Introduction
Glioma is one of the most common types of brain
tumor. Despite the development of current standard therapy, the
prognosis of patients with malignant glioma is poor. All-trans
retinoic acid (ATRA), a physiologically active derivative of
vitamin A, is one of the most potent inducers of differentiation
and has been extensively studied. The biological effects of ATRA
are mediated through retinoic acid receptors (RARs) and retinoic X
receptors. Several studies have shown that ATRA can induce
differentiation and apoptosis in a variety of glioma cells
(1,2), including glioma stem cells (3,4), a
highly tumorigenic and therapy-resistant tumor subpopulation. ATRA
can also induce growth arrest in glioma cells (5). Furthermore, studies have revealed
that ATRA exhibits certain additional anti-glioma effects (6–11).
Li et al (6) reported that
ATRA enhanced the tumoricidal effect of suicide-gene therapy
against medulloblastoma. Other studies have found that ATRA can
significantly enhance the anti-tumor effect of certain
chemoimmunotherapy and cytotoxic chemotherapy drugs, including
interferon-γ and taxol, respectively, on glioma (7–11).
These findings indicate that ATRA may have therapeutic potential in
patients with glioma.
Angiogenesis is a complex biological process, which
involves the degradation of the basement membrane and extracellular
matrix, endothelial cell (EC) proliferation, migration and tube
formation (12). Angiogenesis is
mediated by various regulatory factors, including vascular
endothelial growth factor (VEGF), basic fibroblast growth factor,
hepatocyte growth factor (HGF) and angiopoietin-1 and -2. These
regulatory factors are well-established angiogenic factors, and
their biological effects are mediated through interaction with
their membrane receptors. Angiogenesis is important in numerous
physiological processes, including embryo development, wound
healing and normal growth (13).
However, angiogenesis also has a key role in various pathological
processes, such as inflammation (14), diabetic retinopathy (15) and the formation, development and
recurrence of glioma (16).
Therefore, anti-angiogenesis therapy has been developed as a novel
therapeutic strategy for patients with glioma. Numerous studies
have shown that anti-angiogenesis therapy can significantly inhibit
glioma growth (17,18) and improve the outcome of patients
with glioma (19,20). However, the effect of ATRA on
glioma angiogenesis is yet to be elucidated.
VEGF induces EC proliferation, promotes cell
migration and inhibits apoptosis. VEGF is well established as a key
regulator of angiogenesis and plays an important role in the
formation, development and recurrence of glioma (21–24).
Hypoxia-inducible factor-1α (HIF-1α) is a transcription factor that
is responsible for the induction of genes that regulate numerous
biological processes, including angiogenesis (25). Furthermore, HIF-1α regulates
various genes involved in the different stages of angiogenesis,
including VEGF. VEGF is the most potent endothelial-specific
mitogen and directly participates in angiogenesis by recruiting ECs
and stimulating their proliferation (26).
The present study investigated the effect of ATRA on
angiogenesis by detecting VEGF and HIF-1α expression in two glioma
cell lines, U-87 MG (U87) and SHG44, under normoxia and hypoxia.
The anti-angiogenic effect of ATRA in a rat intracerebral glioma
model was also investigated. The results of this study are likely
to provide an enhanced understanding of the mechanisms underlying
the therapeutic effect of ATRA in malignant glioma.
Materials and methods
Materials
The U87 and SHG44 human glioma cell lines and the C6
rat glioma cell line were purchased from the Cell Resource Center,
Chinese Academy of Sciences (Shanghai, China). A total of 30 male,
specific pathogen-free, Sprague Dawley rats, weighing between 280
and 320 g, were obtained from the Laboratory Animal Center, Medical
College of Xi’an Jiaotong University (Xi’an, China). All animal
procedures were performed in accordance with the Guidance by the
Research Ethics Committee of the Medical College of Xi’an Jiaotong
University.
Cell culture
U87 cells were cultured in Dulbecco’s Modified
Eagle’s Medium (Hyclone, Beijing, China), supplemented with 10%
fetal bovine serum (FBS; Hyclone) in 5% CO2 at 37°C.
SHG44 and C6 cells were cultured in RPMI-1640 (Hyclone),
supplemented with 10% FBS in 5% CO2 at 37°C.
Quantitative polymerase chain reaction
(qPCR) analysis
U87 and SHG44 cells were seeded onto six-well plates
(Corning Inc., Lowell, MA, USA) at a density of 3×105
cells/well. ATRA (Sigma-Aldrich, St. Louis, MO, USA) was dissolved
in dimethyl sulfoxide (DMSO; Sigma-Aldrich) and stored in
light-protected vials at −20°C as a stock solution. The stock
solution was diluted to the desired concentration prior to use. All
experiments were performed under low-light conditions to minimize
ATRA photoisomerization. The cells were incubated in media
containing various concentrations of ATRA (5, 10, 20 and 40 μmol/l)
for 24 h under normoxia. The control group was treated with an
equal volume of the solvent (DMSO) in the culture media.
CoCl2•6H2O (Sigma-Aldrich) was
used to simulate hypoxia in order to investigate the effect of ATRA
on VEGF and HIF-1α expression under hypoxic conditions. U87 and
SHG44 cells were seeded in six-well plates at a density of
3×105 cells/well and were incubated in media containing
various concentrations of ATRA (5, 10, 20 and 40 μmol/l) and 100
μmol/l CoCl2 for 24 h. The hypoxia control group was
treated with an equal volume of the solvent (DMSO) and 100 μmol/l
CoCl2 in the culture media. The normoxia control group
was solely treated with an equal volume of the solvent (DMSO).
The cells were lysed and the total RNA was isolated
using the RNAfast200 kit (Shanghai Fastagen Biotechnology Co.,
Ltd., Shanghai, China) according to the manufacturer’s
instructions. The RNA was reverse transcribed using PrimeScript™ RT
Master Mix (Takara Bio Inc., Dalian, China). qPCR was performed
using SYBR® Premix Ex Taq™II (Takara Bio Inc.) with a
Bio-Rad IQ5 thermal cycler (Bio-Rad Laboratories, Inc., Hercules,
CA, USA) and analyzed with Bio-Rad iQ5 software version 2.0. Gene
expression was compared using the Δcycle threshold (ΔCt) method
(ΔCt=CtTarget−Ctβ-actin), where β-actin
expression was used as an endogenous reference gene. The changes in
target gene expression were evaluated using the 2−ΔΔCt
method (27). All the primers were
designed and synthesized by Takara Bio Inc. (Table I).
 | Table IPrimer sequences for the quantitative
polymerase chain reaction. |
Table I
Primer sequences for the quantitative
polymerase chain reaction.
| Gene | Sequence | Product length
(bp) |
|---|
| HIF-1α | Forward,
5′-TCTGGGTTGAAACTCAAGCAACTG- 3′
Reverse, 5′-CAACCGGTTTAAGGACACATTCTG-3′ | 150 |
| VEGF | Forward,
5′-TCACAGGTACAGGGATGAGGACAC-3′
Reverse, 5′-CAAAGCACAGCAATGTCCTGAAG-3′ | 72 |
| β-actin | Forward,
5′-TGGCACCCAGCACAATGAA-3′
Reverse, 5′-CTAAGTCATAGTCCGCCTAGAAGCA-3′ | 186 |
Western blot analysis
U87 and SHG44 cells were cultured in 25-ml culture
flasks (Corning Inc.) with ATRA at various concentrations under
normoxia or hypoxia for 24 h, in accordance with the aforementioned
methods. The cells were then harvested for the subsequent assays.
The cells were washed twice with phosphate-buffered saline (PBS),
then scraped on ice in 300 μl radioimmunoprecipitation assay lysis
buffer (Beyotime Institute of Biotechnology, Haimen, China) with 1
mmol/l phenylmethylsulfonyl fluoride. The lysates were cleared of
insoluble material using centrifugation and protein concentration
was determined using a Bradford protein assay kit (Beyotime
Institute of Biotechnology). The samples were boiled in 1X SDS-PAGE
sample loading buffer, resolved using SDS-PAGE and transferred to
polyvinylidene fluoride membranes (Bio-Rad Laboratories, Inc.). The
membranes were probed with anti-VEGF (Epitomics, Burlingame, CA,
USA) and anti-GAPDH (Bioworld Technology Inc., St. Louis Park, MN,
USA) antibodies diluted 1:1,000 and 1:5,000, respectively.
Membranes from the hypoxia groups were also probed with anti-HIF-1α
antibodies (Cell Signaling Technology, Inc., Beverly, MA, USA)
diluted 1:1,000. Subsequent to washing in Tris-buffered saline
containing 0.02% Tween 20, the membranes were incubated with a
secondary polyclonal anti-rabbit immunoglobulin G (IgG) antibody
conjugated to horseradish peroxidase (Thermo Fisher Scientific,
Inc., Waltham, MA, USA) diluted 1:2,000. Membranes were developed
using Super Signal™ West Pico chemiluminescent reagent (Pierce
Biotechnology, Inc., Rockford, IL, USA).
Animal studies
A total of 10 μl cell suspension containing
1×106 C6 cells was injected at the coronal suture 3 mm
away from the midline and at a depth of 5 mm into the right frontal
lobe of the rats. Seven days after C6 cell injection, 30
tumor-bearing rats were randomly divided into three groups (10
rats/group). The low- and high-dose groups were treated with
different doses of ATRA (5 and 10 mg/kg/day, respectively) diluted
in corn oil. ATRA was administered using intraperitoneal injection
between days 8 and 21. The control group was treated with an equal
volume of solvent.
On day 22, after two weeks of treatment, all
tumor-bearing rats were sacrificed. Brain and tumor samples were
obtained, and frozen sections were prepared. Immunohistochemistry
was performed to detect the cluster of differentiation (CD)
34-positive cells in order to evaluate the microvessel density
(MVD) in each group using an immunohistochemical assay kit (Boster
Biological Technology Co., Ltd., Wuhan, China). All procedures were
performed according to the manufacturer’s instructions. Briefly,
the sections were fixed in 4% paraformaldehyde for 20 min and 3%
hydrogen peroxide in methanol was then used to quench any
endogenous peroxidase activity. Non-specific protein binding was
blocked using 5% bovine serum albumin for 10 min at room
temperature. The sections were incubated overnight with rabbit
anti-CD34 antibody (Boster Biological Technology Co., Ltd.) diluted
1:100 at 4°C. The negative control sections were incubated with PBS
instead of the antibody. Subsequent to three washes in PBS, the
sections were incubated in biotinylated anti-rabbit IgG antibody at
3°C for 30 min. Following incubation, the sections were washed
three times for 2 min in PBS and antibody location was determined
using a 3,3′-diaminobenzidine substrate kit (Tiangen Biotech Co.,
Ltd., Beijing, China) for 5 min. Normal vascular endothelium was
used as a positive control. MVD was determined as previously
described by Weidner et al (28). Briefly, the tumor sections were
scanned at low magnifications (×40 or ×100) to determine the areas
of most intense tumor angiogenesis, termed the ‘hot spots’.
Following ‘hot spot’ identification, the MVD was calculated by
averaging the number of individual microvessels in five fields at
high magnification (x400).
Statistical analysis
Values are presented as the mean ± standard
deviation and data were analyzed using SPSS 17.0 software (SPSS,
Inc., Chicago, IL, USA). One-way analysis of variance was used to
compare the groups and least significant difference tests were
performed for further inter-group comparisons. A value of P<0.05
was considered to indicate a statistically significant
difference.
Results
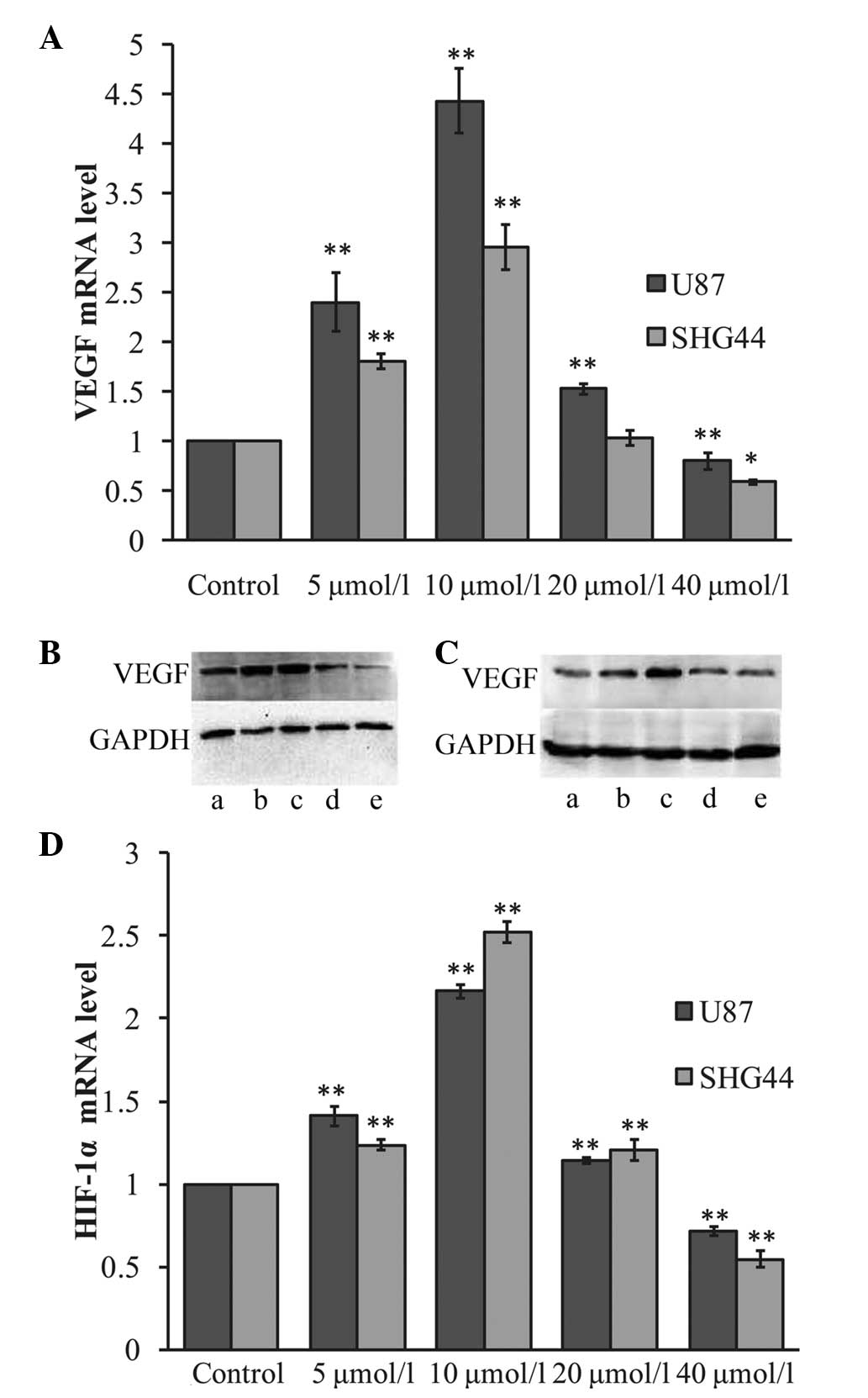
Effects of ATRA on the VEGF and HIF-1α
expression in glioma cells under normoxia
Following incubation with various concentrations of
ATRA for 24 h, VEGF mRNA levels were observed to vary among the
different groups. As shown in Fig.
1A, after 24 h the VEGF mRNA expression was observed to be
significantly upregulated in the U87 and SHG44 glioma cells in the
lower ATRA concentration groups (5 and 10 μmol/l), compared with
that in the control group (P<0.01). Treatment with 5 μmol/l ATRA
was found to increase VEGF mRNA expression ~2.4-fold in the U87 and
1.3-fold in the SHG44 glioma cells. Treatment with 10 μmol/l ATRA
increased VEGF mRNA expression ~4.42-fold in the U87 and 2.24-fold
in the SHG44 glioma cells. Conversely, following treatment with 40
μmol/l ATRA, the VEGF mRNA expression was observed to be
significantly downregulated to 0.80- and 0.77-fold that in the
corresponding control groups, in the U87 (P<0.01) and SHG44
(P<0.05) glioma cells, respectively. Furthermore, following
treatment with 20 μmol/l ATRA, VEGF mRNA levels in the U87 glioma
cells increased 1.53-fold relative to the levels in the control
group (P<0.01); however, no significant difference was observed
in the SHG44 glioma cells.
VEGF protein expression was analyzed using western
blot analysis, as shown in Fig. 1B and
C. In accordance with the changes in VEGF mRNA expression,
treatment with lower concentrations of ATRA (5 and 10 μmol/l) was
observed to upregulate VEGF protein expression in the U87 and SHG44
glioma cells. Furthermore, treatment with high ATRA concentrations
(40 μmol/l) significantly inhibited VEGF expression in the glioma
cell lines.
HIF-1α mRNA expression was also analyzed in the U87
and SHG44 glioma cells following ATRA treatment. As shown in
Fig. 1D, qPCR revealed that,
following treatment with lower concentrations of ATRA (5 and 10
μmol/l), HIF-1α mRNA expression was upregulated in the glioma cell
lines. Treatment with 5 μmol/l ATRA was observed to upregulate
HIF-1α mRNA expression ~1.41- and 1.24-fold in the U87 and SHG44
glioma cells, respectively. Furthermore, treatment with 10 μmol/l
ATRA was found to upregulate HIF-1α mRNA expression ~2.2- and
2.5-fold in the U87 and SHG44 glioma cells, respectively (P<0.01
versus the control group). However, treatment with 40 μmol/l ATRA
was observed to significantly decrease HIF-1α mRNA expression in
glioma cells to 0.71- and 0.55-fold that in the control group in
the U87 and SHG44 glioma cells, respectively (P<0.01). HIF-1α
protein is rapidly degraded under normoxic conditions; therefore,
HIF-1α protein expression was not detected under normoxia.
Effects of ATRA on VEGF and HIF-1α
expression in glioma cells under hypoxia
Hypoxia is one of the primary regulators of
angiogenesis; therefore, the effects of ATRA on VEGF and HIF-1α
expression was analyzed under hypoxia in the U87 and SHG44 cells.
As shown in Fig. 2, hypoxia was
observed to significantly upregulate VEGF protein and mRNA
expression (P<0.01 versus the normoxia control group). However,
hypoxia only increased HIF-1α protein expression, with HIF-1α mRNA
expression unaffected by the hypoxia mimic (P>0.05).
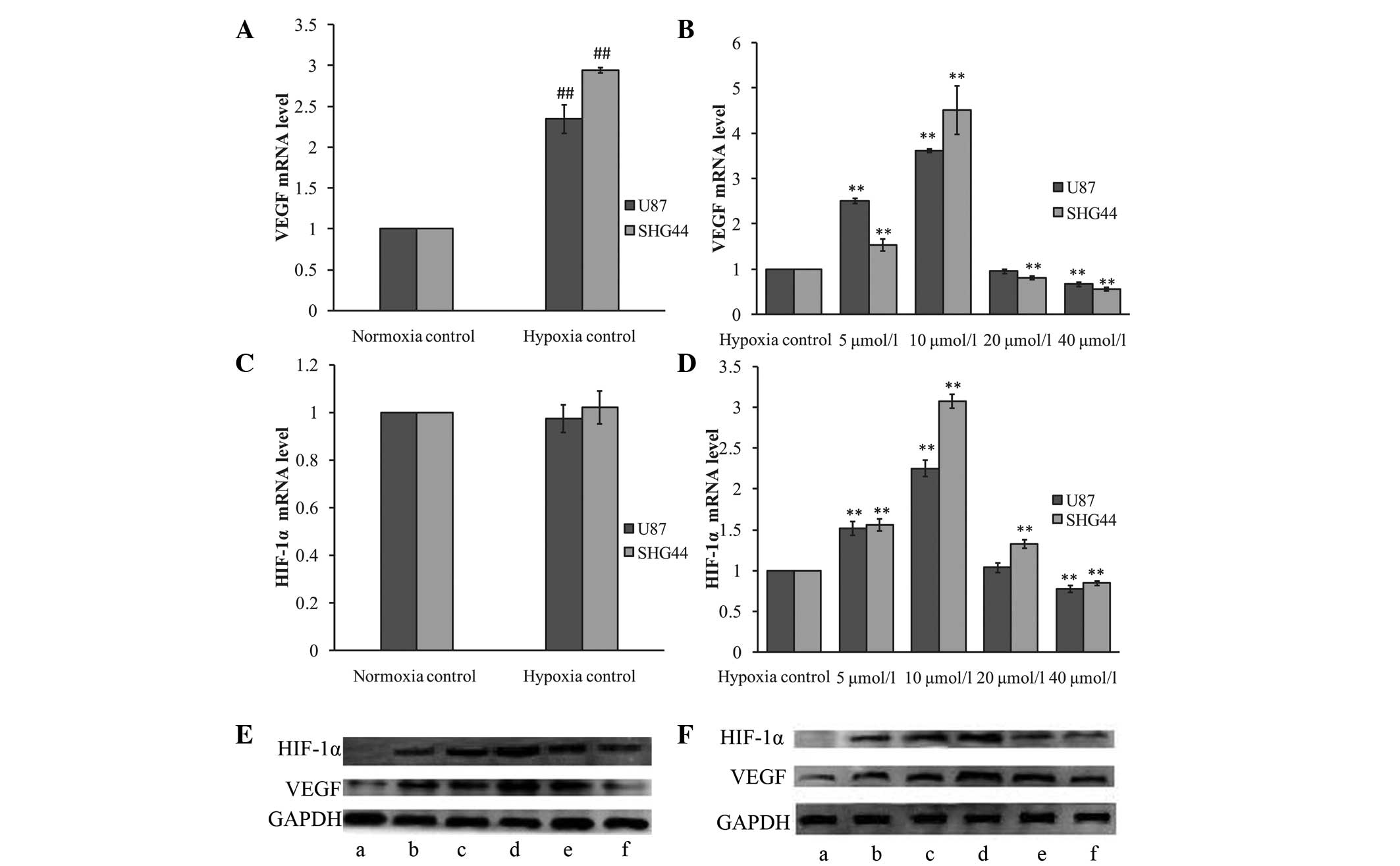 | Figure 2Effects of ATRA on VEGF and HIF-1α
expression under hypoxia. (A and C) Effect of hypoxia mimic on (A)
VEGF and (C) HIF-1α mRNA expression. Hypoxia was simulated using
100 μmol/l CoCl2. The mRNA expression of VEGF, but not
of HIF-1α, was induced by the hypoxia mimic. (B and D) Effects of
ATRA on (B) VEGF and (D) HIF-1α mRNA expression under hypoxia. U87
and SHG44 glioma cells were treated with various concentrations of
ATRA (5, 10, 20 and 40 μmol/l) for 24 h under hypoxia. The hypoxia
control group was treated with an equal volume of solvent (dimethyl
sulfoxide) and 100 μmol/l CoCl2 in the culture media.
The VEGF and HIF-1α mRNA expression increased following treatment
with 5 and 10 μmol/l ATRA in the two glioma cell lines (P<0.01
versus the hypoxia control group). Following treatment with 40
μmol/l ATRA, the VEGF and HIF-1α mRNA expression was significantly
downregulated (P<0.01 versus the hypoxia control group). (E and
F) Western blot analysis of VEGF and HIF-1α protein expression in
(E) U87 and (F) SHG44 glioma cells treated with various
concentrations of ATRA. (a) Normoxia control; (b) hypoxia control;
(c) 5, (d) 10, (e) 20 and (f) 40 μmol/l ATRA.
**P<0.01 versus the hypoxia control group;
##P<0.01 versus the normoxia control group. ATRA,
all-trans retinoic acid; VEGF, vascular endothelial growth factor;
HIF-1α, hypoxia-inducible factor-1α. |
Under hypoxia, the U87 and SHG44 glioma cells
treated with various concentrations of ATRA exhibited a similar
reaction to those under normoxia. VEGF and HIF-1α mRNA and protein
expression was significantly upregulated by lower concentrations of
ATRA (5 and 10 μmol/l), compared with the hypoxia control group
(P<0.01). However, treatment with high concentrations of ATRA
(40 μmol/l) was observed to decrease the expression of VEGF and
HIF-1α (P<0.01).
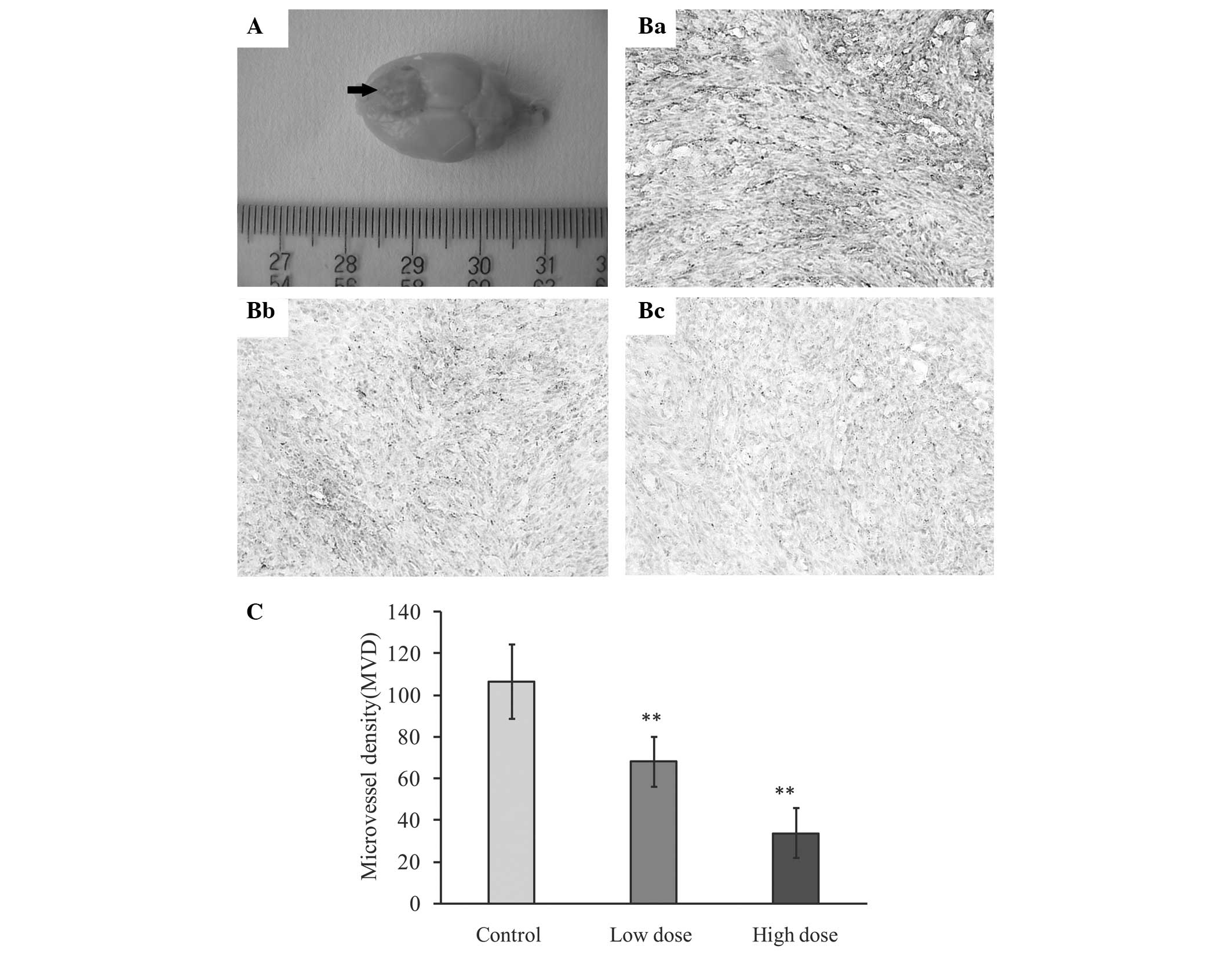
Effect of ATRA on MVD in the rat
intracerebral glioma model
In order to investigate the anti-angiogenic effect
of ATRA on glioma in vivo, CD34 was examined using
immunohistochemistry to assess glioma MVD in each group of
tumor-bearing rats. As shown in Fig.
3, ATRA was found to significantly decrease the number of
CD34-stained glioma microvessels, particularly at higher doses (10
mg/kg/day) (P<0.01 versus the control group). The average MVDs
were 106.56±17.80, 68.04±11.95 and 33.66±12.05 in the control, low-
(5 mg/kg/day) and high- (10 mg/kg/day) dose groups,
respectively.
Discussion
It is well established that VEGF is a key regulator
of angiogenesis and has an important role in the formation,
development and recurrence of glioma. Several studies have reported
that ATRA affects VEGF expression; however, the results are
controversial (29–34). Reports have demonstrated that ATRA
is capable of inhibiting VEGF expression in several cell types,
including human gastric cancer (29), esophageal squamous cell carcinoma
(30) and leukemia (31) cells. However, ATRA has been
reported to have a stimulatory effect on VEGF expression in various
other cell types, such as human umbilical vein ECs (32), human retinal pigment epithelial
cells (33) and retinoblastoma Y78
cells (34). The only consensus
regarding the effect of ATRA on VEGF expression is that the
anti-leukemia effect of ATRA is exerted through the downregulation
of VEGF (31,35–37).
The contradictory results produced by these studies may be a
consequence of differences in cell type, methodology, experimental
conditions and the concentrations of ATRA. However, little is known
concerning the effect of ATRA on VEGF expression in glioma.
In the present study, ATRA was observed to have a
dose-dependent effect on VEGF expression in glioma cells. Lower
concentrations of ATRA were found to significantly increase VEGF
expression at the transcriptional and translational level in glioma
cells. However, high concentrations of ATRA were observed to
significantly decrease the expression of VEGF mRNA and protein.
These converse effects have also been reported in ECs. Saito et
al (32) reported that, at
concentrations between 1 nmol/l and 1 μmol/l, ATRA induced the
expression of VEGF and its receptor, VEGFR-2, in ECs. However, it
has also been reported that 5 μmol/l ATRA decreases VEGF and
VEGFR-2 expression, as well as Akt phosphorylation in ECs (38). In addition to the differences in
methodology and experimental conditions in these two studies, the
different concentrations of ATRA may have contributed to the
contradictory results. The present study revealed that ATRA has a
concentration-dependent effect on VEGF mRNA and protein expression
in glioma cells.
The mechanism underlying the ATRA-induced regulation
of VEGF expression is yet to be elucidated. Evidence suggests that
ATRA increases the protein expression of specificity protein (Sp) 1
through post-transcriptional mechanisms, and that elevated Sp1
protein levels induce VEGF expression (34). It has also been shown that ATRA is
capable of directly inducing VEGF expression through the RAR
signaling pathway (32). However,
it has additionally been suggested that ATRA may decrease VEGF gene
transcription through a direct repeat 1 element located at the
transcription initiation site (31). Furthermore, HIF-1α is well
established as a key regulator of VEGF expression. Studies have
indicated that ATRA regulates VEGF expression through the
regulation of HIF-1α (39,40). Therefore, in the present study, the
effect of ATRA on HIF-1α expression in glioma cells was
investigated.
The effect of ATRA on HIF-1α mRNA expression was
observed to be similar to that on VEGF expression. Lower
concentrations of ATRA significantly increased HIF-1α expression
and high concentrations of ATRA significantly decreased HIF-1α
expression. HIF-1α protein is rapidly degraded under normoxic
conditions and is undetectable; therefore, hypoxia was mimicked
using CoCl2, and the effect of ATRA on VEGF and HIF-1α
expression, particularly HIF-1α protein expression, was
investigated in glioma cells under hypoxia.
Hypoxia can induce angiogenesis in numerous
physiological and pathological processes, primarily through the HIF
pathway. In the present study, mimicking hypoxia was found to
significantly increase HIF-1α protein, but not mRNA, expression.
This phenomenon is associated with the mechanism underlying
CoCl2-mediated hypoxia mimicry (41). Briefly, cobalt displaces iron from
the iron-binding site of the enzyme, thus interrupting with the
degradation of the HIF-1α protein under normoxia. In the present
study, following treatment with different concentrations of ATRA
under hypoxia, similar dose-dependent changes in HIF1-α and VEGF
protein expression were observed. Thus, there is reason to
speculate that the regulation of VEGF expression by ATRA in glioma
cells, may partially be through the regulation of HIF-1α
expression.
The mechanism underlying the regulation of HIF-1α
expression by ATRA remains unclear. It has been suggested that the
ATRA-induced upregulation of HIF-1α expression may involve the
stabilization of HIF-1α mRNA (39). It has also been reported that ATRA
can induce HIF-1α expression through intracrine prostaglandin
E2 signaling (40).
However, the mechanisms underlying the inhibitory effect of high
concentrations of ATRA on HIF-1α expression are yet to be
elucidated, and further research is required.
In the present study, a rat intracerebral glioma
model was generated in order to investigate the effect of ATRA on
the inhibition of glioma angiogenesis in vivo. ATRA has been
reported to inhibit angiogenesis in solid tumors, including
esophageal squamous cell carcinoma (30). In the present study, ATRA was found
to significantly decrease glioma MVD, particularly in the high-dose
group.
The results of the present study suggest that,
despite the increased expression of VEGF induced by lower
concentrations of ATRA in glioma cells in vitro, ATRA has
strong anti-angiogenic effects on glioma in vivo. However,
the mechanisms underlying the anti-angiogenic effects of ATRA on
glioma in vivo are yet to be fully elucidated. The findings
of the present study suggest that the inhibitory effects of high
concentrations of ATRA on VEGF and HIF-1α expression are likely to
be associated with the anti-angiogenic mechanisms. Furthermore,
ATRA has been reported to significantly inhibit the secretion and
expression of HGF in glioma cells (42). HGF is an important pro-angiogenic
cytokine; therefore, the inhibition of HGF may contribute to these
anti-angiogenic effects in vivo. However, ATRA-mediated
differentiation has also been shown to reduce angiogenesis in
stem-like glioma cells in vivo (4). Therefore, the mechanisms underlying
the anti-angiogenic effect of ATRA in vivo are likely to be
complex and require further investigation.
VEGF has an important role in angiogenesis. The
upregulation of VEGF expression in glioma cells induced by lower
concentrations of ATRA may reduce its therapeutic effects on glioma
in vivo. Therefore, as a therapeutic strategy, ATRA has
certain limitation; the combination of ATRA with therapies
targeting VEGF may have greater beneficial effects for the
treatment of glioma.
In conclusion, in the present study, ATRA was found
to have a concentration-dependent effect on the expression of VEGF
and HIF-1α. Furthermore, ATRA was observed to inhibit glioma
angiogenesis in vivo; however, further research is required
in order to reveal its mechanisms.
References
|
1
|
Zang CB, Wächter M, Liu H, et al: Ligands
for PPARgamma and RAR cause induction of growth inhibition and
apoptosis in human glioblastomas. J Neurooncol. 65:107–118. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Tang K, Cao L, Fan SQ, et al: Effect of
all-trans-retinoic acid on C6 glioma cell proliferation and
differentiation. Zhong Nan Da Xue Xue Bao Yi Xue Ban. 33:892–897.
2008.(In Chinese).
|
|
3
|
Karsy M, Albert L, Tobias ME, Murali R and
Jhanwar-Uniyal M: All-trans retinoic acid modulates cancer stem
cells of glioblastoma multiforme in an MAPK-dependent manner.
Anticancer Res. 30:4915–4920. 2010.PubMed/NCBI
|
|
4
|
Campos B, Wan F, Farhadi M, et al:
Differentiation therapy exerts antitumor effects on stem-like
glioma cells. Clin Cancer Res. 16:2715–2728. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Chang Q, Chen Z, You J, et al:
All-trans-retinoic acid induces cell growth arrest in a human
medulloblastoma cell line. J Neurooncol. 84:263–267. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Li S, Gao Y, Pu K, Ma L, Song X and Liu Y:
All-trans retinoic acid enhances bystander effect of suicide-gene
therapy against medulloblastomas. Neurosci Lett. 503:115–119. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Das A, Banik NL and Ray SK: Molecular
mechanisms of the combination of retinoid and interferon-gamma for
inducing differentiation and increasing apoptosis in human
glioblastoma T98G and U87MG Cells. Neurochem Res. 34:87–101. 2009.
View Article : Google Scholar
|
|
8
|
Haque A, Banik NL and Ray SK: Emerging
role of combination of all-trans retinoic acid and interferon-gamma
as chemoimmunotherapy in the management of human glioblastoma.
Neurochem Res. 32:2203–2209. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhang R, Banik NL and Ray SK: Combination
of all-trans retinoic acid and interferon-gamma upregulated
p27(kip1) and down regulated CDK2 to cause cell cycle arrest
leading to differentiation and apoptosis in human glioblastoma LN18
(PTEN-proficient) and U87MG (PTEN-deficient) cells. Cancer
Chemother Pharmacol. 62:407–416. 2008. View Article : Google Scholar
|
|
10
|
Karmakar S, Banik NL, Patel SJ and Ray SK:
Combination of all-trans retinoic acid and taxol regressed
glioblastoma T98G xenografts in nude mice. Apoptosis. 12:2077–2087.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Karmakar S, Banik NL and Ray SK:
Combination of all-trans retinoic acid and paclitaxel-induced
differentiation and apoptosis in human glioblastoma U87MG
xenografts in nude mice. Cancer. 112:596–607. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Distler JH, Hirth A, Kurowska-Stolarska M,
Gay RE, Gay S and Distler O: Angiogenic and angiostatic factors in
the molecular control of angiogenesis. Q J Nucl Med. 47:149–161.
2003.PubMed/NCBI
|
|
13
|
Folkman J and Klagsbrun M: Angiogenic
factors. Science. 235:442–447. 1987. View Article : Google Scholar
|
|
14
|
Muramatsu R, Takahashi C, Miyake S,
Fujimura H, Mochizuki H and Yamashita T: Angiogenesis induced by
CNS inflammation promotes neuronal remodeling through
vessel-derived prostacyclin. Nat Med. 18:1658–1664. 2012.
View Article : Google Scholar
|
|
15
|
Crawford TN, Alfaro DV III, Kerrison JB
and Jablon EP: Diabetic retinopathy and angiogenesis. Curr Diabetes
Rev. 5:8–13. 2009. View Article : Google Scholar
|
|
16
|
Würdinger T and Tannous BA: Glioma
angiogenesis: Towards novel RNA therapeutics. Cell Adh Migr.
3:230–235. 2009.PubMed/NCBI
|
|
17
|
Chen H, Fan K, Wang S, Liu Z and Zheng Z:
Dual targeting of glioma U251 cells with nanoparticles prevents
tumor angiogenesis and inhibits tumor growth. Curr Neurovasc Res.
9:133–138. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Guo SW, Che HM and Li WZ: Anti-tumor
effect of lentivirus-mediated gene transfer of alphastatin on human
glioma. Cancer Sci. 102:1038–1044. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Vredenburgh JJ, Desjardins A, Herndon JE
II, et al: Phase II trial of bevacizumab and irinotecan in
recurrent malignant glioma. Clin Cancer Res. 13:1253–1259. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kreisl TN, Kim L, Moore K, et al: Phase II
trial of single-agent bevacizumab followed by bevacizumab plus
irinotecan at tumor progression in recurrent glioblastoma. J Clin
Oncol. 27:740–745. 2009. View Article : Google Scholar
|
|
21
|
Plate KH, Breier G, Weich HA, Mennel HD
and Risau W: Vascular endothelial growth factor and glioma
angiogenesis: coordinate induction of VEGF receptors, distribution
of VEGF protein and possible in vivo regulatory mechanisms. Int J
Cancer. 59:520–529. 1994. View Article : Google Scholar
|
|
22
|
Bodey B, Siegel SE and Kaiser HE:
Up-regulation of VEGF expression and related neo-angiogenesis in
childhood high-grade gliomas: implications for anti-angiogenic
anti-neoplastic therapy. In Vivo. 20:511–518. 2006.PubMed/NCBI
|
|
23
|
Zagzag D, Lukyanov Y, Lan L, et al:
Hypoxia-inducible factor 1 and VEGF upregulate CXCR4 in
glioblastoma: implications for angiogenesis and glioma cell
invasion. Lab Invest. 86:1221–1232. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Li D, Li XP, Wang HX, et al: VEGF induces
angiogenesis in a zebrafish embryo glioma model established by
transplantation of human glioma cells. Oncol Rep. 28:937–942.
2012.PubMed/NCBI
|
|
25
|
Carmeliet P, Dor Y, Herbert JM, et al:
Role of HIF-1alpha in hypoxia-mediated apoptosis, cell
proliferation and tumour angiogenesis. Nature. 394:485–490. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Conway EM, Collen D and Carmeliet P:
Molecular mechanisms of blood vessel growth. Cardiovasc Res.
49:507–521. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
|
|
28
|
Weidner N, Semple JP, Welch WR and Folkman
J: Tumor angiogenesis and metastasis - correlation in invasive
breast carcinoma. N Engl J Med. 324:1–8. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zhang JP, Chen XY and Li JS: Effects of
all-trans-retinoic on human gastric cancer cells BGC-823. J Dig
Dis. 8:29–34. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Lu TY, Li WC, Chen RY, et al: Inhibition
effects of all trans-retinoic acid on the growth and angiogenesis
of esophageal squamous cell carcinoma in nude mice. Chin Med J
(Engl). 124:2708–2714. 2011.PubMed/NCBI
|
|
31
|
Tee MK, Vigne JL and Taylor RN: All-trans
retinoic acid inhibits vascular endothelial growth factor
expression in a cell model of neutrophil activation. Endocrinology.
147:1264–1270. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Saito A, Sugawara A, Uruno A, et al:
All-trans retinoic acid induces in vitro angiogenesis via retinoic
acid receptor: possible involvement of paracrine effects of
endogenous vascular endothelial growth factor signaling.
Endocrinology. 148:1412–1423. 2007. View Article : Google Scholar
|
|
33
|
Chen JT, Liang JB, Chou CL, Shyu RC and Lu
DW: Retinoic acid induces VEGF gene expression in human retinal
pigment epithelial cells (ARPE-19). J Ocul Pharmacol Ther.
21:413–419. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Akiyama H, Tanaka T, Maeno T, et al:
Induction of VEGF gene expression by retinoic acid through
Sp1-binding sites in retinoblastoma Y79 cells. Invest Ophthalmol
Vis Sci. 43:1367–1374. 2002.PubMed/NCBI
|
|
35
|
Wang C, Chen FY, Gu CH, et al: Effect of
ATRA and DNR on the expression and secretion of VEGF in leukemic
cells. Zhonghua Xue Ye Xue Za Zhi. 25:171–174. 2004.(In
Chinese).
|
|
36
|
Ye J, Liu FQ and Wu YP: The effect of
all-trans retinoid acid and sodium selenite
(Na2SeO3) on VEGF and its receptor expression
in HL-60 cells. Zhongguo Shi Yan Xue Ye Xue Za Zhi. 12:142–146.
2004.(In Chinese).
|
|
37
|
Bai X, Fu JX, Ding KY, Cen JN, Wang W and
Ruan CG: Quantitative analysis of gene expression for vascular
endothelial growth factor and its application. Zhongguo Shi Yan Xue
Ye Xue Za Zhi. 13:548–552. 2005.(In Chinese).
|
|
38
|
Cho DH, Choi YJ, Jo SA, Nam JH, Jung SC
and Jo I: Retinoic acid decreases nitric oxide production in
endothelial cells: a role of phosphorylation of endothelial nitric
oxide synthase at Ser(1179). Biochem Biophys Res Commun.
326:703–710. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Fernández-Martinez AB, Jiménez MI,
Hernández IS, et al: Mutual regulation of hypoxic and retinoic acid
related signalling in tubular proximal cells. Int J Biochem Cell
Biol. 43:1198–1207. 2011.PubMed/NCBI
|
|
40
|
Fernández-Martinez AB, Arenas Jiménez MI
and Lucio Cazaña FJ: Retinoic acid increases hypoxia-inducible
factor-α through intracrine prostaglandin E(2) signaling in human
renal proximal tubular cells HK-2. Biochim Biophys Acta.
1821:672–683. 2012.
|
|
41
|
Ardyanto TD, Osaki M, Tokuyasu N, Nagahama
Y and Ito H: CoCl2-induced HIF-1 alpha expression
correlates with proliferation and apoptosis in MKN-1 cells: A
possible role for the PI3K/Akt pathway. Int J Oncol. 29:549–555.
2006.PubMed/NCBI
|
|
42
|
Chattopadhyay N, Butters RR and Brown EM:
Agonists of the retinoic acid- and retinoid X-receptors inhibit
hepatocyte growth factor secretion and expression in U87 human
astrocytoma cells. Brain Res Mol Brain Res. 87:100–108. 2001.
View Article : Google Scholar : PubMed/NCBI
|