal IgG1), MMP-9 (sc-12759; mouse monoclonal
IgG1), IKBα (sc-203; rabbit polyclonal IgG), phospho (p)-IKBα
(sc-8404; mouse monoclonal IgG2b), CaMKII (sc-9035; rabbit
polyclonal IgG) and p-CaMKIIα (Thr 286) (sc-12886-R; rabbit
polyclonal IgG) were obtained from Santa Cruz Biotechnology, Inc.
(Santa Cruz, CA, USA). Four human gastric cancer cell lines (MKN28,
GBC-SD, BGC-803 and SGC-7901) were obtained from the American Type
Culture Collection (Manassas, VA, USA). The cells were cultured in
RPMI-1640 (Invitrogen Life Technologies, Carlsbad, CA, USA)
containing 10% fetal bovine serum (FBS) at 37°C in a 5%
CO2 atmosphere.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR) analysis of MMP9 mRNA
expression
Total cellular RNA was extracted using TRIzol
reagent (Invitrogen Life Technologies) according to manufacturer
instructions. Single strand cDNA was synthesized from 2 mg total
RNA with the Superscript II system (Life Technologies, Rockville,
MD, USA) using an oligo(dT)12–18 primer (Invitrogen Life
Technologies). The primer sequences were as follows:
5′-TGTACCGCTATGGTTACACTCG-3′ and 5′-GGCAGGGACAGTTGCTTCT-3′ for
human MMP9; and 5′-TGGAGAAAATCTGGCACCACACC-3′ and
5′-GATGGGCACAGTGTGGGTGACCC-3′ for actin (as a control gene).
Primers were synthesized by Sangon Corporation (Shanghai, China).
The synthesis of cDNA was checked by qRT-PCR using the actin
primers. PCR consisted of denaturing (95°C for 15 sec), annealing
(56°C for 30 sec), and extension (72°C for 30 sec) for 30 cycles
using a PCR system (Applied Biosystems, Foster City, CA, USA).
Tissue processing and protein
extraction
The surgically resected gastric cancer tissues were
collected from 20 gastric cancer patients (including, 10
non-metastatic cancer tissues and 10 metastatic cancer tissues with
lymph nodes or vessels) from the Department of Oncology at
Changzheng Hospital, the Second Military Medical University
(Shanghai, China). The samples were acquired following obtaining
informed consent from the patients. All of the experimental
procedures were approved by the Institute Research Ethics Committee
at the Second Military Medical University. All of the fresh
specimens were washed three times with phosphate-buffered saline
(PBS) and dissected, and the protein was extracted from cell
lysates with cell lysis buffer (Cell Signaling Technology, Inc.,
Boston, MA, USA) according to the manufacturer’s instructions.
Western blot analysis
Protein concentration was measured by the
Bicinchoninic Acid protein assay reagent kit (Pierce, Rockford, IL,
USA). The protein (50 μg) from gastric cancer cells and tissue
samples were resolved with 10% SDS-PAGE and subjected to western
blot analysis. The blots were probed with the specific primary
antibodies (1:1,000) followed by the appropriate horseradish
peroxidase-conjugated secondary antibodies (1:2,000; Cell Signaling
Technology Inc., Beverly, MA, USA). The bands were visualized with
SuperSignal West Femto chemiluminescent reagents (Pierce
Biotechnology, Inc., Rockford, IL, USA).
Construct of constitutively active CaMKII
and cell transfection
Constitutively active CaMKII expression plasmid was
cloned with PCR cloning and mutation (H282 is mutated H282R).
Flag-tagged expression vectors of H282R were constructed and
transfected into BGC-803 using Jetpei (Polyplus Transfection,
Illkirch, France) according to the manufacturer’s instructions.
cDNA encoding constitutively active human CaMKIIα (His282 mutated
to Arg, H282R) was amplified by PCR. Primer were:
5′-GGCATCCCTGCATGGGCAGAC-3′ and 5′-ACGGTGGAGCGGTGCGAGAT-3′. PCR was
performed by denaturing the reaction mixture at 94°C for 2 min,
followed by 32 cycles (1 min at 94°C, 1 min at 58°C and 1 min at
72°C).The amplification product was inserted into the Flag-tagged
expression vectors (Invitrogen Life Technologies), and transfected
into BGC 803 using Jetpei (Polyplus Transfection) according to the
manufacturer’s instructions.
Migration and invasion assay
Transwell polycarbonate membranes and
Matrigel-coated invasion chambers (BD Biosciences, San Jose, CA,
USA) were used to determine the effect of CaMKII on cell migration
and invasion, respectively. The BGC-803 and SGC-7901 cells were
starved with free medium, and 5×104 cells were suspended
in RPMI-1640 medium with KN-62 (10 μm) and added to the upper
chamber. The lower chamber was filled with 500 μl of media
containing 10% FBS. Following 24 h culture, non-migrated or
non-invasive cells were scraped from the upper side of the
Transwell membrane filter inserts with a cotton-tipped swab.
Migrated/invasive cells on the lower side were stained with crystal
violet and counted. The number of migrated/invaded cells were
counted in three independent high powered fields (x20) readings
with a light microscope (DFC420C; Leica Microsystems, Wetzlar,
Germany).
Measurement of MMP-9 production by
ELISA
The production of MMP-9 in the culture supernatants
was quantified with ELISA (R&D Systems, Minneapolis, MN, USA)
according to the manufacturer’s instructions, as described
previously (20).
Nuclear factor (NF)-κB activation
assay
The cells were co-transfected with a mixture of
NF-κB luciferase reporter plasmid, pRL-TK-Renilla-luciferase
plasmid and the appropriate additional constructs or KN-62 for 24
h. Total DNA was equalized with an empty control vector. Luciferase
activity was measured with a dual-luciferase reporter assay system
(Promega Corporation, Madison, WI, USA) according to the
manufacturer’s instructions. The data were normalized for
transfection efficiency by measuring firefly luciferase activity
and comparing it with that of Renilla luciferase.
Statistical analysis
Data are expressed as the mean ± standard deviation
and compared using Student’s t-test. P<0.05 was considered to
indicate a statistically significant difference.
Results
CaMKII promotes gastric cancer cell
migration and invasion
The mechanisms underlying cancer metastasis involve
multiple factors and the alteration of various genes. To
investigate the role of CaMKII on the regulation of gastric cancer
cells, constitutively active CaMKII (H282 mutated to R) expression
plasmid with Flag-tagged H282R, was constructed by PCR. Firstly,
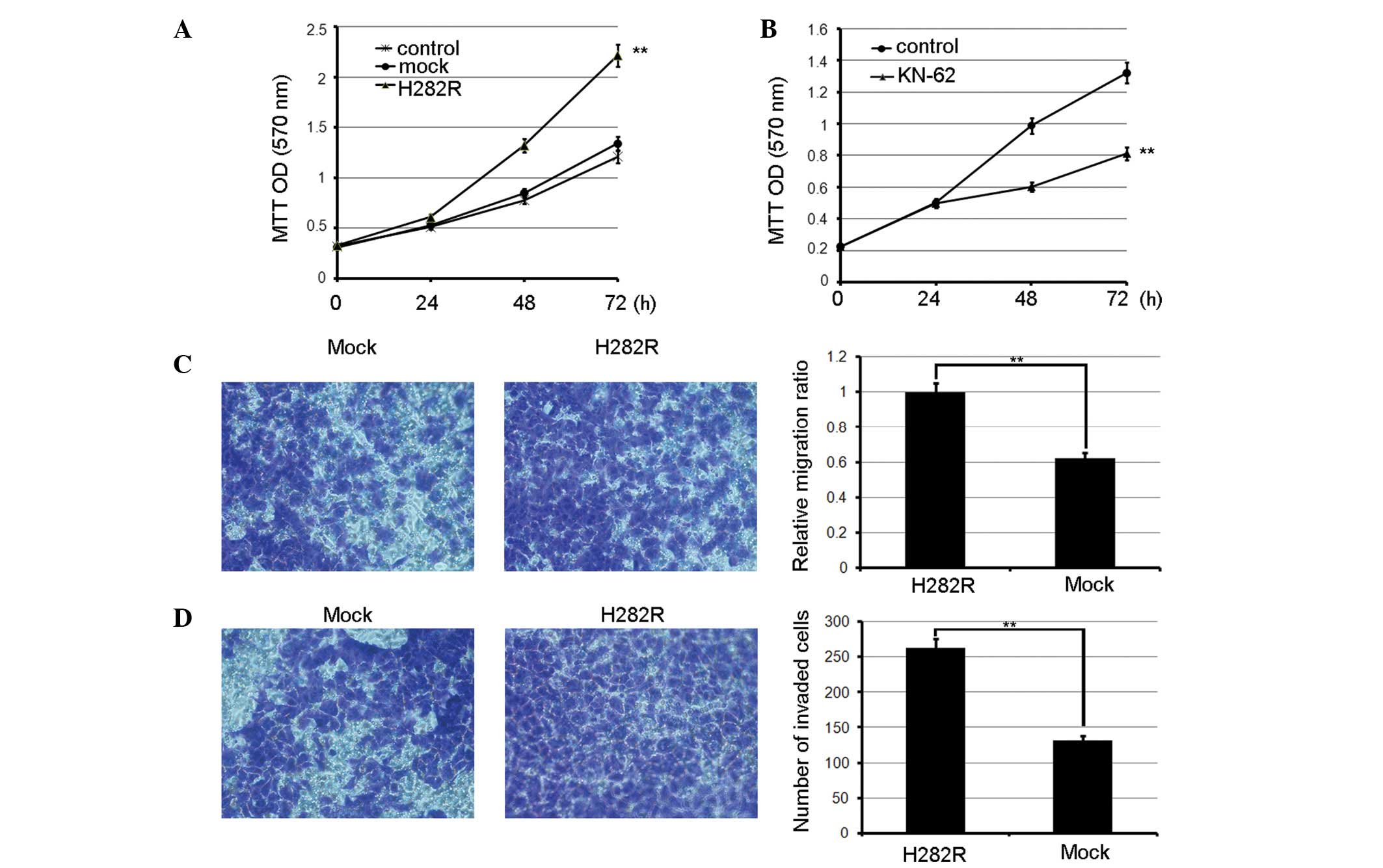
when observing the effects of CaMKII on gastric cancer growth via a
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide assay,
it was noted that H282R overexpression accelerated BGC-803 cell
proliferation compared with the mock transfected cells. Also, the
inhibition of CaMKII activity by KN-62 significantly suppressed
cell growth compared with the control (Fig. 1A and B). Next, to assess the role
of CaMKII in gastric cancer cell invasion and metastasis, it was
observed that greater numbers of BGC-803 cells stably expressing
H282R migrated to the opposite sides of the filters compared with
the control cells treated with KN-62 (Fig. 1C). Similarly, greater numbers of
BGC-803 cells stably expressing H282R invaded the matrigel compared
with the control cells (Fig. 1D).
Therefore, CaMKII was able to promote gastric cancer
metastasis.
CaMKII enhances MMP-9 production of
gastric cancer cells
MMP-9 is known to have a crucial role in cancer cell
metastasis (21,22), thus, it was investigated whether
MMP-9 was involved in CaMKII-mediated promotion of migration and
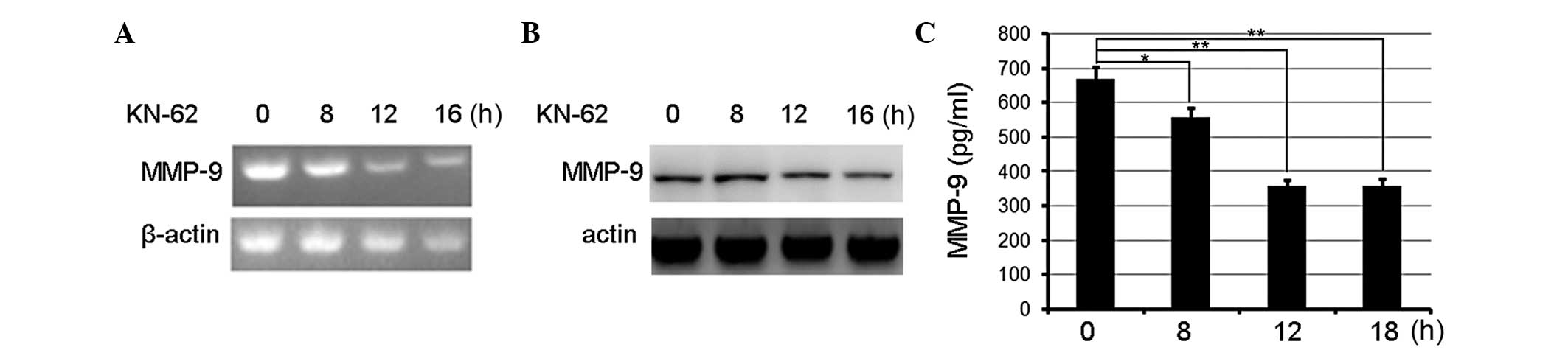
invasiveness. Firstly, the MMP-9 expression in KN-62-treated
gastric cancer cells was measured and it was observed that the
inhibition of CaMKII activity by KN-62 decreased MMP-9 expression
in a time-dependent manner and peaked following treatment for 16 h
as measured by PCR (Fig. 2A).
Immunoblotting assays confirmed that KN-62 decreased the MMP-9
expression at the protein level (Fig.
2B). In addition, MMP-9 was released from KN-62-treated cells
according to ELISA. MMP-9 production was decreased in KN-62-treated
BGC-803 cells compared with the controls (Fig. 2C). Therefore, CaMKII inhibition
suppressed MMP-9 expression and production.
CaMKII enhances NF-κB transcription and
Akt activation, thereby promoting MMP-9 production
Several signaling pathways participate in cancer
metastasis, including MAPK, phosphoinositide 3-phospate (PI3K)-Akt
and NF-κB. CaMKII was recently demonstrated to be a central
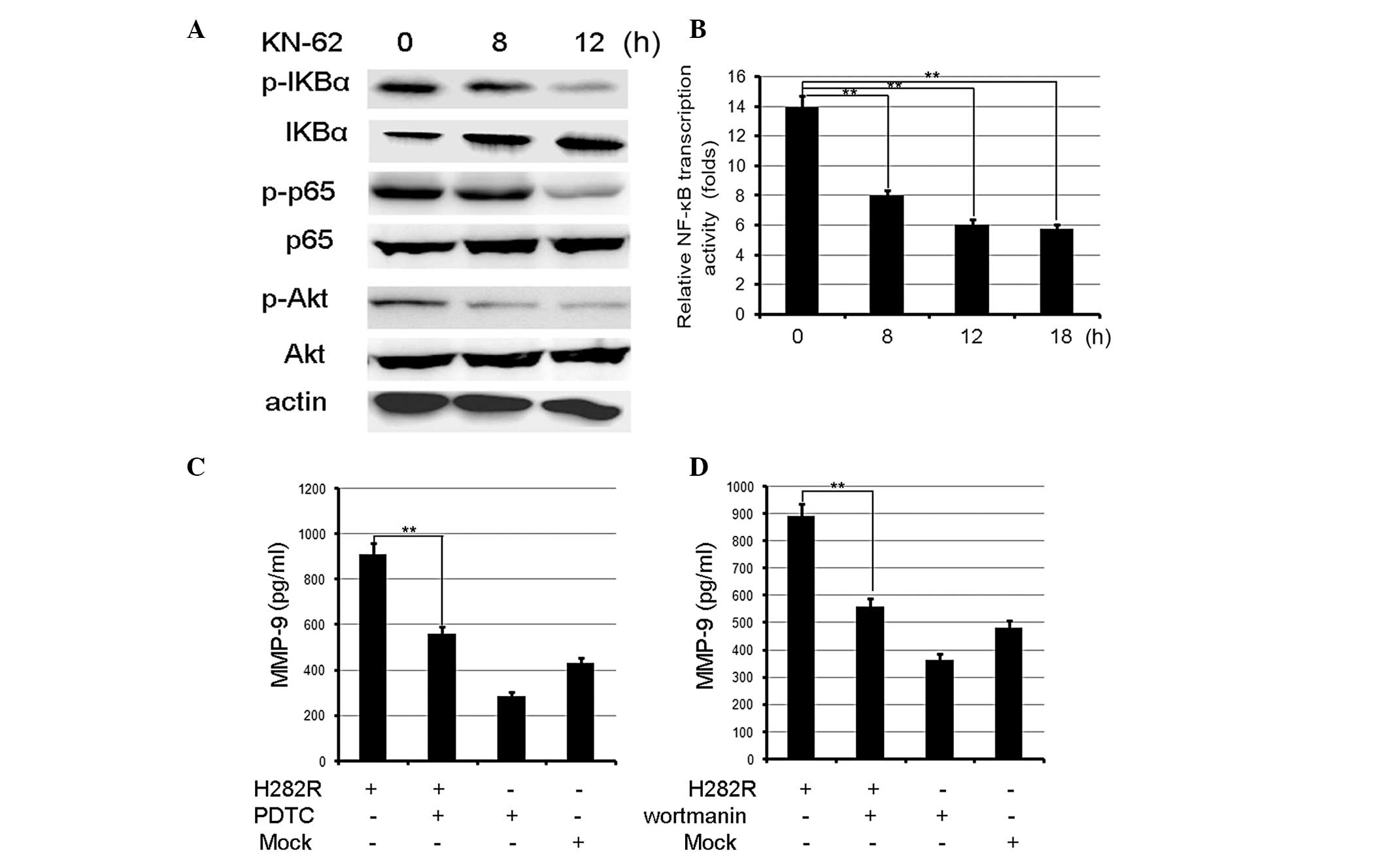
regulator of NF-κB activation in lymphocytes (23). To examine whether CaMKII affects
NF-κB transcription activity in gastric cancer cells,
phosphorylated IKBα, total IKBα and active NF-κB (p-p65) levels
were measured by western blot analysis. As demonstrated in Fig. 3A, KN-62 decreased IKBα and p65
phosphorylation, and increased IKBα in BGC-803 cells. Using a
luciferase reporter assay, it was identified that NF-κB
transcription activation was inhibited by KN-62 (Fig. 3B). In addition, Akt activation was
suppressed in KN-62-treated BGC-803 cells (Fig. 3A). Therefore, CaMKII increased
NF-κB transcription and Akt activation in gastric cancer cells.
NF-κB is a key transcription factor for MMP-9
expression in physiological and pathological conditions (24), it was therefore important to
examine whether NF-κB is involved in CaMKII-mediated upregulation
of MMP-9 expression. It was identified that the overexpression of
H282R increased MMP-9 production, and that PDTC, a specific NF-κB
inhibitor, partly inhibited the H282R-mediated upregulation of
MPP-9 production (Fig. 3C). It was
also investigated whether Akt is involved in CaMKII-mediated
upregulation of MMP-9 expression, it was identified that wortmanin,
a specific Akt inhibitor, decreased H282R-mediated upregulation of
MPP-9 production (Fig. 3D).
Therefore, CaMKII upregulated MMP-9 in gastric cancer cells and
this was dependent on NF-κB and Akt activation.
CaMKII activity was enhanced in
metastatic gastric cancer
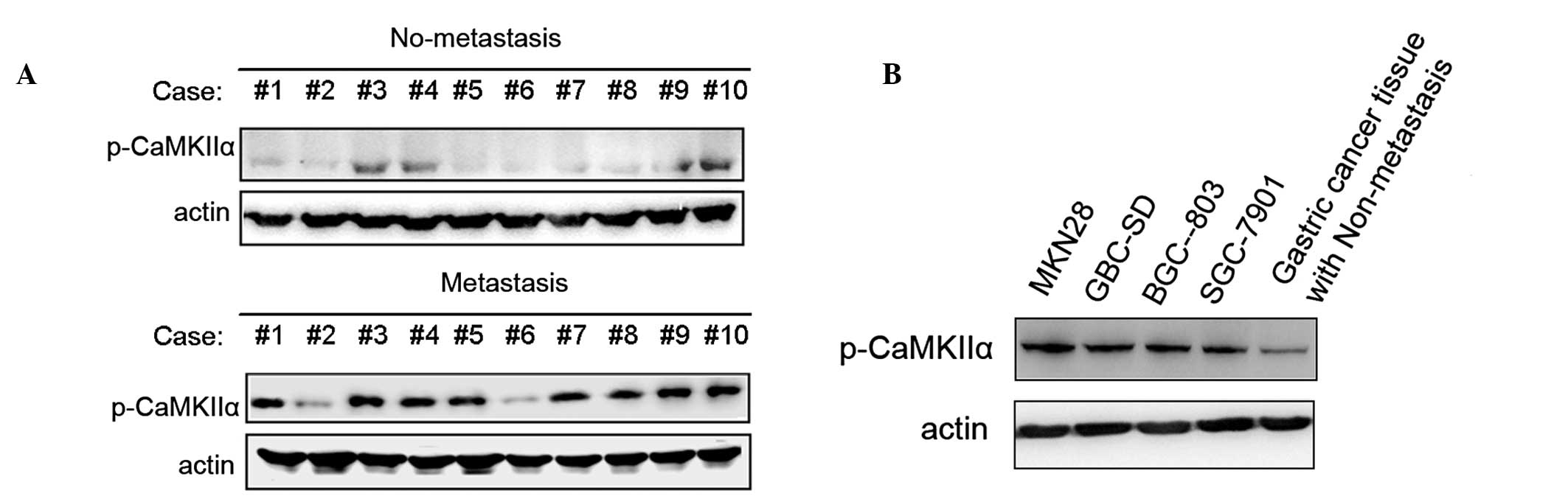
Next, the activation of CaMKII in gastric cancer
tissues was observed. It was noted that 70% of the cases of gastric
cancer with metastasis, including lymph node and/or lymphatic
vessel metastasis, had higher CaMKII phosphorylation (at Thr286)
compared with 20% of the cases of non-metastatic cancer (Fig. 4A). Next, CaMKII phosphorylation in
diverse gastric cancer cell lines, which have greater capability of
invasiveness was examined. The CaMKII phosphorylation was increased
in four higher metastatic gastric cancer cell lines (MNK28, GBC-SD,
BGC-803 and SGC-7901; Fig. 4B).
These findings further confirm that CaMKII may be involved in
gastric cancer metastasis and therefore targeting this kinase may
represent a strategy for preventing gastric cancer metastasis.
Discussion
Cancer metastasis is an obstacle to cancer therapy
and a leading cause of mortality for gastric cancer patients
(2). To overcome and improve
treatment outcomes, understanding the biological mechanisms of
gastric cancer metastasis is required. To the best of our
knowledge, the present study demonstrates for the first time that
CaMKII promotes gastric cancer metastasis by upregulating
NF-κB/Akt-dependent MMP-9 production.
To investigate the molecular mechanisms underlying
pro-gastric cancer metastatic properties of CaMKII, MMP-9, a
classic metastatic-prompting gene implicated in numerous types of
human cancer for its ability to cleave various extracellular matrix
molecules, was examined (25,26).
CaMKII has been reported to regulate MMP-9 activity, as conferred
by the evidence that CaMKII promotes MMP-9 expression in
cardiomyocytes (27) and promotes
vascular smooth muscle migration via the regulation of MMP-9
activity (15). In the present
study, inhibition of CaMKII decreased MMP-9 expression and
production, and the overexpression of H282R or constitutively
activate CaMKII, increased MMP-9 production, which was consistent
with the results of previous studies (15,27).
Furthermore, CaMKII activity was observed to be increased in
samples of metastatic gastric cancer, which also confirms the
pro-metastatic biological function of CaMKII. Therefore, CaMKII
enhances gastric cancer cell metastasis by increasing MMP-9
expression and production.
MMP-9 production is regulated by multiple signaling
pathways (28–30). Previous studies have demonstrated
that NF-κB is a key regulator of MMP-9 (31). The present data indicate that
CaMKII upregulated MMP-9 expression, and inhibition of NF-κB
activation eliminated CaMKII-mediated MMP-9 production. This
suggests that CaMKII increased MMP-9 expression by enhancing NF-κB
activation. A binding site for NF-κB has been reported to be the
promoter of the MMP-9 gene, contributing to MMP-9 expression
(32), a concept that supports the
present findings that NF-κB-dependent CaMKII-mediated MMP-9
expression patterns. In addition, these data demonstrate that
CaMKII-mediated MMP-9 expression was partly dependent on Akt
activation. However, Further studies are required to elucidate the
details of this regulatory mechanism
In conclusion, CaMKII enhanced gastric cancer cell
metastasis by upregulating NF-κB-/Akt-dependent MMP-9 production.
To the best of our knowledge, this is the first study that
demonstrates that CaMKII is able to regulate gastric cancer
metastasis. These data provide the theoretical basis for the
development of CaMKII inhibitors to treat gastric cancer
metastasis.
Acknowledgements
The authors would like to thank Dr Guanzhen Yu
(Department of Pathology, Changzheng Hospital, Shanghai, China) for
providing the gastric cancer specimens and technique assistance.
The authors would also like to thank the Letpub group for providing
language aid. This study was supported by a grants from the
National Natural Science Foundation of China (grant no.
31070789).
Abbreviations:
|
CaMKII
|
calcium/calmodulin-dependent protein
kinase II
|
|
MAPK
|
mitogen-activated protein kinase
|
|
NF-κB
|
nuclear factor-κB
|
|
MMP
|
matrix metalloproteinase
|
|
MTT
|
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide
|
References
|
1
|
Jemal A, Siegel R, Ward E, Hao Y, Xu J,
Murray T and Thun MJ: Cancer statistics. CA Cancer J Clin.
58:71–96. 2008.
|
|
2
|
Ahmad SA, Berman RS and Ellis LM: Biology
of colorectal liver metastases. Surg Oncol Clin N Am. 12:135–150.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Cairns RA, Khokha R and Hill RP: Molecular
mechanisms of tumor invasion and metastasis: an integrated view.
Curr Mol Med. 3:659–671. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hudmon A and Schulman H: Neuronal
Ca2+/calmodulin-dependent protein kinase II: the role of
structure and autoregulation in cellular function. Annu Rev
Biochem. 71:473–510. 2002.
|
|
5
|
Erickson JR, He BJ, Grumbach IM and
Anderson ME: CaMKII in the cardiovascular system: sensing redox
states. Physiol Rev. 91:889–915. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Braun AP and Schulman H: The
multifunctional calcium/ calmodulin-dependent protein kinase: from
form to function. Annu Rev Physiol. 57:417–445. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Coultrap SJ, Buard I, Kulbe JR, Dell’Acqua
ML and Bayer KU: CaMKII autonomy is substrate-dependent and further
stimulated by Ca2+/calmodulin. J Biol Chem.
285:17930–17937. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ducibella T, Schultz RM and Ozil JP: Role
of calcium signals in early development. Semin Stem Cell Dev Biol.
17:324–332. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zayzafoon M: Calcium/calmodulin signaling
controls osteoblast growth and differentiation. J Cell Biochem.
97:56–70. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wang C, Li N, Liu X, Zheng Y and Cao X: A
novel endogenous human CaMKII inhibitory protein suppresses tumor
growth by inducing cell cycle arrest via p27 stabilization. J Biol
Chem. 283:11565–11574. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ma S, Yang Y, Wang C, et al: Endogenous
human CaMKII inhibitory protein suppresses tumor growth by inducing
cell cycle arrest and apoptosis through down-regulation of the
phosphatidylinositide 3-kinase/Akt/HDM2 pathway. J Biol Chem.
284:24773–24782. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Gardoni F, Mauceri D, Marcello E, Sala C,
Di Luca M and Jeromin A: SAP97 directs the localization of Kv4.2 to
spines in hippocampal neurons: regulation by CaMKII. J Biol Chem.
282:28691–28699. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Erickson JR, Joiner ML, Guan X, Kutschke
W, Yang J, Oddis CV, Bartlett RK, Lowe JS, O’Donnell SE,
Aykin-Burns N, et al: A dynamic pathway for calcium-independent
activation of CaMKII by methionine oxidation. Cell. 133:462–474.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Rokhlin OW, Taghiyev AF, Bayer KU, Bumcrot
D, Koteliansk VE, Glover RA and Cohen MB:
Calcium/calmodulin-dependent kinase II plays an important role in
prostate cancer cell survival. Cancer Biol Ther. 6:732–742. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Scott JA, Xie L, Li H, Li W, He JB,
Sanders PN, Carter AB, Backs J, Anderson ME and Grumbach IM: The
multifunctional Ca2+/calmodulin-dependent kinase II
regulates vascular smooth muscle migration through matrix
metalloproteinase 9. Am J Physiol Heart Circ Physiol.
302:H1953–H1964. 2012.
|
|
16
|
Mercure MZ, Ginnan R and Singer HA: CaM
kinase II delta2-dependent regulation of vascular smooth muscle
cell polarization and migration. Am J Physiol Cell Physiol.
294:C1465–C1475. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yang M and Kahn AM: Insulin-inhibited and
stimulated cultured vascular smooth muscle cell migration are
related to divergent effects on protein phosphatase-2A and
autonomous calcium/calmodulin-dependent protein kinase II.
Atherosclerosis. 196:227–233. 2008. View Article : Google Scholar
|
|
18
|
Chen JH, Huang SM, Chen CC, Tsai CF, Yeh
WL, Chou SJ, Hsieh WT and Lu DY: Ghrelin induces cell migration
through GHS-R, CaMKII, AMPK, and NF-κB signaling pathway in glioma
cells. J Cell Biochem. 112:2931–2941. 2011.PubMed/NCBI
|
|
19
|
Cuddapah VA and Sontheimer H: Molecular
interaction and functional regulation of ClC-3 by
Ca2+/calmodulin-dependent protein kinase II (CaMKII) in
human malignant glioma. J Biol Chem. 285:11188–11196. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Asahi M, Asahi K, Jung JC, del Zoppo GJ,
Fini ME and Lo EH: Role for matrix metalloproteinase 9 after focal
cerebral ischemia: effects of gene knockout and enzyme inhibition
with BB-94. J Cereb Blood Flow Metab. 20:1681–1689. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Galis ZS, Sukhova GK, Kranzhöfer R, Clark
S and Libby P: Macrophage foam cells from experimental atheroma
constitutively produce matrix-degrading proteinase. Proc Natl Acad
Sci USA. 92:402–406. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Klein G, Vellenga E, Fraaije MW, Kamps WA
and de Bont ES: The possible role of matrixmetalloproteinase
(MMP)-2 and MMP-9 in cancer, e.g acute leukemia. Crit Rev Oncol
Hematol. 50:87–100. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ishiguro K, Green T, Rapley J, Wachtel H,
Giallourakis C, Landry A, Cao Z, Lu N, Takafumi A, Goto H, Daly MJ
and Xavier RJ: Ca2+/calmodulin-dependent protein kinase
II is a modulator of CARMA1-mediated NF-kappaB activation. Mol Cell
Biol. 26:5497–5508. 2006.PubMed/NCBI
|
|
24
|
Chou YC, Sheu JR, Chung CL, Chen CY, Lin
FL, Hsu MJ, Kuo YH and Hsiao G: Nuclear-targeted inhibition of
NF-kappaB on MMP-9 production by N-2-(4-bromophenyl) ethyl
caffeamide in human monocytic cells. Chem Biol Interact.
184:403–412. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Deryugina EI and Quigley JP: Matrix
metalloproteinases and tumor metastasis. Cancer Metastasis Rev.
25:9–34. 2006. View Article : Google Scholar
|
|
26
|
Egeblad M and Werb Z: New functions for
the matrix metalloproteinases in cancer progression. Nat Rev
Cancer. 2:161–174. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
27
|
He BJ, Joiner ML, Singh MV, et al:
Oxidation of CaMKII determines the cardiotoxic effects of
aldosterone. Nat Med. 17:1610–1618. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Cheng CY, Hsieh HL, Hsiao LD and Yang CM:
PI3-K/Akt/JNK/NF-κB is essential for MMP-9 expression and outgrowth
in human limbal epithelial cells on intact amniotic membrane. Stem
Cell Res. 9:9–23. 2012.PubMed/NCBI
|
|
29
|
Liang KC, Lee CW, Lin WN, Lin CC, Wu CB,
Luo SF and Yang CM: Interleukin-1beta induces MMP-9 expression via
p42/p44 MAPK, p38 MAPK, JNK, and nuclear factor-kappaB signaling
pathways in human tracheal smooth muscle cells. J Cell Physiol.
211:759–770. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Bond M, Chase AJ, Baker AH and Newby AC:
Inhibition of transcription factor NF-kappaB reduces matrix
metalloproteinase-1, -3 and -9 production by vascular smooth muscle
cells. Cardiovasc Res. 50:556–565. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Bond M, Fabunmi RP, Baker AH and Newby AC:
Synergistic upregulation of metalloproteinase-9 by growth factors
and inflammatory cytokines: an absolute requirement for
transcription factor NF-kappa B. FEBS Lett. 435:29–34. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Rhee JW, Lee KW, Kim D, Lee Y, Jeon OH,
Kwon HJ and Kim DS: NF-kappaB-dependent regulation of matrix
metalloproteinase-9 gene expression by lipopolysaccharide in a
macrophage cell line RAW 264.7. J Biochem Mol Biol. 40:88–94. 2007.
View Article : Google Scholar : PubMed/NCBI
|