Introduction
Osteoarthritis (OA) is a prevalent articular disease
in the elderly (1,2), and is characterized by a series of
pathological changes in the structure and function of the joints,
mainly due to a degenerative process that takes place in the
articular cartilage (3). The
chondrocyte is the only cell type present in mature cartilage, and
is responsible for extracellular signaling and the maintenance of
cartilage homeostasis. Changes in chondrocyte function are critical
for the degradation of articular cartilage and serve an important
function in the pathogenesis of OA (4,5).
Several studies have reported that there is reduced proliferative
activity in osteoarthritic chondrocytes and thus promoting
chondrocyte proliferation may be an efficient strategy to treat or
delay the progression of OA (6,7).
Due to the fact that the incidence of knee
osteoarthritis in individuals aged >65 years old is 60–70%, with
the incidence rate reaching 85% in the population of those aged
>75 years (8), OA has had a
major economic and social impact on populations and health-care
systems worldwide (9,10). Although non-steroidal
anti-inflammatory drugs (NSAIDs) have been widely prescribed to
reduce joint pain and stiffness, the inflammatory component of OA
is usually minimal. Thus, the requirement for the anti-inflammatory
effect of NSAIDs in OA is controversial (11). Hyaluronic acid is easily applied by
intra-articular injection; however, it has a short half-life and
repeated intra-articular injections increase the chances of joint
infection (12). Advanced OA is
currently only managed by surgical replacement of the joints,
however, there remain difficulties regarding the degree of
invasion, cost and long-term prognosis (13). These disadvantages call for an
evaluation of the risks and benefits of the therapies for OA and
the requirement for less toxic options. An increasing number
patients suffering from OA turn to complementary and alternative
medicine treatments, including Chinese herbal medicine (14).
Chinese herbal medicine, a major modality in
traditional Chinese medicine (TCM) that has been practiced for
thousands of years in China and other countries in Asia, has
advanced in the treatment of OA, including improving clinical
findings and inhibiting inflammatory reactions and cartilage
degeneration (15,16). In vivo and in vitro
studies have also indicated that Chinese herbal formulas produce
multiple comprehensive effects against OA (17–19).
Duhuo Jisheng Decoction (DHJSD), initially documented in the book
Bei Ji Qian Jin Yao Fang (20), is composed of the following
ingredients: Angelica pubescens; Saposhnikovia
divaricata; Ligusticum chuanxiong; Achyranthes
bidentata; Loranthus parasiticus; Gentiana
macrophylla; Eucommia ulmoides; Angelicae
sinensis; Poria cocos; Codonopsis pilosula; radix
Rehmannia preparata; radix Paeoniae alba; Asarum
sieboldii; Glycyrrhiza uralensis; and Cinnamomum
cassia. It has been widely used for treating OA (21), and a previous study indicated that
DHJSD contains drug- and lead-like compounds with potential synergy
and polypharmacology against OA (22). An in vivo study demonstrated
that treatment with DHJSD promotes the progression of chondrocytes
from G1 to S phase (23), and this may be one of the
mechanisms underlying its use in OA. In addition, previous studies
have reported that chondrocytes treated with IL-1β produce a
particularly effective cell model of the mechanisms involved in
degenerative arthropathies (24).
In order to further elucidate the precise mechanism
of DHJSD in OA, a serum pharmacological method was employed to
investigate its effects on the proliferation of IL-1β-induced
chondrocytes in vitro in the current study.
Materials and methods
Reagents
Fetal bovine serum, Dulbecco’s modified Eagle’s
medium and trypsin were purchased from Hyclone Laboratories, Inc.
(Logan, UT, USA). A cyclin D1 antibody was purchased from Abcam
(Cambridge, MA, USA). Cyclin-dependent kinase 4 (CDK4), Rb and p16
antibodies were obtained from Santa Cruz Biotechnology, Inc. (Santa
Cruz, CA, USA). Anti-collagen type II antibody was obtained from
Merck Millipore (Darmstadt, Germany). The WesternBreeze
Chemiluminescent Immunodetection kit was obtained from Invitrogen
Life Technologies (Carlsbad, CA, USA). Cyclin D1, CDK4, Rb, p16 and
β-actin primers were purchased from Sangon Biotech Co., Ltd.
(Shanghai, China). Table I
displays the primer sequences. IL-1β and collagenase II were
purchased from Sigma-Aldrich (St. Louis, MO, USA).
 | Table IPrimer sequences. |
Table I
Primer sequences.
| Gene | Primer
sequence | Amplicon length
(bp) | Annealing
temperature (°C) |
|---|
| Cyclin D1 | sense, 5′-GAC ACC
AAT CTC CTC AAC GAC-3′
antisense, 5′-AGA CAA GAA ACG GTC CAG GTA G-3′ | 216 | 55 |
| CDK4 | sense, 5′-CCT ACG
GAC ATA CCT GGA CAA-3′
antisense, 5′-GAG GCA ATC CAA TGA GAT CAA-3′ | 404 | 55 |
| Rb | sense, 5′-CTT TAT
TGG CCT GTG CTC TTG-3′
antisense, 5′-ATT CCA TGA TTC GAT GCT CAC-3′ | 225 | 55 |
| p16 | sense, 5′-GCT CTC
CTG CTC TCC TAT GGT-3′
antisense, 5′-AGA AGT TAT GCC TGT CGG TGA-3′ | 268 | 55 |
| β-actin | sense, 5′-GGG AAG
TGC TGG ATA G-3′
antisense, 5′-GTG ATG TTT CGG ATG G-3′ | 453 | 55 |
Animals
A total of 144 healthy Sprague Dawley rats of
average gender (two-month-old, 230–250 g) and 60 Sprague Dawley
rats of either gender (four-week-old, 90–110 g) were purchased from
Shanghai SLAC Laboratory Animal Co., Inc., Shanghai, China and
raised in a sterile environment. Experiments involving the animals
complied with the Guidance Suggestions for the Care and Use of
Laboratory Animals (2006) by the Ministry of Science and
Technology, China (25).
Preparation of DHJSD-containing
serum
The 144 two-month-old rats were randomly divided
into two groups: The DHJSD group (n=108) treated with a dose of 9.3
g/kg/day DHJSD, which is the equivalent dosage used clinically for
humans (26) and the blank group
(n=36) treated with an equivalent dose of saline. All drugs and
saline were administered via gastric gavage twice a day in the
morning and afternoon for 7 consecutive days. The two doses were
given 2 h apart on the seventh day. The animals were anesthetized
by intraperitoneal injection of 2 ml/kg 2% pentobarbital sodium
(Sigma-Aldrich), and arterial blood of the DHJSD group was
collected from the abdominal aorta at 1, 2 and 3 h after the final
dose in the DHJSD group and at 2 h in the blank group. The
collected blood was placed in a 37°C thermostatic water bath for 30
min and centrifuged at 3,000 r/min for 15 min. The serum fraction
was isolated, heat inactivated in a 56°C thermostatic water bath
for 30 min and then filtered through a 0.22 μm filter. The
resulting drug-containing serum was then aliquoted and stored at
−20°C.
Isolation, culture and verification of
chondrocytes
The chondrocytes were isolated, cultured and
verified as previously described (27,28).
The cells used in the current experiments were successfully
verified and counted with a hemocytometer and adjusted to
105 cells/ml.
Determination of chondrocyte viability by
MTT assay
Cell viability was assessed by MTT colorimetric
assay. The second-generation chondrocytes were seeded into 96-well
plates at a density of 1.0×105 cells/ml in 0.1 ml
medium. The cells were treated with a range of concentrations
(10–30%) of 1, 2 and 3-h DHJSD serum for 24, 36, 48, 60 and 72 h.
At the end of the treatment, 100 μl 0.5 mg/ml MTT was added to each
well and the samples were incubated for an additional 4 h at 37°C.
The purple/blue MTT formazan precipitate was dissolved in 100 μl
dimethylsulfoxide (Sigma-Aldrich) and the optical density (OD)
value of each well was measured at 490 nm wavelength using a
microplate reader (BioTek, Winooski, VT, USA).
IL-1β-induced degenerative chondrocyte
model
The degenerative chondrocyte model was established
as previously described (29,30).
Briefly, third generation chondrocytes were exposed to 10 ng/ml
IL-1β for 24 h, and then washed with 1X phosphate-buffered saline
(PBS). The successful establishment of the degenerative chondrocyte
model was verified by optical microscopy and immunohistochemical
analyses.
Observation of cellular morphological
changes
The second generation chondrocytes were seeded into
6-well plates at a density of 1.0×105 cells/ml in 2 ml
medium. The cells were treated with 10 ng/ml IL-1β for 24 h. The
changes in cell morphology were observed using a phase-contrast
microscope (Olympus Corporation, Tokyo, Japan) and images were
captured at a magnification of ×100.
Immunohistochemical assay
The second-generation chondrocytes were cultured on
glass coverslips in 6-well plates at a density of
1.0×105 cells/ml in 2 ml medium. Following treatment
with 10 ng/ml IL-1β for 24 h, the cells were washed with PBS three
times for 5 min and fixed in 4% paraformaldehyde (Sigma-Aldrich)
for 20 min. The antigen retrieval buffer (10 mM sodium citrate; pH
6.0) was preheated to 95°C in a coverglass staining jar placed in a
water bath at 95°C. The coverslips were heated at 95°C for 10 min.
Endogenous peroxidase activity of the sections was quenched by
incubation in PBS containing 3% H2O2 for 10
min following three washes in PBS. Immunohistochemical staining was
performed using the Vectastain Elite ABC kit (Vector Laboratories,
Inc., Burlingame, CA, USA) according to the manufacturer’s
instructions. Briefly, following blocking with normal serum in PBS,
the coverslips were treated with the anti-collagen type II antibody
at a concentration of 1:250 overnight at 4°C. The coverslips were
incubated with a biotinylated anti-rabbit IgG antibody (Cell
Signaling Technology, Inc., Beverly, MA, USA) for 60 min and then
treated with the ABC reagent for 60 min. Next, the cultures were
treated with DAB (Vector Laboratories, Inc.) for 3 min and
subsequently dehydrated with increasing concentrations of ethanol
solutions, cleared with xylene (Sigma-Aldrich) and mounted on a
coverslip using neutral gum.
Cell treatment and grouping
Following IL-1β induction for 24 h, the chondrocytes
were randomly divided into two groups: The DHJSD group and the
blank serum group. After treatment with the appropriate serum, cell
proliferation levels were detected using MTT assay and DNA
staining, followed by fluorescence-activated cell sorting (FACS)
analysis. The mRNA and protein levels of cyclin D1, CDK4, Rb and
p16 were measured by reverse transcription (RT) followed by
semi-quantitative polymerase chain reaction (PCR) analysis and
western blotting, respectively.
Determination of viability of
IL-1β-induced chondrocytes by MTT assay
Following treatment with 10% DHJSD 2-h serum for 24,
36, 48, 60 and 72 h, the viability of IL-1β-induced chondrocytes
was assessed by MTT colorimetric assay. The protocol was as in the
previous description.
Detection of cell cycle distribution in
IL-1β-induced chondrocytes by flow cytometric analysis
Subsequent to treatment, the cell cycle distribution
of the IL-1β-induced chondrocytes was determined by flow cytometric
analysis by FACS with a BD FACSCalibur cytometer (BD Biociences,
Franklin Lakes, NJ, USA) and a cell cycle assay kit. Propidium
iodide staining was performed according to the manufacturer’s
instructions. The percentage of cells in the different phases was
calculated by ModFit LT software, version 3.0 (Verity Software
House, Inc., Topsham, ME, USA), and the numbers of cells in the
G0/G1, S and G2/M phases were
determined.
RT and semi-quantitative PCR
analysis
Following treatment with 10% DHJSD 2-h serum for 24,
48 and 72 h, the IL-1β-induced chondrocytes were washed with PBS
and total RNA was isolated with TRIzol® reagent
(Invitrogen Life Technologies). Oligo (dT) primers (1 μg) were used
for the reverse transcription of the RNA template using SuperScript
II reverse transcriptase (Invitrogen, Grand Island, NY, USA)
according to the manufacturer’s instructions. The obtained cDNA was
used to determine the relative expression of cyclin D1, CDK4, Rb
and p16 by PCR using Taq DNA polymerase (Thermo Fisher Scientific,
Pittsburgh, PA, USA) and β-actin was used as an internal control.
The primer sequences and the annealing temperature used in the
reactions are listed in Table I.
The amplified products were analyzed by 1.5% agarose gel
electrophoresis. Optical density ratios for cyclin D1, CDK4, Rb and
p16 to β-actin were used for the semi-quantitative analyses.
Western blot analysis
Following treatment with 10% DHJSD 2-h serum for 24,
48 and 72 h, the IL-1β-induced chondrocytes were lysed with
mammalian cell lysis buffer containing protease and phosphatase
inhibitor cocktails (EMD Millipore Corporation, San Diego, CA,
USA), and the lysates were separated by 12% SDS-PAGE gel under a
reducing condition at 100 V for 1 h. Subsequent to electrophoresis,
proteins were transferred to polyvinylidine fluoride membranes
(Sigma-Aldrich) in 5% w/v non-fat dry milk using a semidry blotting
system. The membranes were blocked for 30 min with agitation at
room temperature in SuperBlock T20 (TBS) blocking buffer (Thermo
Fisher Scientific Inc., Rockford, IL, USA). The membranes were
washed in Tris-buffered saline with 0.25% Tween-20 (TBST) (Baoman
Biotechnology, Shanghai, China) and exposed to primary antibodies
against cyclin D1 (1:400), CDK 4 (1:400), pRb (1:500) and p16
(1:600) overnight at 4°C. β-actin (1:1,000) was also measured as an
internal control for protein loading. The membranes were then
washed in TBST, and incubated with secondary horseradish
peroxidase-conjugated antibodies (Beijing Zhongshan Golden Bridge
Biotechnology, Beijing, China) at 1:2,500 dilution for 1 h at room
temperature and the membranes were washed again in TBST. Finally,
the antibody-bound protein bands were detected with enhanced
chemiluminescence, and images were captured using a ChemiDoc XRS+
(Bio-Rad Laboratories, Hercules, CA, USA). The grayscale value
ratio of the target protein to the internal control was used to
measure the relative concentration of cyclin D1, CDK4, pRb and
p16.
Statistical analysis
The data were analyzed using SPSS, version 13.0
(SPSS, Inc., Chicago, IL, USA). Statistical data are expressed as
the mean ± standard deviation. Statistical analysis of the data was
performed with Student’s t-test and one-way analysis of variance.
P<0.05 was considered to indicate a statistically significant
difference.
Results
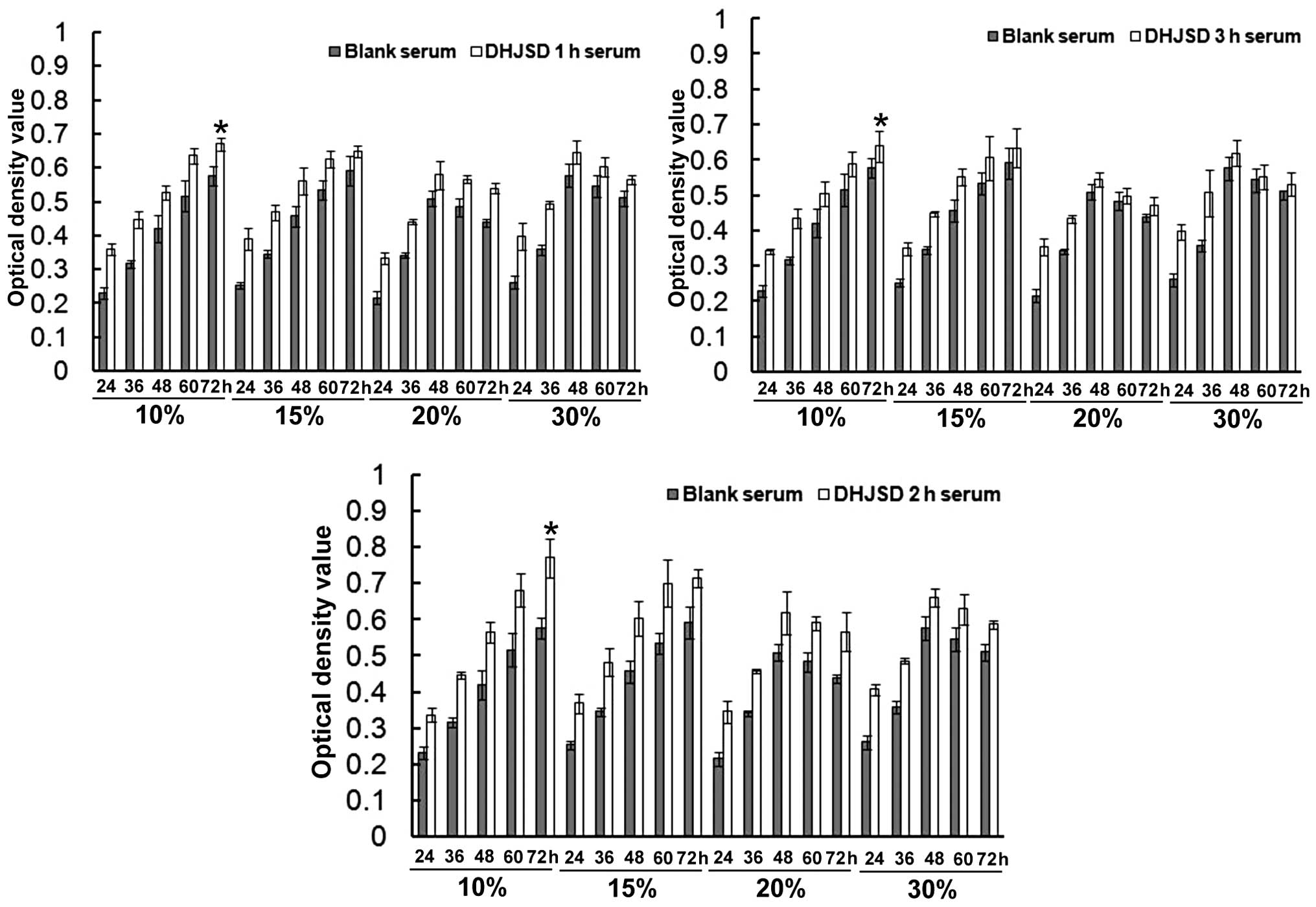
Effect of DHJSD serum on chondrocyte
viability
As presented in Fig.
1, the 10 and 15% concentrations of all DHJSD sera were able to
promote the proliferation of chondrocytes in a time-dependent
manner. The 20 and 30% concentrations of the three DHJSD sera also
promoted the proliferation of chondrocytes, but reached a peak at
48 h and reduced at 72 h. The proliferation was most significant
subsequent to 10% DHJSD sera treatments for 72 h (P<0.01,
compared with the blank serum groups). When comparing the different
sampling time DHJSD sera, it was identified that the optimum
proliferation was produced by the groups treated with the 2-h DHJSD
serum. Therefore, the 10% concentration of the DHJSD 2-h serum was
used in the following experiments.
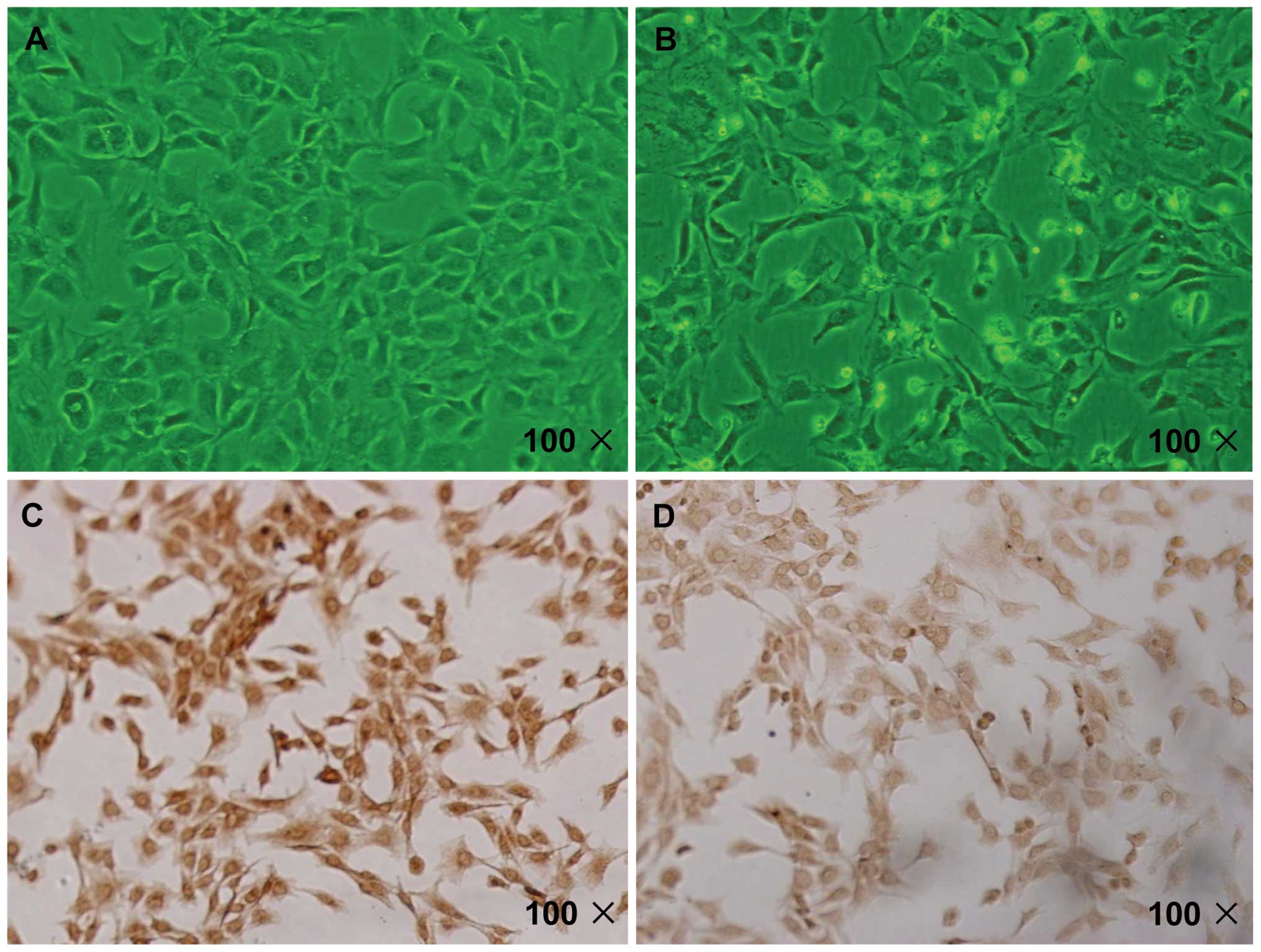
Degenerative chondrocyte model
verification
As presented in Fig. 2A
and B, compared with the normal chondrocytes, the IL-1β-induced
chondrocytes were larger with finger-like protrusions at the edge,
and the cell membrane and cytoplasm was not clear. In addition, the
IL-1β-induced cells were polygonal in shape, and had declining
refractive indices. Type II collagen, a protein specific to
chondrocytes, was stained brown/yellow in the chondrocyte cytoplasm
by immunocytochemical staining. As indicated in Fig. 2C and D, the staining became paler
in the IL-1β-induced group.
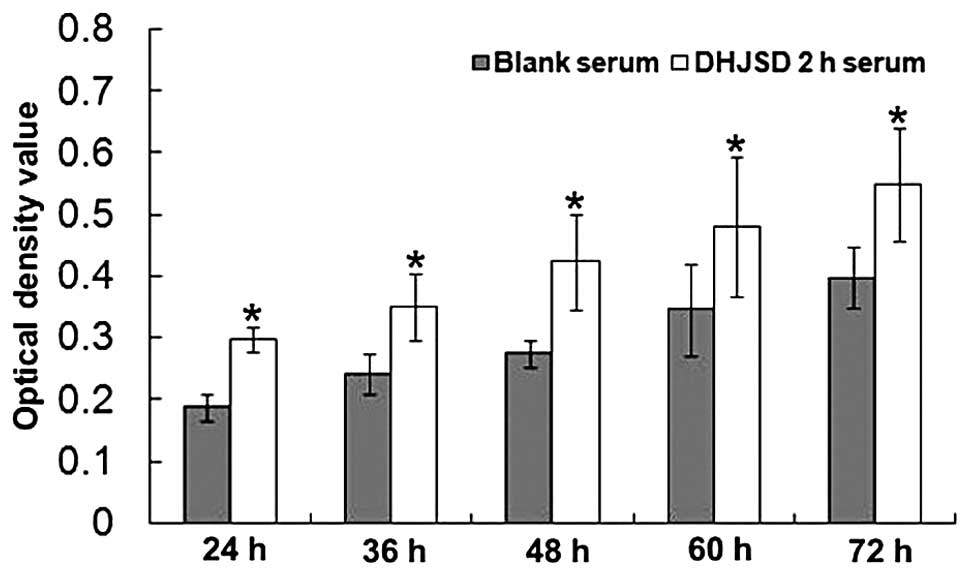
DHJSD 2-h serum promotes the growth of
IL-1β-induced chondrocytes
The effect of DHJSD 2-h serum on the viability of
IL-1β-induced chondrocytes was determined by MTT assay. As
displayed in Fig. 3, treatment
with DHJSD 2-h serum and blank serum led to a gradual increase in
cell viability in a time-dependent manner. The difference between
the optical densities of the blank serum and the DHJSD 2-h serum
groups at all measured time-points (24, 36, 48, 60 and 72 h) were
significant (P<0.01); the promotional effect was greater in the
DHJSD 2-h serum group (P<0.01, compared with the blank serum
group). These results suggest that DHJSD 2-h serum promotes the
growth of IL-1β-induced chondrocytes in a time-dependent
manner.
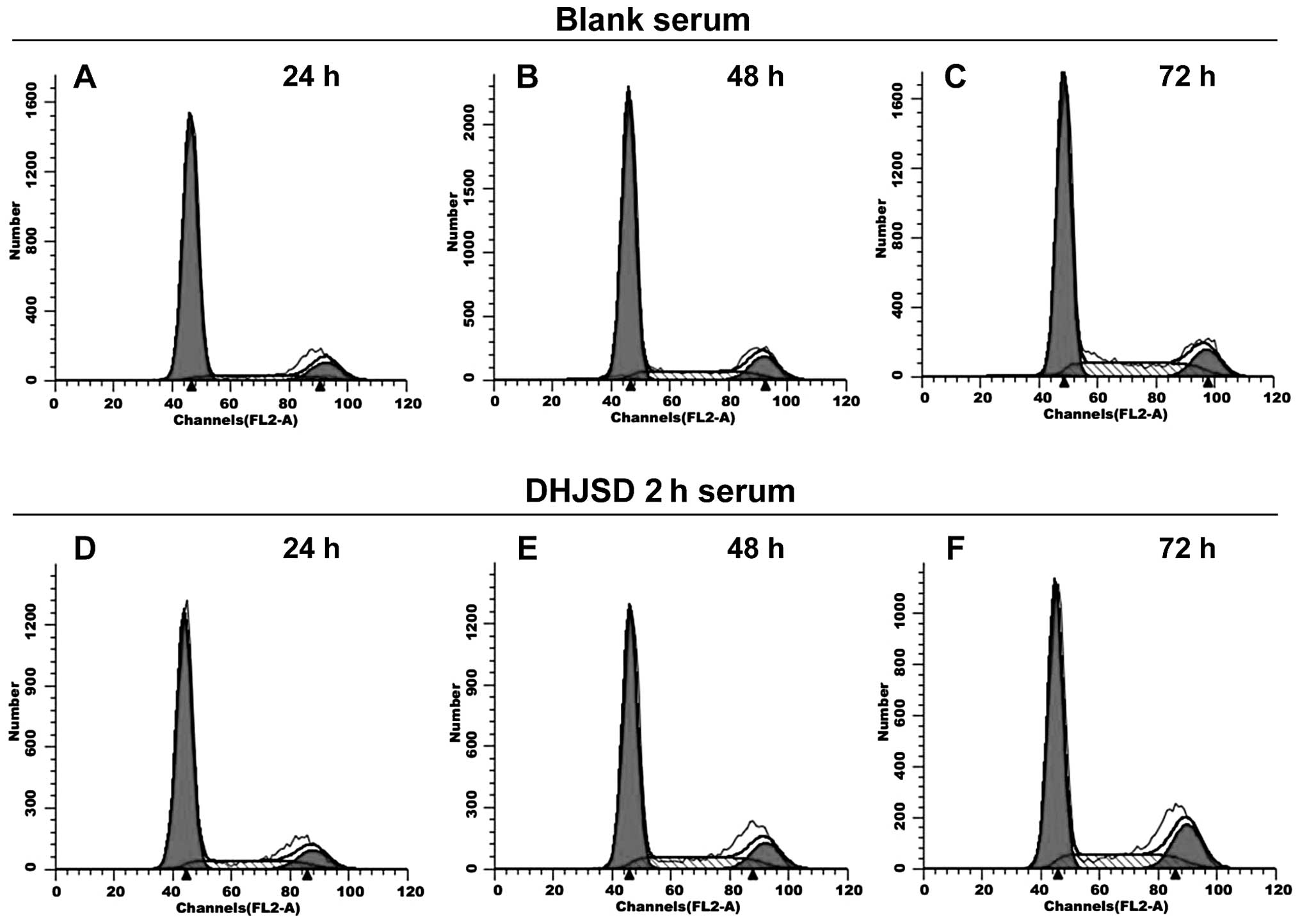
FACS analysis
To further verify the previous results, the effect
of DHJSD 2-h serum on the cell cycle in IL-1β-induced chondrocytes
was evaluated. As presented in Fig.
4 and Table II, the
percentage of G0/G1 phase cells reduced in a
time-dependent manner in the DHJSD 2-h serum group and the blank
serum group. In the DHJSD 2-h serum group, the percentages of
G0/G1 phase cells were 72.97±1.13, 65.13±1.35
and 59.18±2.12% following treatment for 24, 48 and 72 h,
respectively. These were significantly lower than the corresponding
percentages in the blank serum group (79.59±1.69, 71.33±1.11 and
63.86±2.32%, respectively; P<0.01 or P<0.05). However, the
percentages of S phase cells exhibited the opposite trend, and
increased in a time-dependent manner. In the DHJSD 2-h serum group,
the percentages of S phase cells were 16.91±0.64, 22.28±2.45 and
28.05±2.63% following treatment for 24, 48 and 72 h, respectively.
These were significantly higher than the corresponding percentages
of the blank serum group (09.92±2.00, 17.02±2.96 and 24.20±1.05%,
respectively; P<0.01 or P<0.05). The proliferation indices in
the DHJSD 2-h serum group were 27.03±1.13, 34.87±1.35 and
40.82±2.11% following treatment for 24, 48 and 72 h, respectively.
These were higher than the corresponding indices of the blank serum
group (20.41±1.69, 28.67±1.11 and 36.14±2.32, respectively). These
data demonstrate that DHJSD serum has the ability to promote
proliferation by promoting G1/S phase transition.
 | Table IICell cycle distribution detected by
fluorescence-activated cell sorting (%). |
Table II
Cell cycle distribution detected by
fluorescence-activated cell sorting (%).
| Group | h |
G0/G1 | S |
G2/M | Proliferation
index |
|---|
| Blank serum | 24 | 79.59±1.69 | 09.92±2.00 | 10.49±0.33 | 20.41±1.69 |
| 48 | 71.33±1.11a | 17.02±2.96a | 11.65±2.08 | 28.67±1.11 |
| 72 | 63.86±2.32b | 24.20±1.05b | 11.94±1.51 | 36.14±2.32 |
| DHJSD 2-h
serum | 24 | 72.97±1.13a | 16.91±0.64a | 10.13±1.57 | 27.03±1.13 |
| 48 | 65.13±1.35b,e | 22.28±2.45c,f | 12.59±1.11 | 34.87±1.35 |
| 72 | 59.18±2.12d,g | 28.05±2.63d,h | 12.76±1.83 | 40.82±2.11 |
Effect of DHJSD 2-h serum on the mRNA
expression of cyclin D1, CDK4, Rb and p16 in IL-1β-induced
chondrocytes
As presented in Fig. 5A
and B, the mRNA expression levels of cyclin D1, CDK4 and Rb
gradually increased, while those of p16 reduced, in a
time-dependent manner in the DHJSD 2-h and blank serum group. A
significant difference was indicated between the DHJSD and blank
serum groups at all time points (P<0.01). In the DHJSD 2-h serum
group, the expression levels of cyclin D1, CDK4 and Rb mRNA were
higher than those in the blank serum group following treatment for
24, 48 or 72 h (P<0.01 or P<0.05). However, the expression of
p16 exhibited the opposite trend, and its expression level was
lower in the DHJSD 2-h serum group compared with the blank serum
group at all time points (P<0.01 or P<0.05).
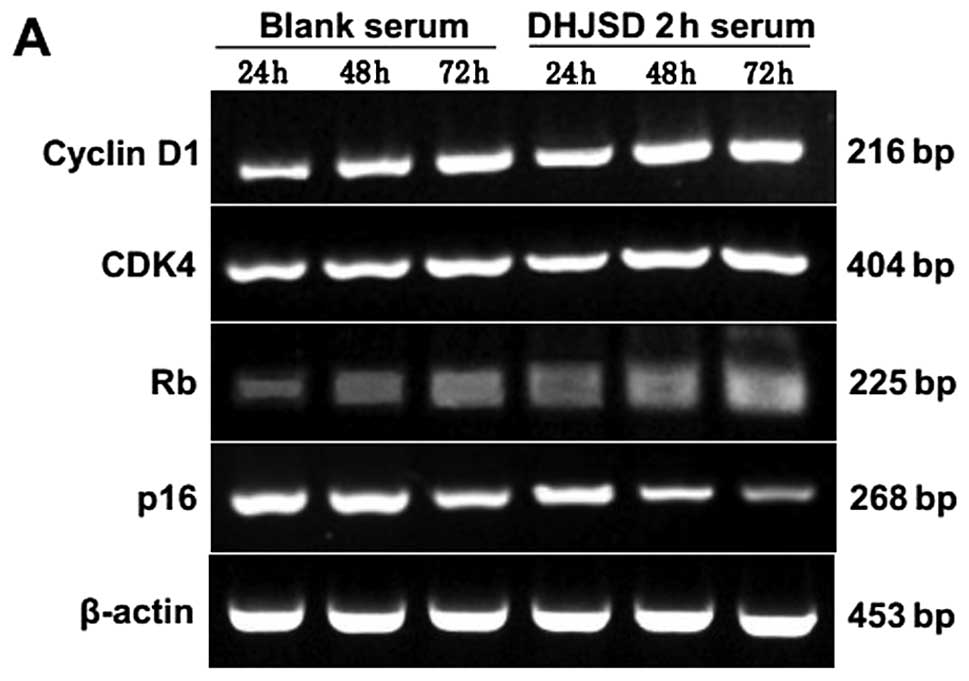 | Figure 5Effect of DHJSD 2-h serum on the mRNA
expression of cyclin D1, CDK4, Rb and p16 in IL-1β-induced
chondrocytes. Following treatment with 10% DHJSD 2-h serum and
blank serum for 24, 48 and 72 h, the cells were collected and the
mRNA levels of cyclin D1, CDK4, Rb and p16 were determined by
RT-PCR. β-actin was used as the internal control. (A) The images
are representative blots. (B) Data are presented as the mean ±
standard deviation of three independent experiments.
*P<0.01 and **P<0.05 vs. blank serum 24
h; ▲P<0.01, ▲▲P<0.05 vs. blank serum 48
h; ⋆P<0.01, ⋆⋆P<0.05 vs. blank serum 72
h. DHJSD, Duhuo Jisheng Decoction; CDK4, cyclin-dependent kinase 4;
Rb, retinoblastoma tumor suppressor protein; IL-1β, interleukin-1β;
RT-PCR, reverse transcription-polymerase chain reaction. |
Effect of DHJSD 2-h serum on the protein
expression of cyclin D1, CDK4, pRb and p16 in IL-1β-induced
chondrocytes
The protein expression patterns of cyclin D1, CDK4,
pRb and p16 were similar to their respective mRNA levels (Fig. 6A and B). The protein expression
levels of cyclin D1, CDK4 and pRb were increased, and those of p16
were reduced in a time-dependent manner in both the DHJSD 2-h and
blank serum groups. However, the protein expression levels of
cyclin D1, CDK4 and pRb in DHJSD 2-h serum group were significantly
higher, and those of p16 were significantly lower, as compared with
the blank serum group at all the time periods (P<0.01 or
P<0.05).
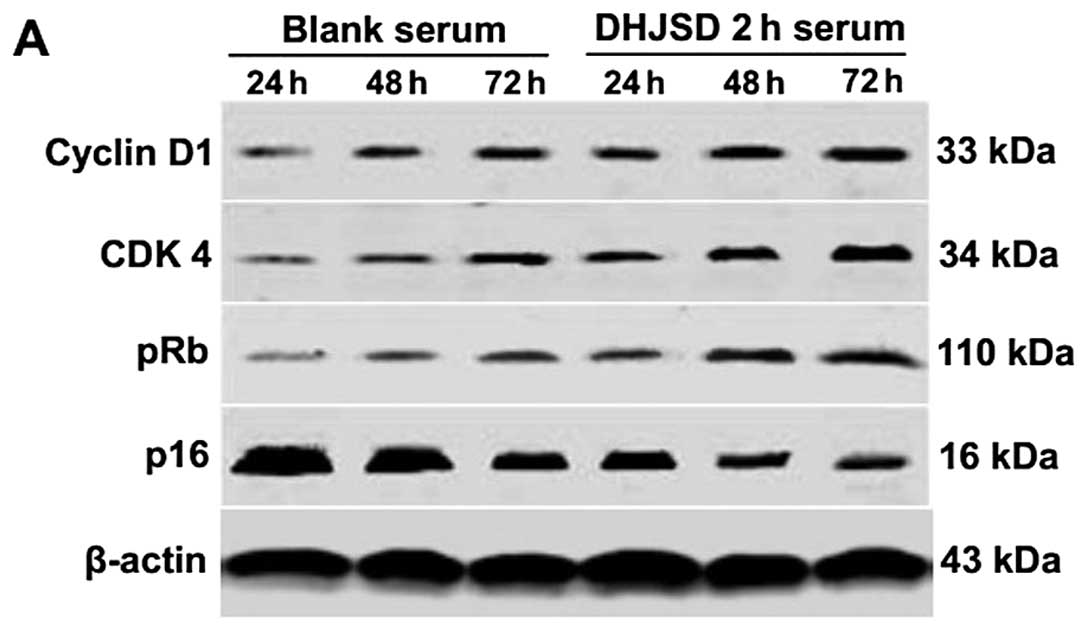 | Figure 6Effect of DHJSD 2-h serum on the
protein expression of cyclin D1, CDK4, pRb and p16 in IL-1β-induced
chondrocytes. Following treatment with 10% DHJSD 2-h serum and
blank serum for 24, 48 and 72 h, the cells were collected. The
protein levels of cyclin D1, CDK4, pRb and p16 were determined by
western blotting. β-actin was used as the internal control. (A)
Representative images of western blots. (B) Data are presented as
the mean ± standard deviation of three independent experiments.
*P<0.01, **P<0.05 vs. blank serum 24 h;
▲P<0.01 and ▲▲P<0.05 vs. blank serum 48
h; ⋆P<0.01 and ⋆⋆P<0.05 vs. blank serum
72 h. DHJSD, Duhuo Jisheng Decoction; CDK4, cyclin-dependent kinase
4; pRb, phosphorylated retinoblastoma tumor suppressor protein;
IL-1β, interleukin-1β; RT-PCR, reverse transcription-polymerase
chain reaction. |
Discussion
OA is a major degenerative disease affecting
millions of individuals. The ability of articular cartilage to
self-repair is limited by a low tissue turnover rate and the
avascular nature of the cartilage, meaning that OA is an
irreversible disease (31). With
regards to therapeutic strategies for OA, there are a large number
of active research and drug discovery programs aimed at identifying
structure-modifying methods of inhibiting joint destruction in OA.
Current drug therapies are only able to reduce symptoms; however,
none of these approaches have significant efficacy as a
disease-modifying anti-OA treatment (32). Chinese herbal medicine is a major
modality in TCM, which has been practiced for thousands of years in
China and other Asian countries, and is used for the treatment of
arthritis and related disorders such as Bi syndrome (33,34).
DHJSD is a traditional Chinese herbal formula which has been widely
used for OA treatment; however, the molecular mechanisms underlying
the therapeutic effects of DHJSD on OA remain unknown. Thus, the
present study was designed to investigate whether the treatment of
OA with DHJSD affected the proliferation of degenerative
chondrocytes, and the possible underlying molecular mechanism. It
was demonstrated that DHJSD-containing serum promoted IL-1β-induced
chondrocytes proliferation through the p16-cyclin D1/CDK4-Rb
pathway.
Serum pharmacology, suggested in a study by Tashino
(35), is a novel method to study
traditional Chinese herbs. It allows one to avoid the various
disadvantages of adding drugs directly to cells (36). Various ingredients are absorbed
into the blood through the gastrointestinal tract and transformed
into bioactive ingredients following oral administration of
traditional Chinese herbs. Cells treated with serum containing
traditional Chinese medicines in vitro are in a similar
condition to cells in vivo (37). Therefore, serum pharmacology
experiments on Chinese herbal medicines may produce reliable
consistency with corresponding experiments in vivo (38). Based on the pharmacokinetics, it is
well-established that drugs have different effects on the body over
different time periods of treatment. Thus, in the present study,
arterial blood was collected from the abdominal aorta at 1, 2 and 3
h subsequent to the final dose of DHJSD. In order to identify the
ideal intervention conditions, the proliferation of chondrocytes
was detected by MTT assay following treatment with a range of
concentrations of the DHJSD 1, 2 and 3-h sera for 24, 36, 48, 60
and 72 h. The results demonstrated that the ideal condition was the
10% concentration of 2-h DHJSD serum, which was used in the
proceeding experiments.
The mechanisms that lead to cartilage degradation
primarily involve the excessive production of matrix
metalloproteinases (MMPs), including collagenases and stromelysins
(39). Chondrocytes largely
contribute to this enhancement by secreting high levels of MMPs in
response to cytokines, primarily IL-1β. These cytokines
synergically enhance pro-MMP secretion and modulate the activation
or inhibition systems, leading to an increase in the proteolytic
activity of cartilage (40). In
vitro, IL-1β modifies the normal metabolic functions of
chondrocytes, and provokes an imbalance between the catabolic and
anabolic events, leading to an excess of cartilage resorption
(40,41). Park et al (42) also reported that the IL-1β-treated
construct can be a simplified model in a closed system to simulate
pathological OA cartilage. Therefore, IL-1β was used in the current
study to reproduce the degenerative chondrocyte model, and a
concentration of 10 ng/ml IL-1β was selected for use, as in a
previous study (29,30). In the present study, the cells
displayed typical morphologies of degenerative chondrocytes
following IL-1β stimulation; numerous chondrocytes became larger
with finger-like protrusions at the edges, and the cell membrane
and cytoplasm became unclear. It was also demonstrated that the
expression of the chondrocyte-specific protein type II collagen was
reduced, which is in accordance with previous findings (43). In view of these results, the
IL-1β-stimulated chondrocyte model was indicated to provide a
suitable context in which to study the effects of DHJSD 2-h serum
on the proliferation of degenerative chondrocytes.
In the present study, it was demonstrated that DHJSD
2-h serum and blank serum treatment led to a time-dependent
increase in the viability of IL-1β-induced chondrocytes, and the
potentiating effect was significantly greater in the DHJSD 2-h
serum group (P<0.01, vs. the blank serum group). These results
suggest that DHJSD 2-h serum promotes IL-1β-induced chondrocyte
growth in a time-dependent manner. Notably, FACS analysis, which
measures the DNA content of cells and is more sensitive to cell
cycle changes than the MTT method, was utilized in the current
study. The percentages of G0/G1 phase cells
reduced in a time-dependent manner in the DHJSD 2-h serum group.
The percentage of S phase cells exhibited the opposite trend, and
increased in a time-dependent manner. These data demonstrate that
DHJSD 2-h serum promotes IL-1β-induced chondrocyte proliferation by
promoting G1/S phase transition.
Previous studies have indicated that the p16-cyclin
D1/CDK4-Rb pathway serves a central function in the G1/S
phase transition; this feedback-regulating network determines the
process of the cell cycle (44).
Briefly, extracellular signals induce the expression of cyclin D1
in cells entering the cell cycle and this binds to and activates
CDK4 (45,46). The ensuing complexes in turn lead
to the phosphorylation of retinoblastoma (Rb), resulting in its
dissociation from transcription factors, which are predominant
members of the E2F family, and activate a number of
genes required for the progression of the cell cycle to the S phase
(45). p16, a member of the INK4
family of CDK inhibitors, inhibits CDK4, maintaining Rb in its
unphosphorylated E2F-associated state, and thereby
preventing G1/S phase transition (47,48).
In the current study, it was demonstrated that DHJSD 2-h serum
enhanced cyclin D1, CDK4 and Rb, and reduced p16 mRNA expression
levels in IL-1β-induced chondrocytes, indicating that DHJSD 2-h
serum promotes the progression of chondrocytes from the
G1 to the S phase by influencing cyclin D1, CDK4, Rb and
p16. In order to further confirm the results, the effects of DHJSD
2-h serum on the protein expression of cyclin D1, CDK4, pRb and p16
were determined by western blotting analysis. The results revealed
that the protein expression of cyclin D1, CDK4 and Rb were
increased, and the expression level of p16 was reduced following
DHJSD serum treatment, which is in accordance with the observed
patterns of mRNA expression.
In conclusion, the current study demonstrated that
DHJSD-containing serum of rats has the ability to promote
proliferation in IL-1β-induced chondrocytes, through the promotion
of G1/S transition via modulating the expressions of
cyclin D1, CDK4, Rb and p16. These data provide a better
understanding of the effects and mechanisms of DHJSD in the
treatment of OA. However, it is unclear which of the composites of
this classic herbal medicine contributes to the pro-proliferative
effect. Therefore, further study of the individual components of
DHJSD is required in future, in order to clarify these
mechanisms.
Acknowledgements
The present study was supported by the National
Natural Science Foundation of China (grant no. 81373818).
References
|
1
|
Felson DT: Clinical practice.
Osteoarthritis of the knee. N Engl J Med. 354:841–848. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Zhang W, Nuki G, Moskowitz RW, et al:
OARSI recommendations for the management of hip and knee
osteoarthritis: part III: Changes in evidence following systematic
cumulative update of research published through January 2009.
Osteoarthritis Cartilage. 18:476–499. 2010. View Article : Google Scholar
|
|
3
|
Qiu G: Osteoarthritis diagnosis and
treatment guidelines. Chinese J Joint Surgery. 1:281–285. 2007.
|
|
4
|
Shortkroff S and Yates KE: Alteration of
matrix glycosaminoglycans diminishes articular chondrocytes’
response to a canonical Wnt signal. Osteoarthritis Cartilage.
15:147–154. 2007.PubMed/NCBI
|
|
5
|
Chan BY, Fuller ES, Russell AK, et al:
Increased chondrocyte sclerostin may protect against cartilage
degradation in osteoarthritis. Osteoarthritis Cartilage.
19:874–885. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Huang JG, Xia C, Zheng XP, et al:
17β-Estradiol promotes cell proliferation in rat osteoarthritis
model chondrocytes via PI3K/Akt pathway. Cell Mol Biol Lett.
16:564–575. 2011.
|
|
7
|
Kashiwagi A, Schipani E, Fein MJ, Greer PA
and Shimada M: Targeted deletion of Capn4 in cells of the
chondrocyte lineage impairs chondrocyte proliferation and
differentiation. Mol Cell Biol. 30:2799–2810. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Sarzi-Puttini P, Cimmino MA, Scarpa R, et
al: Osteoarthritis: an overview of the disease and its treatment
strategies. Semin Arthritis Rheum. 35(Suppl 1): 1–10. 2005.
View Article : Google Scholar
|
|
9
|
Badley EM, Rasooly I and Webster GK:
Relative importance of musculoskeletal disorders as a cause of
chronic health problems, disability, and health care utilization:
findings from the 1990 Ontario Health Survey. J Rheumatol.
21:505–514. 1994.
|
|
10
|
Felson DT, Lawrence RC, Dieppe PA, et al:
Osteoarthritis: new insights. Part 1: the disease and its risk
factors. Ann Intern Med. 133:635–646. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Bradley JD, Brandt KD, Katz BP, Kalasinski
LA and Ryan SI: Comparison of an antiinflammatory dose of
ibuprofen, an analgesic dose of ibuprofen, and acetaminophen in the
treatment of patients with osteoarthritis of the knee. N Engl J
Med. 325:87–91. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lohmander LS, Dalén N, Englund G, et al:
Intra-articular hyaluronan injections in the treatment of
osteoarthritis of the knee: a randomised, double blind, placebo
controlled multicentre trial. Ann Rheum Dis. 55:424–431. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Berenbaum F: New horizons and perspectives
in the treatment of osteoarthritis. Arthritis Res Ther. 10(Suppl
2): S12008. View
Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ahmed S, Anuntiyo J, Malemud CJ and Haqqi
TM: Biological basis for the use of botanicals in osteoarthritis
and rheumatoid arthritis: a review. Evid Based Complement Alternat
Med. 2:301–308. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Parkman CA: Alternative therapies for
osteoarthritis. Case Manager. 12:34–36. 2001. View Article : Google Scholar
|
|
16
|
Teekachunhatean S, Kunanusorn P,
Rojanasthien N, et al: Chinese herbal recipe versus diclofenac in
symptomatic treatment of osteoarthritis of the knee: a randomized
controlled trial [ISRCTN70292892]. BMC Complement Altern Med.
4:192004.PubMed/NCBI
|
|
17
|
Setty AR and Sigal LH: Herbal medications
commonly used in the practice of rheumatology: mechanisms of
action, efficacy, and side effects. Semin Arthritis Rheum.
34:773–784. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Chun SC, Jee SY, Lee SG, Park SJ, Lee JR
and Kim SC: Anti-inflammatory activity of the methanol extract of
moutan cortex in LPS-activated Raw264.7 cells. Evid Based
Complement Alternat Med. 4:327–333. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wu M and Gu Z: Screening of bioactive
compounds from moutan cortex and their anti-inflammatory activities
in rat synoviocytes. Evid Based Complement Alternat Med. 6:57–63.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Sun SM: Bei Ji Qian Jin Yao Fang. 8. 1st
edition. People’s Medical Publishing Press; Beijing: pp. 166–167.
1982
|
|
21
|
Lai JN, Chen HJ, Chen CC, Lin JH, Hwang JS
and Wang JD: Duhuo jisheng tang for treating osteoarthritis of the
knee: a prospective clinical observation. Chin Med. 2:42007.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zheng CS, XXJ, Ye HZ, Wu GW, Li XH, Huang
SP and Liu XX: Computational approaches for exploring the potential
synergy and polypharmacology of Duhuo Jisheng Decoction in the
therapy of osteoarthritis. Mol Med Report. 7:1812–1818.
2013.PubMed/NCBI
|
|
23
|
Wu G, Chen W, Fan H, et al: Duhuo Jisheng
Decoction promotes chondrocyte proliferation through accelerated
G1/S transition in osteoarthritis. Int J Mol Med. 32:1001–1010.
2013.PubMed/NCBI
|
|
24
|
Panico AM, Cardile V, Garufi F, Puglia C,
Bonina F and Ronsisvalle G: Effect of hyaluronic acid and
polysaccharides from Opuntia ficus indica (L.) cladodes on
the metabolism of human chondrocyte cultures. J Ethnopharmacol.
111:315–321. 2007.PubMed/NCBI
|
|
25
|
The Ministry of Science and Technology of
the People’s Republic of China. Guidance Suggestions for the Care
and Use of Laboratory Animals. 2006.
|
|
26
|
Huang J, Huang X, Chen Z, Zheng Q and Sun
R: Dose conversion among different animals and healthy volunteers
in pharmacological study. Chin J Clin Pharmacol Ther. 9:1069–1072.
2004.(In Chinese).
|
|
27
|
Li XH, Du M, Liu XX, et al: Millimeter
wave treatment inhibits NO-induced apoptosis of chondrocytes
through the p38MAPK pathway. Int J Mol Med. 25:393–399.
2010.PubMed/NCBI
|
|
28
|
Li XH, Wu MX, YHZ, Chen WL, Lin JM, Zheng
LP and Liu XX: Experimental study on the suppression of sodium
nitroprussiate-induced chondrocyte apoptosis by tougu xiaotong
capsule-containing serum. Chin J Integr Med. 17:436–443. 2011.(In
Chinese).
|
|
29
|
Sanchez C, Mathy-Hartert M, Deberg MA,
Ficheux H, Reginster JY and Henrotin YE: Effects of rhein on human
articular chondrocytes in alginate beads. Biochem Pharmacol.
65:377–388. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Wu SQ, Otero M, Unger FM, et al:
Anti-inflammatory activity of an ethanolic Caesalpinia
sappan extract in human chondrocytes and macrophages. J
Ethnopharmacol. 138:364–372. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Mankin HJ: The response for articular
cartilage to mechanical injury. J Bone Joint Surg Am. 64:460–466.
1982.PubMed/NCBI
|
|
32
|
Brandt KD and Mazzuca SA: Lessons learned
from nine clinical trials of disease-modifying osteoarthritis
drugs. Arthritis Rheum. 52:3349–3359. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Bensky D, Gamble A and Stöger E: Chinese
Herbal Medicine: Materia Medica. 3rd edition. Eastland Press;
Seattle, WA: 2004
|
|
34
|
Ho LJ and Lai JH: Chinese herbs as
immunomodulators and potential disease-modifying antirheumatic
drugs in autoimmune disorders. Curr Drug Metab. 5:181–192. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Tashino S: ‘Serum pharmacology’ and ‘serum
pharmaceutical chemistry’: from pharmacology of Chinese traditional
medicines to start a new measurement of drug concentration in
blood. Ther Drug Monit Res. 5:54–64. 1988.
|
|
36
|
Liu N, Liu JT, Ji YY and Lu PP: Dahuang
zhechong pill containing serum inhibited platelet-derived growth
factor-stimulated vascular smooth muscle cells proliferation by
inducing G1 arrest partly via suppressing protein kinase C
α-extracellular regulated kinase 1/2 signaling. Chin J Integr Med.
18:371–377. 2012.(In Chinese).
|
|
37
|
Dang XY, Dong L, Shi HT and Zou BC:
Effects of serum containing Chinese medicine Sanpi Pingwei formula
on proliferation and apoptosis of human SGC-7901 cells. Chin J
Integr Med. 19:119–126. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Wang WJ, Li DJ, Li J and Zhou WJ: An in
vitro study on neuroprotective effects of serum containing
Gengnianchun decoction and its main monomers against amyloid beta
protein-induced cellular toxicity. J Chin Integr Med. 8:67–73.
2010.(In Chinese).
|
|
39
|
Henrotin Y, Sanchez C and Reginster JY:
The inhibition of metalloproteinases to treat osteoarthritis:
reality and new perspectives. Expert Opin Ther Patents. 12:29–43.
2002. View Article : Google Scholar
|
|
40
|
Tetlow LC, Adlam DJ and Woolley DE: Matrix
metalloproteinase and proinflammatory cytokine production by
chondrocytes of human osteoarthritic cartilage. Arthritis Rheum.
44:585–594. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Martel-Pelletier J, Di Battista J and
Lajeunesse D: Biochemical factors in joint articular tissue
degradation in osteoarthritis. Osteoarthritis: Clinical and
Experimental Aspects. Reginster J-YL, Pelletier JP,
Martel-Pelletier J and Henrotin YE: Springer; Berlin: pp. 156–187.
1999, View Article : Google Scholar
|
|
42
|
Park K, Hoffmeister B, Han DK and Hasty K:
Therapeutic ultrasound effects on interleukin-1beta stimulated
cartilage construct in vitro. Ultrasound Med Biol. 33:286–295.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Tan L, Peng H, Osaki M, Choy BK, Auron PE,
Sandell LJ and Goldring MB: Egr-1 mediates transcriptional
repression of COL2A1 promoter activity by interleukin-1beta. J Biol
Chem. 278:17688–17700. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Gibson SL, Dai CY, Lee HW, et al:
Inhibition of colon tumor progression and angiogenesis by the
Ink4a/Arf locus. Cancer Res. 63:742–746. 2003.PubMed/NCBI
|
|
45
|
Massagué J: G1 cell-cycle control and
cancer. Nature. 432:298–306. 2004.
|
|
46
|
Musgrove EA, Caldon CE, Barraclough J,
Stone A and Sutherland RL: Cyclin D as a therapeutic target in
cancer. Nat Rev Cancer. 11:558–572. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Li J, Poi MJ and Tsai MD: Regulatory
mechanisms of tumor suppressor P16(INK4A) and their relevance to
cancer. Biochemistry. 50:5566–5582. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Witkiewicz AK, Knudsen KE, Dicker AP and
Knudsen ES: The meaning of p16(ink4a) expression in tumors:
functional significance, clinical associations and future
developments. Cell Cycle. 10:2497–2503. 2011. View Article : Google Scholar : PubMed/NCBI
|