Introduction
Vitamin A (VA) is an essential fat-soluble
micronutrient. Retinoic acid (RA) is the main active metabolite of
VA in vivo, and it exists in three isomers:
All-trans-retinoic acid (ATRA), 13-cis-retinoic acid
(13-cis-RA) and 9-cis-retinoic acid
(9-cis-RA). ATRA is able to regulate the transcriptional
activity of downstream target genes through retinoic acid receptor
(RAR)-mediated signal transduction. RARs consist of two families,
the RARs and the retinoid X receptors (RXRs), with each family
composed of three isotypes: α, β and γ. The RARα subtype has an
important role in neuronal development (1–3).
A previous study by our group identified that
neonatal rats with VA deficiency during the gestational period
exhibited a reduction in the expression of RARα, coupled with
deficits in learning and memory function in adulthood (4). It has also been demonstrated that a
VA deficiency reduces the recovery ability of learning and memory
function in pups following hypoxic-ischemic brain damage (HIBD),
which is regulated by the RARα signaling pathway (5). The RARα signaling pathway is
activated by ATRA, which triggers a variety of biological
functions. Several previous studies investigated the mechanism of
apoptosis in neuronal cells and the effect of antagonizing neuronal
apoptosis in brain damage (6–14).
However, whether VA has an anti-apoptotic function and the
mechanisms underlying these effects in neuronal cells have remained
elusive.
The present study aimed to establish an oxygen and
glucose deprivation (OGD) model in vitro to investigate
whether ATRA is neuroprotective by suppressing the apoptosis of
neuronal cells. It was revealed that ATRA treatment at 4 μmol/l
suppresses apoptosis of PC12 cells injured by OGD through the RARα
signaling pathway. The study of ATRA may facilitate and promote the
clinical application of VA as an adjuvant therapy for patients with
brain damage.
Materials and methods
Culturing PC12 cells
PC12 cells were purchased from the Cell Bank of
Shanghai Institute of Cell Biology, Chinese Academy of Sciences
(Shanghai, China). The cells were cultured in Dulbecco’s modified
Eagle’s medium (DMEM; Hyclone, Logan, UT, USA) containing 10% horse
serum (HS; Gibco-BRL, Grand Island, NY, USA), 5% fetal bovine serum
(FBS; Gibco-BRL), 100 units/ml penicillin and 100 μg/ml
streptomycin at 37°C in a 5% CO2 incubator. At 90%
confluence, the PC12 cells were digested with TrypLE (Gibco-BRL)
and passaged at a ratio of 1:2 every 3–4 days.
Construction of an in vitro OGD
model
The PC12 cells were seeded and grown to ~90%
confluence in a six-well plate. To establish the OGD model, the
culture medium of PC12 cells was changed to Earle’s balanced salt
solution (EBSS; Hyclone; 11.6 mmol/l NaCl, 5.4 mmol/l KCl, 0.8
mmol/l MgSO4, 10 mmol/l NaH2PO4,
26.2 mmol/l NaHCO3, 1.8 mmol/l CaCl2, 10 mg/l
phenol red and no glucose; pH 7.4) following washing of the cells
twice with D-Hank’s solution, which was prepared by resolving
Hank’s balanced salt mixture (Hycyclone Laborotories, Inc., Omaha,
Nebraska, USA) with double distilled water. The cells were then
placed in an incubator (Thermo Forma 3111; Thermo Scientific;
Waltham, MA, USA) with 5% O2 and 95% N2 at
37°C for 6 h.
ATRA treatment
ATRA (R2625; Sigma, St. Louis, MO, USA) was added to
the culture medium (DMEM) of PC12 cells at final concentrations of
0.5, 2, 4 and 20 μmol/l for 24 h. Next, the PC12 cells pre-treated
with each concentration of ATRA were immediately injured by OGD.
PC12 cells without ATRA treatment served as a positive control.
RNA interference of RARα
A recombinant adenovirus of RARα-small interfering
(si)RNA was designed and constructed in our laboratory (5). Three types of RARα-siRNA adenovirus
were pooled to transfect into PC12 cells for 36 h. A pair of
unspecific siRNA was labeled with a red fluorescent protein, RFP,
and served as a negative control. The infection efficiency and
consistency of Ad-siRARα and Ad-RFP were confirmed using
fluorescent microscopy (Nijon TE2000-A; Nikon, Tokyo, Japan).
RNA isolation and quantitative polymerase
chain reaction (qPCR) analysis
Total RNA was isolated using an RNA extraction kit
(Genemega Inc., San Diego, CA, USA) and reverse-transcribed into
cDNA using the PrimeScript® RT reagent kit (DRR037A;
Takara Bio, Inc., Shiga, Japan) using a Bio-Rad My Cycler (Bio-Rad
Laboratories, Inc., Hercules, CA, USA) according to the
manufacturer’s instructions. The single-stranded cDNA was diluted
5- to 10-fold and used as the PCR template. The cDNA was quantified
using real-time PCR with the StepOne v2.1 Real-Time PCR instrument
(ABI; Redwood City, CA, USA) and RealMasterMix (SYBR Green; Tiangen
Biotech Co., Ltd., Beijing, China). The procedure was performed as
follows: Denaturation at 95°C for 10 min, followed by 45 cycles at
95°C for 15 sec, 60°C for 60 sec and 72°C for 30 sec. Melting curve
analysis and gel electrophoresis were utilized to ensure that a
single PCR product was amplified in each reaction. The PCR primers
were designed using Primer Premier 5.0 software (Premier Biosoft
International, Paolo Alto, CA, USA). The primer sequences for RARα,
B-cell lymphoma 2-associated X (Bax), B-cell lymphoma 2 (Bcl-2) and
β-actin are summarized in Table
I.
 | Table IPrimer sequences for polymerase chain
reaction. |
Table I
Primer sequences for polymerase chain
reaction.
| Gene | Primer sequences |
|---|
| RARα | F:
5′CAGGAGGGAGAAGGCAGTGAC3′
R: 5′ATGGCTTGAGTTCGGAGGACAG3′ |
| Bax | F:
5′AAGTAGAAGAGGGCAACCAC3′
R: 5′GATGGCAACTTCAACTGGG3′ |
| Bcl-2 | F:
5′CGGGAGAACAGGGTATGA3′
R: 5′CAGGCTGGAAGGAGAAGAT3′ |
| β-actin | F:
5′GCATAGCCACGCTTGTTCTTGAAG3′
R: 5′GAACCGCTCATTGCCGATAGTG3′ |
The data were presented as the Ct value and the
ratio of the relative quantity of the target gene to β-actin was
calculated. A semi-quantitative PCR reaction was conducted using
the following protocol: 94°C for 20 sec, 68°C for 30 sec and 70°C
for 20 sec for 16 cycles, with a 1°C decrease per cycle, followed
by 25–32 cycles at 94°C for 20 sec, 56°C for 30 sec and 70°C for 20
sec. The PCR products were resolved on 1.5% agarose gels. All of
the samples were normalized to the endogenous levels of β-actin.
All operations were conducted in accordance with the standard
protocol.
Western blot analysis
The cells were collected from dishes or wells and
lysed in radioimmunoprecipitation assay (RIPA) buffer containing
phenylmethanesulfonyl fluoride (Biotake Co., Ltd., Taiwan, China).
The cell lysates were collected and the protein concentration was
determined using a bicinchoninic acid protein concentration
determination kit (Biotake Co., Ltd., Beijing, China). The cell
lysates were purified by 10% SDS-Page (Beyotime, China), with ~20
μg of total protein loaded per lane. Following electrophoresis, the
proteins were transferred to an Immobilon-P membrane (Millipore,
Billerica, MA, USA). Following blocking with 5% FBS in
Tris-buffered saline/Tween-20 (TBST) buffer at room temperature for
1 h, the membrane was probed overnight with the primary antibodies,
goat polyclonal anti-RARα (28767; 1:200–1:2,000; Abcam, Cambridge,
MA, USA), mouse monoclonal anti-Bax (B8429; 1:30–1:200; Sigma),
mouse monoclonal anti-Bcl-2 (sc-7382; 1:100–1:1,000; Santa Cruz
Biotechnology, Inc., Santa Cruz, CA, USA) and mouaw monoclonoal
β-actin (sc-130065; 1:100–1:1,000; Santa Cruz Biotechnology, Inc.),
respectively, at 4°C and was then incubated with the appropriate
secondary antibody conjugated with horseradish peroxidase (Santa
Cruz Biotechnology, Inc.) at room temperature for 1 h. The protein
was detected using an enhanced chemiluminescent substrate kit of
chemiluminescent HRP substrate (Millipore, Billerica, MA, USA) and
recorded using a Kodak 440CF Image Station (Kodak, Rochester, NY,
USA).
Measurement of apoptosis
The apoptotic rate of the PC12 cells was determined
using an Annexin V-enhanced green fluorescent protein
(EGFP)/propidium iodide (PI) Apoptosis Detection kit (KGA103;
KeyGen Biotech, Nanjing, China) and flow cytometric analysis.
Following OGD treatment, the cells were collected, washed twice
with cold PBS and resuspended in 400 μl of 1X Annexin V binding
buffer at a concentration of ~1×106 cells/ml. A total of
5 μl of Annexin V-EGFP solution was added to the cell suspension
and incubated for 15 min at 2–8°C in the dark. Next, 10 μl of PI
was added to the mixture for 5 min. The stained cells were analyzed
using flow cytometry (FacsCalibur; BD Biosciences, Franklin Lakes,
NJ, USA) within 1 h. Annexin V labeled with a fluorophore
identified cells at the early stage of apoptosis, and PI, a
fluorescent nucleic acid binding dye, stained cells in the middle
and late stages of apoptosis. The apoptotic rate was calculated as
the percentage of Annexin V-positive and PI-negative cells divided
by the total number of cells in the imaged region.
Measurement of the mitochondrial membrane
potential (MMP)
The MMP change of the PC12 cells was measured using
a mitochondrial membrane potential assay kit with JC-1 (C2006;
Beyotime Institute of Biotechnology, Shanghai, China) and flow
cytometric analysis. JC-1 is widely used as a fluorescent probe to
detect the MMP. When the MMP is high, the dye assembles in a
dimeric configuration in the mitochondrial matrix and emits a red
fluorescent signal. When the MMP is low, the dye exists in a
monomeric configuration and emits a green fluorescent signal.
Therefore, changes in the different fluorescent signals emitted by
JC-1 reflect changes in the MMP. The percentage of fluorescent
intensity reflects changes in the MMP and the increase in the
percentage of green fluorescence intensity suggests a decrease in
the MMP. A decrease in the MMP is an early indicator of apoptosis.
The MMP measurement was performed according to the manufacturer’s
instructions. Briefly, 1–6×105 cells were incubated in a
mixture of 0.5 ml culture medium and 0.5 ml JC-1 staining working
fluid at 37°C for 20 min. Following centrifugation, the cells were
washed twice with cold JC-1 staining buffer (1×) and resuspended
using JC-1 staining buffer for analysis via flow cytometry within 1
h.
Statistical analysis
Values are expressed as the mean ± standard error of
measurements. Significant differences between samples were analyzed
using a one-way analysis of variance (ANOVA), a repeated-measures
ANOVA and the Student-Newman-Keuls test (SNK-q). All statistical
analyses were computed using GraphPad Prism 5.0 (GraphPad Software,
Inc., La Jolla, CA, USA) software by professional staff. P≤0.05 was
considered to indicate a statistically significant difference.
Results
Construction of an OGD model in PC12
cells
The PC12 cell line was derived from transplantable
rat pheochromocytoma. The PC12 cells in culture exhibited clearly
visible spindle-or oval-shaped cell bodies, while they exhibited
shrinkage, light refraction with decreased, chrysanthemum-like cell
body shrinkage and an increase in floating cells following injury
with OGD when compared with the normal PC12 cells (Fig. 1A). Flow cytometric analysis
indicated that the apoptotic rate of PC12 cells following OGD
treatment increased to 18.51±1.42%, which was significantly
different from that in the uninjured group (8.08±0.90%; Fig. 1B and C). Flow cytometry was also
used to analyze the changes in the MMP in the PC12 cells. It was
identified that the percentage of green fluorescence intensity of
JC-1 in PC12 cells also significantly increased, from 4.14±1.47% in
the untreated group to 14.47±3.73% in the OGD-injured group
(Fig. 1D and E), suggesting that
OGD injury decreased the MMP in PC12 cells. The mRNA and protein
expression of apoptosis-associated-factor Bax was upregulated
following ODG injury. The mRNA expression of anti-apoptotic factor
Bcl-2 in the OGD-injured group was marginally increased when
compared with the uninjured group (Fig. 1F); however, the protein expression
of Bcl-2 was not significantly different (Fig. 1G). These data demonstrated that the
OGD model in vitro effectively induced apoptosis of PC12
cells.
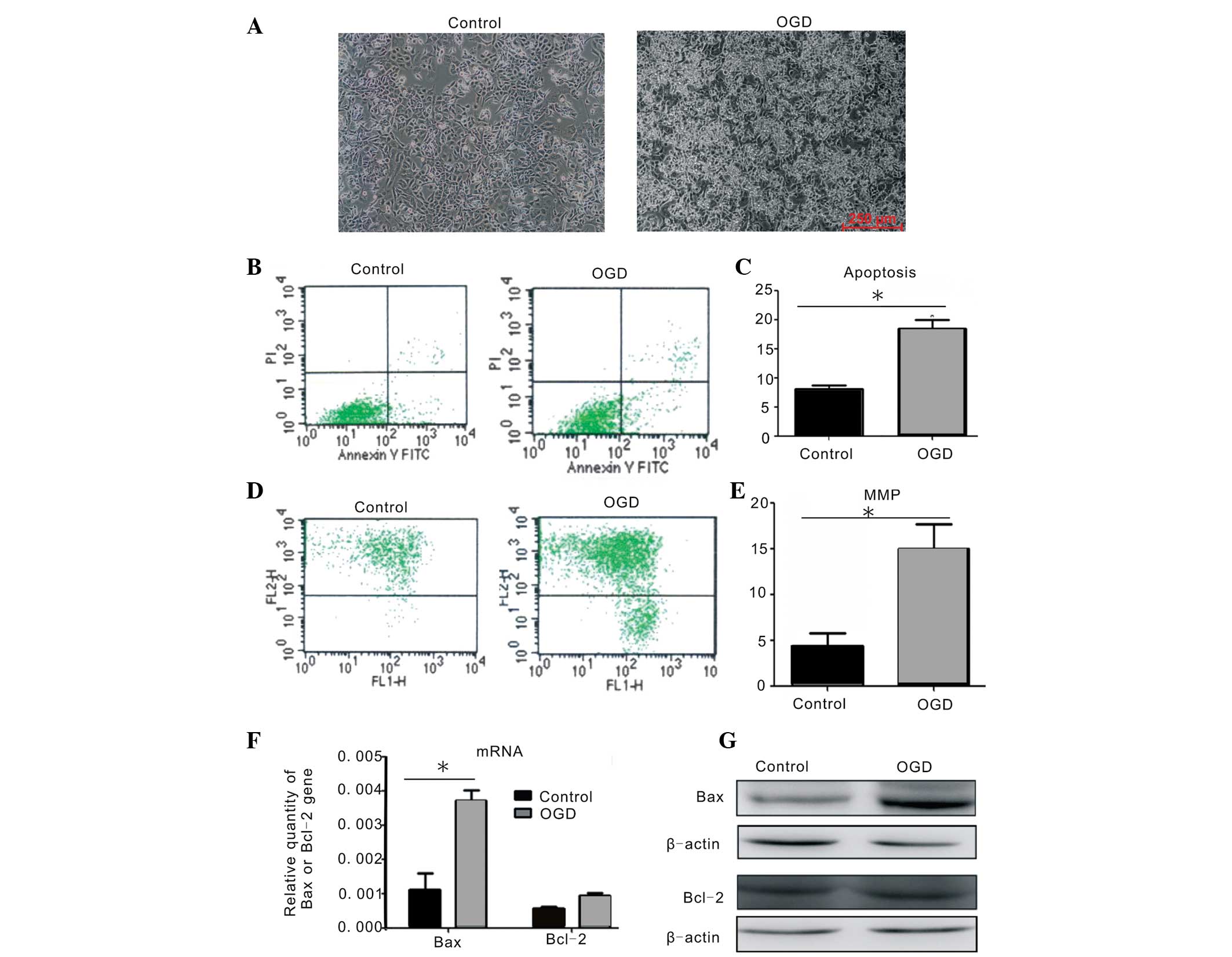 | Figure 1Apoptosis in PC12 cells injured by
OGD. (A) Morphological changes in PC12 cells with or without OGD
injury. Scale bar, 250 μm (magnification, ×100). (B) The percentage
of apoptosis in injured PC12 cells following OGD treatment as
measured by flow cytometry. (C) Quantitative comparison of the
percentage of apoptotic cells in the control and OGD-injured PC12
cells (*P<0.05 compared with control group, by
one-way ANOVA). (D) Percentage of green fluorescent intensity of
JC-1 in the PC12 cells using a mitochondrial membrane potential
assay kit and flow cytometric analysis. (E) Quantitative comparison
of the percentage of green fluorescence intensity in the PC12 cells
with or without OGD treatment (*P<0.05 compared with
control group, by one-way ANOVA). (F–G) Changes in mRNA and protein
expression of Bax and Bcl-2 in PC12 cells following OGD injury as
measured using quantitative polymerase chain reaction and western
blott analysis. These results were confirmed in at least three
independent experiments and normalized to β-actin
(*P<0.05 vs. control, by one-way ANOVA). OGD, oxygen
glucose deprivation; MMP, mitochondrial membrane potential; ANOVA,
analysis of variance; Bcl-2, B-cell lymphoma 2; Bax,
Bcl-2-associated X. |
Effect of ATRA treatment on PC12 cells
injured by OGD
To examine the effect of ATRA, the PC12 cells were
pretreated with four different concentrations prior to OGD injury.
As revealed in Fig. 2A, the
apoptotic rate of PC12 cells following OGD was 14.01±1.26 and
11.48±1.53% in the 2 and 4 μmol/l ATRA groups, respectively, which
was significantly decreased compared with the OGD-treated group
(P<0.05). However, the apoptotic rate of the 0.5 μmol/l
ATRA-treated group was equal to that of the OGD-treated group,
whereas the ratio was significantly increased following 20 μmol/l
ATRA treatment (P<0.05). The percentage of green fluorescent
intensity reversely indicating MMP levels was in parallel with the
changes in apoptotic rates (Fig.
2B). Pretreatment with 4 μmol/l ATRA increased the MMP and
decreased the apoptotic rate to a greater extent than 2 μmol/l
ATRA; however, the differences were not statistically significant.
The 4 μmol/l ATRA treatment was determined to be the optimal
anti-apoptotic concentration of ATRA to use in the subsequent
experiments, as it caused apoptosis more effectively.
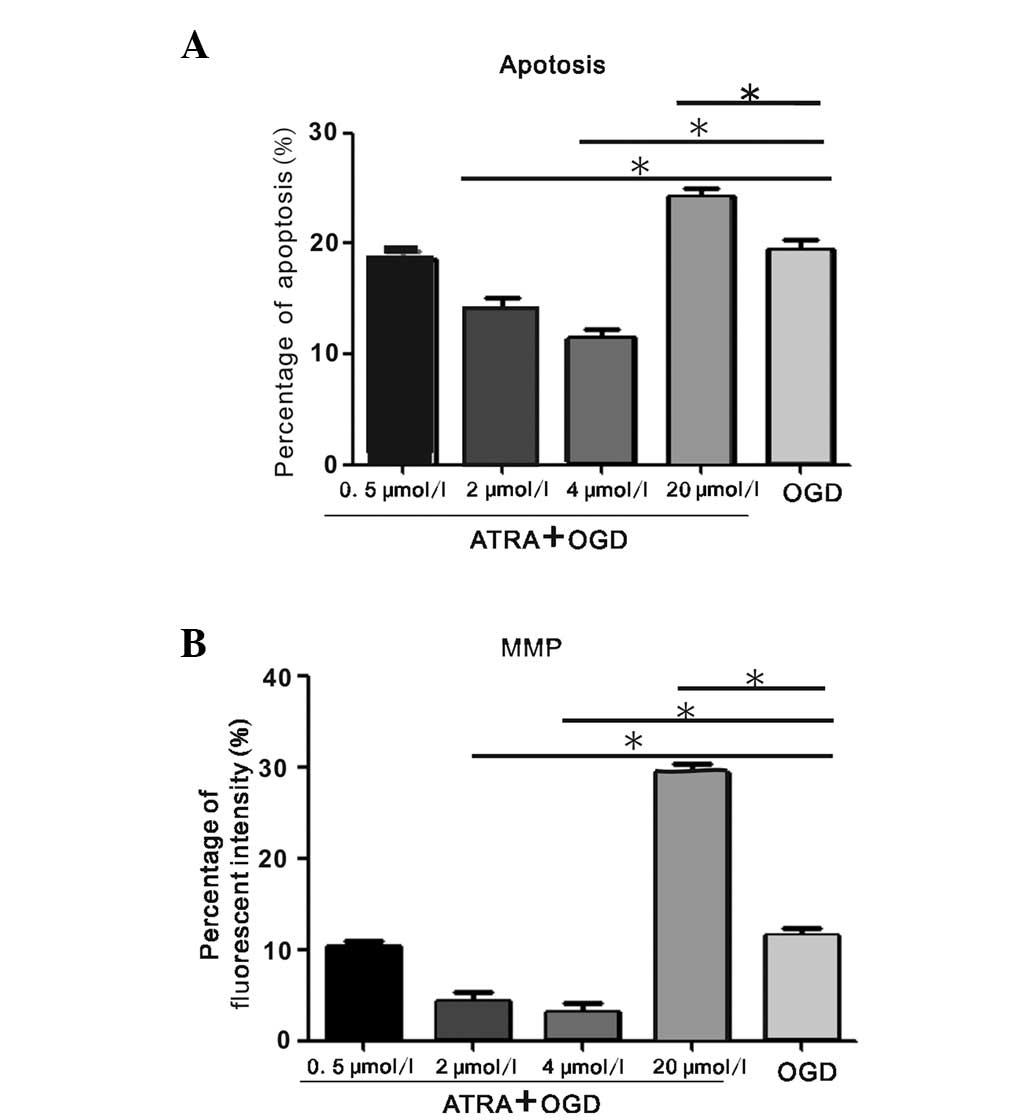 | Figure 2Detection of apoptosis and MMP change
in PC12 cells injured by OGD following ATRA treatment. (A)
Quantification of the apoptotic rate in OGD-injured PC12 cells
following treatment with ATRA at four concentrations (0.5, 2, 4 and
20 μmol/l) as measured by FACS (*P<0.05 vs. the OGD
group without ATRA treatment, by one-way ANOVA). (B) Quantification
of the percentage of green fluorescence intensity reversely
correlated with the MMP in OGD-injured PC12 cells following ATRA
treatment at four concentrations (0.5, 2, 4 and 20 μmol/l) as
measured by FACS (*P<0.05 vs. the OGD group without
ATRA treatment, by one-way ANOVA). The above data were obtained
from at least three independent experiments and the reported values
are expressed as the mean ± standard error of the mean. MMP,
mitochondrial membrane potential; OGD, oxygen glucose deprivation;
ATRA, all-trans-retinoic acid; ANOVA, analysis of variance; FACS,
fluorescence-activated cell sorting. |
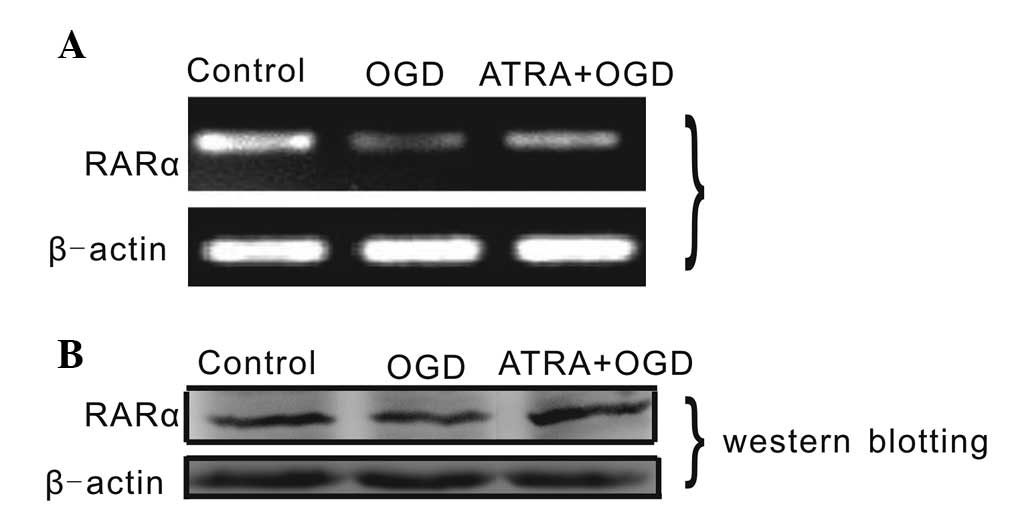
RARα mRNA and protein expression levels
are altered following OGD treatment with ATRA at 4 μmol/l
To examine the effects of ATRA on PC12 cells, the
changes in RARα expression levels were detected by qPCR and western
blot analysis (Fig. 3). The mRNA
expression of RARα in OGD-injured PC12 cells increased following
treatment with 4 μmol/l ATRA, whereas the expression decreased
following OGD injury without any other treatment. The protein
expression levels of RARα in OGD-injured PC12 cells treated with 4
μmol/l ATRA were higher than those in OGD-injured cells without
ATRA treatment. This finding suggested that the levels of RARα
expression are affected by OGD and 4 μmol/l ATRA, and that RARα is
involved in ATRA-mediated signaling during OGD.
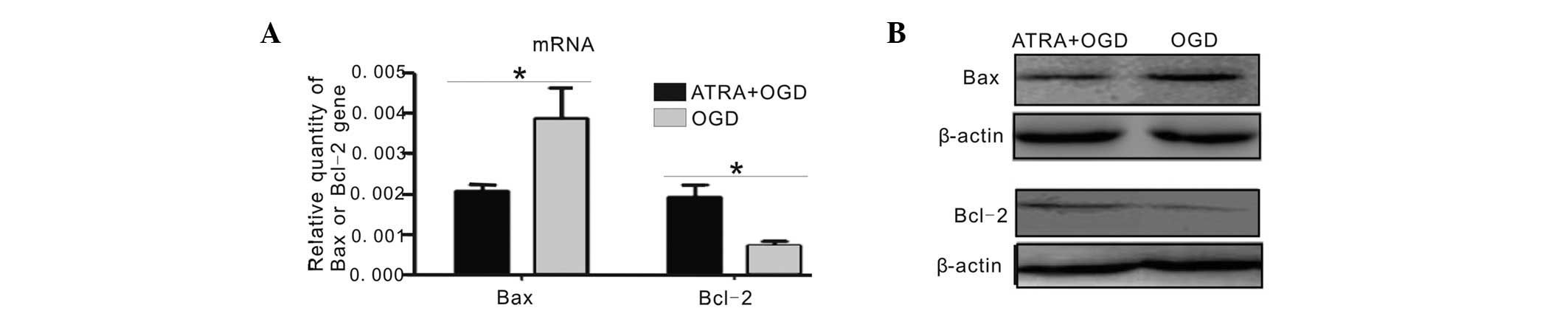
Anti-apoptotic effect of 4 μmol/l
ATRA
To examine the anti-apoptotic effect of 4 μmol/l
ATRA, the expression of apoptosis-associated factors was examined.
As revealed in Fig. 4A, when
compared with the OGD-injured cells without ATRA treatment, the
mRNA expression of Bax was significantly reduced by 4 μmol/l ATRA
treatment following OGD injury (P<0.05). Furthermore, the mRNA
expression of Bcl-2 was significantly increased in the 4 μmol/l
ATRA+OGD group (P<0.05). The changes in the expression levels of
Bax and Bcl-2 were consistent with the changes in their protein
expression, as detected by western blot analysis (Fig. 4B). These results suggested that a 4
μmol/l ATRA pre-treatment for 24 h may protect the PC12 cells from
the induction of apoptosis caused by OGD.
RARα regulates the anti-apoptotic effect
of ATRA treatment on PC12 cells injured by OGD
The expression levels of RARα were altered by ATRA
in PC12 cells following OGD injury. To investigate whether RARα
regulates the anti-apoptotic effect of ATRA treatment, the
OGD-injured PC12 cells were infected with Ad-siRARα. The mRNA and
protein expression levels of RARα were inhibited in the PC12 cells
with OGD+ATRA treatment by Ad-siRARα when compared with those in
cells transfected with the Ad-RFP control vector (Fig. 5A). Following transfection with
Ad-siRARα, the percentages of apoptotic cells and the green
fluorescence intensity reversely correlated with the MMP were
significantly higher than those of the Ad-RFP group following 4
μmol/l ATRA+OGD treatment (Fig. 5B and
C). Furthermore, the PC12 cells transfected with Ad-siRARα had
increased mRNA expression levels of Bax and decreased mRNA
expression levels of Bcl-2 following the 4 μmol/l ATRA+OGD
treatment (Fig. 5D). The changes
in Bax and Bcl-2 protein expression were similar to the changes in
mRNA expression in the PC12 cells transfected with Ad-siRARα and
treated with 4 μmol/l ATRA+OGD (Fig.
5E). These data indicated that pre-treatment with 4 μmol/l ATRA
suppressed apoptosis in OGD-injured PC12 cells through the RARα
signaling pathway.
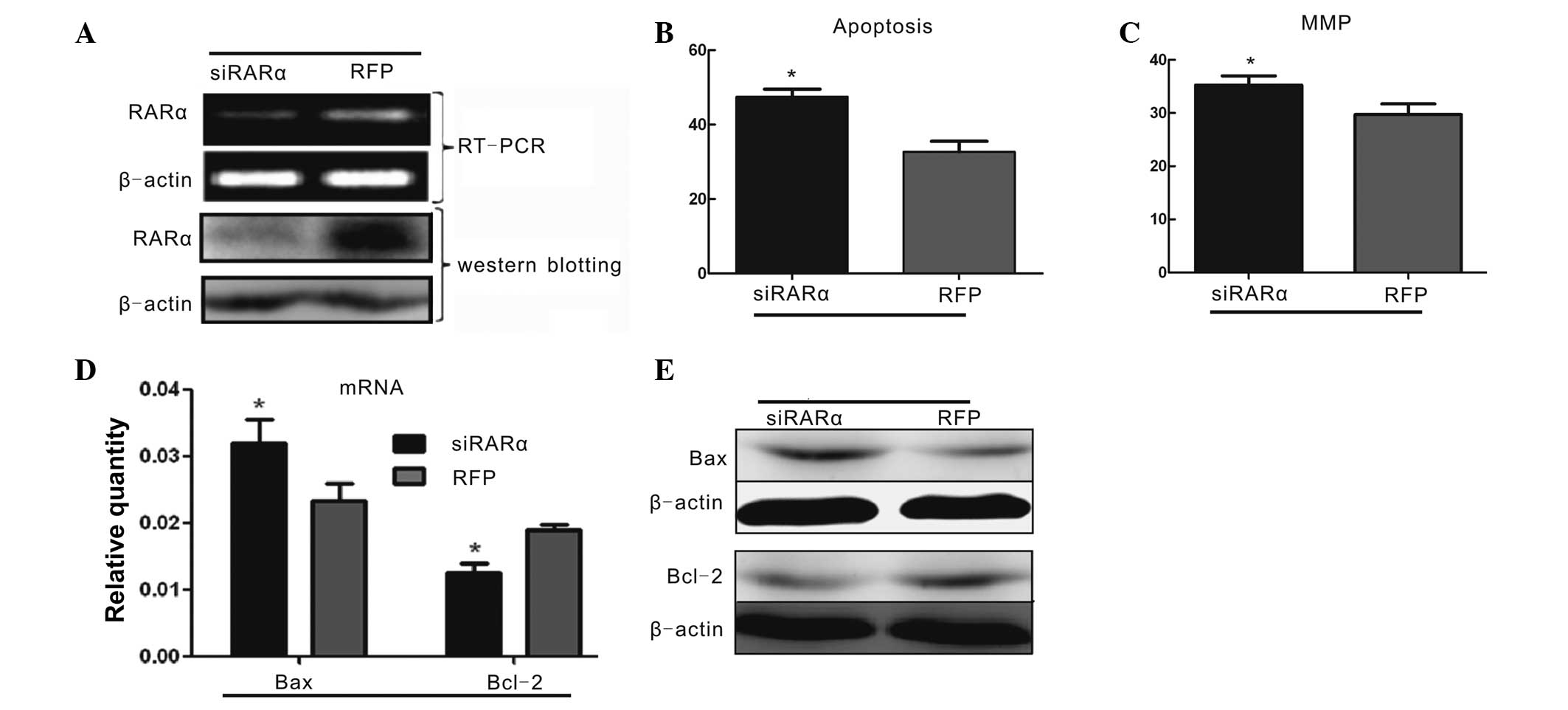 | Figure 5Ad-siRARα blocks the anti-apoptotic
effects of 4 μmol/l ATRA on PC12 cells treated by OGD. (A)
Ad-siRARα significantly inhibited mRNA and protein expression of
RARα in PC12 cells treated by OGD and 4 μmol/l ATRA. (B)
Quantitative comparison of the percentage of apoptosis in
OGD+ATRA-treated PC12 cells following transfection with either
Ad-siRARα or RFP. Fluorescence-activated cell sorting results were
confirmed in at least three independent experiments. Values are
expressed as the mean ± SEM (*P<0.05 vs. the
RFP+ATRA+OGD group, by one-way ANOVA). (C) Quantification of the
percentage of green fluorescence intensity of JC-1 reversely
correlated with the MMP in the OGD+ATRA-treated PC12 cells
following transfection with either Ad-siRARα or RFP. These tests
were performed in at least three independent experiments. Values
are expressed as the mean ± SEM (*P<0.05 vs. the
RFP+ATRA+OGD group, by one-way ANOVA). (D–E) Changes in the
expression levels of Bax and Bcl-2 following Ad-siRARα transfection
of the OGD+ATRA-treated PC12 cells, as measured by quantitative
polymerase chain reaction and western blot analysis. Data from at
least three independent experiments were analyzed and normalized to
β-actin. Values are expressed as the mean ± SEM
(*P<0.05 vs. the RFP+ATRA+OGD group, by one-way
ANOVA). RARα, retinoic acid receptor α; OGD, oxygen glucose
deprivation; ATRA, all-trans-retinoic acid; RFP, red fluorescent
protein; MMP, mitochondrial membrane potential; SEM, standard error
of the mean; ANOVA, analysis of variance; Bcl-2, B-cell lymphoma 2;
Bax, Bcl-2-associated X; siRNA, small interfering RNA; Ad,
adenovirus. |
Discussion
Vitamin A is an essential fat-soluble micronutrient
that contributes to development and growth. RA, which includes
ATRA, 13-cis-retinoic acid (13-cis-RA) and 9-cis-retinoic
acid (9-cis-RA), is a main active metabolite of VA in
vivo. ATRA can regulate the transcriptional activity of
downstream genes through retinoic acid nuclear receptor-mediated
signal transduction. VA deficiency during pregnancy downregulates
the expression of RARα and NR1 through Ca2+ signaling
and decreases the learning and memory function of offspring
(4). Jacobs et al (15) found that RA promotes neurogenesis
and that RA deficiency leads to lower survival of nerve cells in
vivo. RA also affects the proliferation and apoptosis of
damaged nerve cells (16).
Shinozaki et al (17) found
that precursor nerve cells differentially weakened in the
hippocampal dentate gyrus granule cell layer if RA was deprived in
adult rats. However, the regulatory effects of VA on the
anti-apoptotic mechanisms of neuron cells remain elusive.
In the present study, an in vitro OGD model
in PC12 cells was successfully established, which exhibited an
increase in the percentage of apoptosis and a decrease in the
levels of MMP, but also significantly enhanced levels of Bax
expression. These results demonstrated that OGD damage leads to
acute apoptosis in PC12 cells. Furthermore, it was identified that
2 and 4 μmol/l ATRA significantly suppressed the apoptosis of PC12
cells injured by OGD and effectively maintained MMP stability. In
addition, 4 μmol/l ATRA upregulated Bcl-2 expression and
downregulated Bax expression. However, 0.5 μmol/l ATRA was not able
to reverse the apoptosis that occurred in OGD-injured PC12 cells
due to its low concentration, and 20 μmol/l ATRA produced increased
levels of apoptosis in PC12 cells due to toxicity at such a high
concentration. These results indicated that ATRA had different
biological effects at different concentrations. Numerous studies
have demonstrated that ATRA is correlated with apoptosis (18–21).
In the present study, 4 μmol/l ATRA treatment more effectively
suppressed apoptosis in the PC12 cells following OGD treatment and
it was optimal to further reveal the mechanism of ATRA
regulation.
ATRA receptors include two types, RARs and RXRs,
which both contain three subtypes (α, β and γ). Most commonly,
these receptors form homo- or heterodimers that combine with
region-specific RARs elements (RARE) in the promoter region of a
target gene to regulate its transcription. RARα is a main receptor
in hippocampal development in the rat, particularly in learning and
memory function. A previous in vivo study by our group
demonstrated that VA levels affect learning and memory function via
the RARα pathway (22). In the
present study, OGD injury decreased the levels of RARα expression
in PC12 cells, and the expression of RARα increased when ATRA was
added to PC12 cells following OGD treatment, suggesting that the
ATRA signaling pathway may regulate apoptosis following OGD damage.
Katsuki et al (23)
demonstrated that ATRA upregulates RARα expression and enhances
BDNF reactivity to protect the dopaminergic neural system from
damage.
To confirm a regulatory role of RARα, an Ad-siRARα
recombinant adenovirus was used. It was revealed that Ad-siRARα
significantly blocked RARα expression in PC12 cells following ATRA
and OGD treatment. In PC12 cells subjected to ATRA and OGD
treatment, inhibition of RARα significantly increased the
percentage of apoptosis, decreased the MMP, and also upregulated
Bax expression and downregulated Bcl-2 expression. These findings
demonstrated that the anti-apoptotic properties of ATRA may be
regulated through the RARα signaling pathway. Therefore, future
studies will focus on the regulatory effect of RARα on the
anti-apoptotic Bcl-2 gene and the pro-apoptotic Bax gene.
In conclusion, an in vitro OGD model in PC12
cells was successfully established in the present study.
Pretreatment with ATRA at a concentration of 2–4 μmol/l had an
anti-apoptotic effect on OGD-injured PC12 cells via the RARα
signaling pathway, suggesting a new method of adjuvant therapy for
patients with brain damage.
Acknowledgements
This study was supported by grants from the National
Natural Science Foundation of China (grant nos. 30830106 and
81100454 to Professor Tingyu Li and nos. 30872670 and 81271385 to
Dr Jie Chen) and the Project of Chongqing Municipal Health Bureau,
China (no. 2009-1-41 to Dr Jie Chen).
Abbreviations:
|
HIBD
|
hypoxia-ischemia brain damage
|
|
VA
|
vitamin A
|
|
RA
|
retinoic acid
|
|
PC12 cells
|
rheochromocytoma cells
|
|
OGD
|
oxygen glucose deprivation
|
|
RFP
|
red fluorescent protein
|
|
RARα
|
retinoic acid receptor α
|
|
MMP
|
mitochondrial membrane potential
|
References
|
1
|
Janesick A, Shiotsugu J, Taketani M and
Blumberg B: RIPPLY3 is a retinoic acid-inducible repressor required
for setting the borders of the pre-placodal ectoderm. Development.
139:1213–1224. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Hale LA, Tallafuss A, Yan YL, Dudley L,
Eisen JS and Postlethwait JH: Characterization of the retinoic acid
receptor genes raraa, rarab and rarg during zebrafish development.
Gene Expr Patterns. 6:546–555. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Grandel H, Lun K, Rauch GJ, et al:
Retinoic acid signalling in the zebrafish embryo is necessary
during pre-segmentation stages to pattern the anterior-posterior
axis of the CNS and to induce a pectoral fin bud. Development.
129:2851–2865. 2002.PubMed/NCBI
|
|
4
|
Zhang X, Chen K, Chen J, Liu YX, Qu P and
Li TY: Effect of marginal vitamin A deficiency during pregnancy on
retinoic acid receptors and N-methyl-D-aspartate receptor
expression in the offspring of rats. J Nutr Biochem. 22:1112–1120.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Jiang W, Yu Q, Gong M, et al: Vitamin A
deficiency impairs postnatal cognitive function via inhibition of
neuronal calcium excitability in hippocampus. J Neurochem.
121:932–943. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Jiang T, Gao L, Shi J, Lu J, Wang Y and
Zhang Y: Angiotensin-(1–7) modulates renin-angiotensin system
associated with reducing oxidative stress and attenuating neuronal
apoptosis in the brain of hypertensive rats. Pharmacol Res.
67:84–93. 2013.
|
|
7
|
Raveendran AT and Skaria PC: Learning and
cognitive deficits in hypoxic neonatal rats intensified by BAX
mediated apoptosis: protective role of glucose, oxygen, and
epinephrine. Int J Neurosci. 123:80–88. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Cheng O, Li Z, Han Y, Jiang Q, Yan Y and
Cheng K: Baicalin improved the spatial learning ability of global
ischemia/reperfusion rats by reducing hippocampal apoptosis. Brain
Res. 1470:111–118. 2012. View Article : Google Scholar
|
|
9
|
Wang YQ, Wang L, Zhang MY, et al:
Necrostatin-1 suppresses autophagy and apoptosis in mice traumatic
brain injury model. Neurochem Res. 37:1849–1858. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Hill JW, Poddar R, Thompson JF, Rosenberg
GA and Yang Y: Intranuclear matrix metalloproteinases promote DNA
damage and apoptosis induced by oxygen-glucose deprivation in
neurons. Neuroscience. 220:277–290. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Piao CS, Loane DJ, Stoica BA, et al:
Combined inhibition of cell death induced by apoptosis inducing
factor and caspases provides additive neuroprotection in
experimental traumatic brain injury. Neurobiol Dis. 46:745–758.
2012. View Article : Google Scholar
|
|
12
|
Chaung WW, Wu R, Ji Y, et al: Peripheral
administration of human adrenomedullin and its binding protein
attenuates stroke-induced apoptosis and brain injury in rats. Mol
Med. 17:1075–1083. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Tang LH, Xia ZY, Zhao B, Wei XD, Luo T and
Meng QT: Phosphocreatine preconditioning attenuates apoptosis in
ischemia-reperfusion injury of rat brain. J Biomed Biotechnol.
2011:1070912011.PubMed/NCBI
|
|
14
|
Jin W, Ni H, Dai Y, et al: Effects of
tert-butylhydroquinone on intestinal inflammatory response and
apoptosis following traumatic brain injury in mice. Mediators
Inflamm. 2010:5025642010.PubMed/NCBI
|
|
15
|
Jacobs S, Lie DC, DeCicco KL, et al:
Retinoic acid is required early during adult neurogenesis in the
dentate gyrus. Proc Natl Acad Sci USA. 103:3902–3907. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Maden M: Retinoic acid in the development,
regeneration and maintenance of the nervous system. Nat Rev
Neurosci. 8:755–765. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
17
|
Shinozaki Y, Sato Y, Koizumi S, Ohno Y,
Nagao T and Inoue K: Retinoic acids acting through retinoid
receptors protect hippocampal neurons from oxygen-glucose
deprivation-mediated cell death by inhibition of c-jun-N-terminal
kinase and p38 mitogen-activated protein kinase. Neuroscience.
147:153–163. 2007. View Article : Google Scholar
|
|
18
|
Karmakar S, Banik NL and Ray SK:
Combination of all-trans retinoic acid and paclitaxel-induced
differentiation and apoptosis in human glioblastoma U87MG
xenografts in nude mice. Cancer. 112:596–607. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Keedwell RG, Zhao Y, Hammond LA, et al: A
retinoid-related molecule that does not bind to classical retinoid
receptors potently induces apoptosis in human prostate cancer cells
through rapid caspase activation. Cancer Res. 64:3302–3312. 2004.
View Article : Google Scholar
|
|
20
|
Thin TH, Li L, Chung TK, Sun H and Taneja
R: Stra13 is induced by genotoxic stress and regulates
ionizing-radiation-induced apoptosis. EMBO Rep. 8:401–407. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Li J, Orr B, White K, et al: Chmp 1A is a
mediator of the anti-proliferative effects of all-trans retinoic
acid in human pancreatic cancer cells. Mol Cancer. 8:72009.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Jiang W, Wen EY, Gong M, et al: The
pattern of retinoic acid receptor expression and subcellular,
anatomic and functional area translocation during the postnatal
development of the rat cerebral cortex and white matter. Brain Res.
1382:77–87. 2011. View Article : Google Scholar
|
|
23
|
Katsuki H, Kurimoto E, Takemori S, et al:
Retinoic acid receptor stimulation protects midbrain dopaminergic
neurons from inflammatory degeneration via BDNF-mediated signaling.
J Neurochem. 110:707–718. 2009. View Article : Google Scholar
|