Introduction
Hepatic fibrosis is a common pathology in China that
severely impacts the health of affected individuals and poses a
significant risk of morbidity. Fibrosis of the liver occurs
predominantly through the accumulation of collagen, proteoglycans
and other macromolecules within the extracellular matrix (ECM).
Both the quantity and composition of the ECM are often markedly
altered in patients with liver fibrosis (1,2).
Chronic liver disease is known to progress from mild to severe
inflammation, and then to fibrosis and cirrhosis. Hepatic fibrosis
therefore occurs between chronic liver disease and cirrhosis, and
constitutes a dynamic and bidirectional stage (3). In the present study, porcine serum
(PS)-induced hepatic fibrosis was used as a unique model of liver
fibrosis in the absence of obvious hepatocyte injury or
inflammatory cell infiltration (4,5).
This liver fibrosis model, induced by an immunological reaction,
was similar to models generated by repeat injections of equine
serum, egg yolk and human albumin, but not rat serum (6). Previous research has indicated that
hepatic fibrosis is a complex pathological process involving
numerous cytokines and cell signaling pathways (7). The activation of hepatic stellate
cells (HSCs), which produce collagen, is considered to be induced
in a paracrine manner through the mediation of various factors
released from necrotic hepatocytes, Kupffer cells or endothelial
cells (8). Domitrovic and Jakovac
(9) suggested that liver fibrosis
decreased through the inactivation of HSCs under the control of
fibrogenic cytokines. Transforming growth factor (TGF)-β1 has a
pivotal role in liver fibrosis, and can activate HSCs, the
principal cellular source of excess ECM during hepatic fibrosis,
though the TGF-β/Smad signaling pathway (10).
Colchicine has been widely used in clinical practice
for the treatment of acute gout and other immunological diseases,
and therefore was used as a positive control in the present study.
In China, it is believed that patients with liver fibrosis have
been treated with herbal medicines for thousands of years.
Traditional Chinese medicine is still extensively used for the
treatment of liver disease. In recent years, considerable attention
has been paid to the use of traditional Chinese medicine in the
treatment of liver fibrosis and cirrhosis (11). Acremoniumterricola milleretal
mycelium (AMM) is isolated from Acremonium terricola, in
submerged fermentation. AMM consists of a number of beneficial
components, including Cordyceps polysaccharide, palmitic acid and
unsaturated fatty acids, and has been used for its
anti-inflammatory and antioxidant effects, as well as for
regulation of the immune system. This Chinese medicine has been
reported to exert various pharmacological effects on animals both
in vitro and in vivo (12). Our previous study demonstrated that
AMM has protective effects on carbon tetrachloride-induced liver
fibrosis in rats (13). The
present study was performed to determine whether AMM has any
beneficial effects on PS-induced immunological hepatic
fibrosis.
Materials and methods
Materials
AMM was supplied by Anhui Haikui Biotechnology
Company (Hefei, China). Colchicine was obtained from Sigma-Aldrich
(St. Louis, MO, USA). PS was purchased from Zhengzhou YiKang
Biotechnology Company (Zhengzhou, China). Commercial kits used to
assay alanine transaminase (ALT), aspartate transaminase (AST),
superoxide dismutase (SOD), glutathione peroxidase (GSH-Px) and
hydroxyproline (Hyp) were obtained from Nanjing Jiancheng Institute
of Biotechnology (Nanjing, China). The hyaluronic acid (HA),
laminin (LN) and procollagen type III (PCIII) radioimmunoassay kits
were purchased from Beijing North Institute of Biotechnology
(Beijing, China). Mouse anti-α-smooth muscle actin (α-SMA) and
anti-β-actin antibodies, as well as rabbit anti-phosphorylated-
(p-)Smad2, anti-Smad2, anti-p-Smad3, anti-Smad3 and anti-Smad7
antibodies were purchased from Bioworld Technology, Inc., (St.
Louis Park, MN, USA).
Animals
Male Sprague Dawley rats, weighing 130–150 g, were
provided by the Experimental Animal Center of Anhui Province
(Hefei, China). Animals were housed five per cage, with access to
water and food ad libitum, and maintained at a constant
temperature (22±1°C) and humidity (50±20%) under a 12-h light/dark
cycle. Animal treatment and maintenance were carried out in
accordance with the guidelines for the humane treatment of animals
set by the Association of Laboratory Animal Sciences and the Center
for Laboratory Animal Sciences, Anhui Medical University (Hefei,
China).
Experimental design
After 1 week of acclimation, the animals were
subjected to experimentation. Sixty adult male Sprague Dawley rats
were randomly divided into six groups, as follows: Control, model
(PS-treated), PS plus colchicine (0.1 mg/kg) and PS plus AMM (175,
350 and 700 mg/kg, respectively). All groups, with the exception of
the control, received PS intraperitoneally twice per week for 18
weeks. The colchicine and AMM groups were treated with colchicine
(0.1 mg/kg, intragastrically) and AMM (175, 350 or 700 mg/kg,
intragastrically) each day, respectively, at the beginning of the
injection of PS. The control and model groups were administered the
same volume of vehicle.
The procedure of dividing the animals into groups
and for the generation of the PS-induced model of liver fibrosis
was based on the method described previously, with certain
modifications (14,15). Animals were weighed once per week,
and 24 h after the final injection of PS all animals were
sacrificed under anesthesia with ether. Blood samples were
collected from the abdominal aorta and centrifuged (3,000 × g for
10 min), and the serum was stored at −80°C until further analysis.
The liver was subsequently washed in situ with ice-cold
isotonic saline, then removed and divided into two portions; one
portion was fixed for histopathology and the other was immediately
frozen at −80°C until required.
Hepatotoxicity studies
To assess hepatotoxicity, the serum levels of ALT
and AST were measured using commercial kits according to the
manufacturer’s instructions (Nanjing Jiancheng Institute of
Biotechnology).
Hepatic Hyp
The Hyp content in fresh liver samples was measured
in accordance with the methods described by Jamall et al
(16). The content of hepatic Hyp
was determined using a Hyp kit following the manufacturer’s
instructions (Nanjing Jiacheng Bioengineering Institute).
Oxidative stress
The livers were thawed and washed with normal saline
to remove blood and clots. Homogenates were centrifuged (1,000 × g
for 10 min, 4°C) and aliquots of the supernatants were then used to
assay the expression levels of SOD and GSH-Px.
Serum fibrotic markers
The serum levels of HA, LN and PCIII were assayed
using a radioimmunoassay kit (North Institute of Biotechnology,
Beijing, China) according to the manufacturer’s instructions.
Histopathology
A portion of the liver specimens of each rat was
fixed in 10% neutral buffered formalin and embedded in paraffin.
Sections measuring 4 μm were then cut and stained with hematoxylin
and eosin (H&E) and Sirius Red. The stained slides were
examined independently by two pathologists with no prior knowledge
of their source. The histological grade of hepatic fibrosis was
assessed based on the New Inuyama staging system (17): 0, no fibrosis (normal liver and
absence of fibrosis); I, fibrosis present (collagen fibers present
that extend from the portal triad or the central vein to the
peripheral region); II, mild fibrosis (mild collagen fiber presence
with extension without compartment formation); III, moderate
fibrosis (moderate collagen fibers present with moderate
pseudo-lobe formation); IV, severe fibrosis (severe collagen fiber
presence with thickening of the partial compartments and frequent
pseudo-lobe formation) (18). The
percentage of area occupied by collagen fibrosis was calculated by
dividing the number of red collagen fibroses by the total number of
collagen fibroses. At least five fields were selected under light
microscopy at ×200 magnification to determine positive collagen
fibrosis, and the average was calculated.
Immunohistochemistry
Immunohistochemical analyses were performed on
paraffin sections incubated with primary mouse monoclonal α-SMA
antibody, diluted 1:100, at 4°C overnight. The sections were then
incubated with biotinylated secondary goat anti-mouse
immunoglobulin G antibody (Santa Cruz Biotechnology, Inc., Santa
Cruz, CA, USA), prior to the sections being counterstained with
hematoxylin. Normal mouse antiserum was used as a negative control
in place of the primary antibody, and consistently demonstrated no
antigenic reaction. The numbers of α-SMA-positive and -negative
HSCs were visualized using diaminobenzidine (Dako, Tokyo, Japan).
Five fields were randomly selected from each section and the number
of positive cells for each antibody was counted under light
microscopy at ×400 magnification. The percentage of α-SMA-positive
HSCs was calculated by dividing the number of HSCs counted in each
slide by the total number of cells, and an average number for each
group was calculated.
Western blotting
Frozen liver tissue (100 mg) was washed several
times in distilled water and homogenized in extraction buffer (25
mM HEPES, 400 mM KCl, 1 mM EDTA and 1.5 mM MgCl2). The
homogenate was then centrifuged at 12,000 × g for 10 min at 4°C,
and the supernatant was transferred to a fresh tube and stored at
−80°C. The concentration of protein homogenate was determined using
a bicinchoninic acid protein assay kit according to the
manufacturer’s instructions (ZSGB-BIO, Beijing, China). Proteins
were assayed using a Tanon-4200 automatic digital gel imaging
system (Tianneng, Shanghai, China) (19). Protein samples (50–150 μg) were
separated by SDS-PAGE and transferred to polyvinylidene difluoride
membranes for 2 h at 100 V (Millipore, Bedford, MA, USA). The
membranes were then incubated overnight at 4°C under agitation with
the following primary antibodies (diluted 1:800 in Tris-buffered
saline with Tween): Anti-p-Smad2, -p-Smad3, -Smad2, -Smad3, and
-Smad7 (Bioworld Technology, Inc.). The membranes were subsequently
incubated with secondary antibody (Santa Cruz Biotechnology, Inc.)
for 2 h at room temperature, and the immunoreactive proteins were
visualized using enhanced chemiluminescence (Amersham Pharmacia
Biotech, Piscataway, NJ, USA) and autoradiography. Gel reverse
zymography was performed on the liver of five randomly selected
rats per group. Quantity One® software (Bio-Rad,
Hercules, CA, USA) was used to quantify the band densities.
Statistical analysis
Data are expressed as the mean ± standard deviation.
Data were analyzed by a one-way analysis of variance, and the
Student’s t-test was used for two-group comparisons. A Ridit test
was used for the statistical analysis of qualitative data.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Serum markers of liver damage and
antioxidant status
The effects of treatment with AMM on the PS-induced
elevation of serum markers due to hepatic damage are shown in
Table I. The serum activities of
AST and ALT were both increased in the PS-treated group as compared
with those in the control group, but the difference did not reach
statistical significance (P>0.05). The activities of SOD and
GSH-Px in the PS-treated group were significantly lower as compared
with those in the control group (P<0.001). AMM (350 and 700
mg/kg) only moderately reduced the AST and ALT activities relative
to the control group (P>0.05) but significantly enhanced SOD and
GSH-Px activities as compared with the PS-treated group (P<0.05
and P<0.01, respectively) (Table
I).
 | Table IEffects of AMM on serum markers of
liver damage and antioxidant status in PS-induced hepatic
fibrosis. |
Table I
Effects of AMM on serum markers of
liver damage and antioxidant status in PS-induced hepatic
fibrosis.
| Group | Dose (mg/kg) of
AMM/colchicine | ALT (U/l) | AST (U/l) | SOD (U/mg) | GSH-Px (U/mg) |
|---|
| Control | - | 56.08±12.26 | 64.50±15.76 | 134.02±17.23 | 123.03±16.18 |
| PS-treated | - | 67.35±13.68 | 81.13±18.84 | 97.61±15.25a | 64.95±10.89a |
| PS+AMM | 175 | 63.31±14.43 | 74.59±13.52 | 108.35±11.05 | 71.04±15.78 |
| 350 | 61.31±12.51 | 71.23±12.94 | 117.43±19.85b | 85.32±18.06b |
| 700 | 59.74±14.62 | 69.35±14.12 | 125.45±26.21b | 100.73±17.56c |
| PS+colchicine | 0.1 | 62.16±15.26 | 73.36±17.72 | 127.67±22.58c | 83.79±13.34c |
Hepatic Hyp content and serum fibrotic
marker levels
The Hyp content and HA, LN and PCIII levels were
markedly increased in the PS-treated group as compared with those
in the control group (P<0.01). AMM (350 and 700 mg/kg)
significantly attenuated the increase in the content of Hyp, and
reduced the levels of serum HA, LN and PCIII as compared with the
PS-treated group (P<0.01 and P<0.001) (Table II).
 | Table IIEffects of AMM on Hyp content and HA,
LN and PCIII levels in PS-induced hepatic fibrosis. |
Table II
Effects of AMM on Hyp content and HA,
LN and PCIII levels in PS-induced hepatic fibrosis.
| Group | Dose (mg/kg) of
AMM/colchicine | Hyp (mg/g) | HA (ng/ml) | LN (ng/ml) | PCIII (μg/l) |
|---|
| Control | - | 64.48±6.47 | 117.49±19.65 | 54.81±17.94 | 56.31±8.85 |
| PS-treated | - |
112.69±18.13a |
324.13±64.23a |
147.22±34.02a | 92.65±13.22a |
| PS+AMM | 175 | 98.13±16.09 | 269.82±59.04 | 129.64±15.49 | 83.52±10.26 |
| 350 | 85.27±15.97b |
228.83±53.67c |
102.29±19.13b | 73.41±8.09b |
| 700 | 78.29±11.95c |
186.77±43.69c | 87.73±17.53c | 65.83±7.45c |
| PS+colchicine | 0.1 | 89.27±15.49b |
221.82±51.63c | 90.65±18.05c | 68.59±8.94c |
Histopathological changes in the
liver
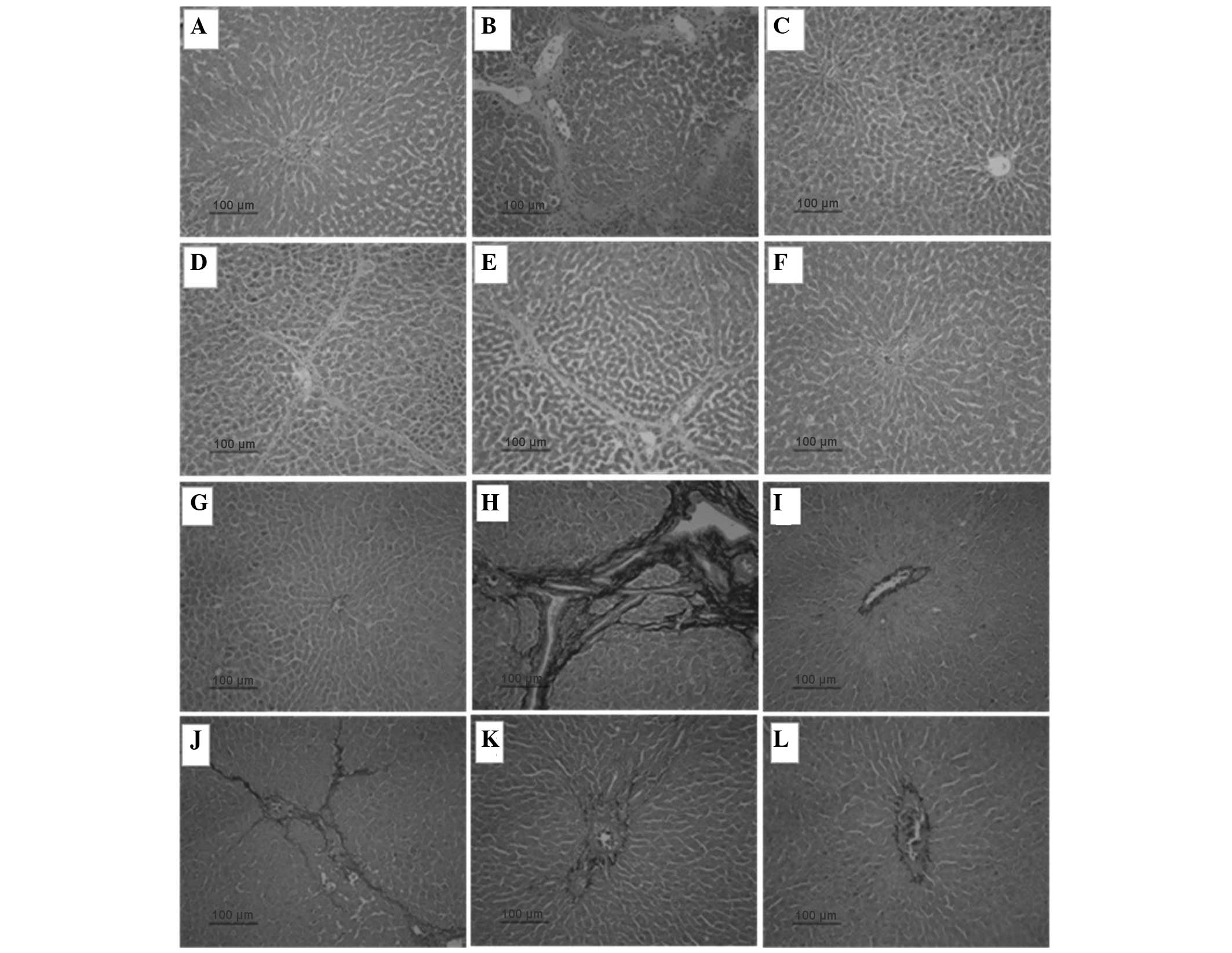
The histopathological changes in the liver are shown
in Fig. 1. The structure of the
liver tissue was normal in the control group, with no detectable
changes in the histology (Fig 1A).
The livers from the control group stained with Sirius Red stain
showed traces of collagen only in the walls of major blood vessels
(Fig. 1G). PS administration
resulted in an extensive accumulation of collagen in the liver
tissue. Fibrosis predominantly developed around the central veins
near the hepatic capsule, and complete septal fibrosis was
observed; furthermore, pseudo-lobe formation was apparent in severe
cases. Only a small number of inflammatory cells were found around
the portal area and central vein, without notable hepatocyte
necrosis (Fig. 1B and H). AMM (350
and 700 mg/kg) treatment resulted in a dose-dependent decrease in
fibrotic deposits with short fibrous septa, and markedly reduced
the pathological changes as compared with the PS-treated group. The
structure of the liver tissue remained relatively normal (Fig. 1E, F, K and L).
The PS-treated group showed a high degree of
fibrosis. AMM (350 and 700 mg/kg) and colchicine (0.1 mg/kg)
treatment resulted in a marked improvement in the histological
scores in comparison with the PS-treated group (Table III).
 | Table IIIEffects of AMM on pathological
grading in PS-induced hepatic fibrosis. |
Table III
Effects of AMM on pathological
grading in PS-induced hepatic fibrosis.
| Groups | Dose (mg/kg) of
AMM/colchicine | Pathological
grading of hepatic fibrosis (n) | Collagen area
(%) |
|---|
|
|---|
| 0 | I | II | III | IV |
|---|
| Control | - | 8 | 0 | 0 | 0 | 0 | 1.37±0.32 |
| PS-treated | - | 0 | 0 | 1 | 4 | 3 | 10.13±1.35a |
| PS+AMM | 175 | 0 | 0 | 3 | 3 | 2 | 9.25±0.83 |
| 350 | 0 | 2 | 3 | 3 | 0 | 8.73±0.79b |
| 700 | 0 | 2 | 3 | 2 | 0 | 7.32±0.57c |
| PS+colchicine | 0.1 | 0 | 3 | 3 | 2 | 0 | 8.01±0.63c |
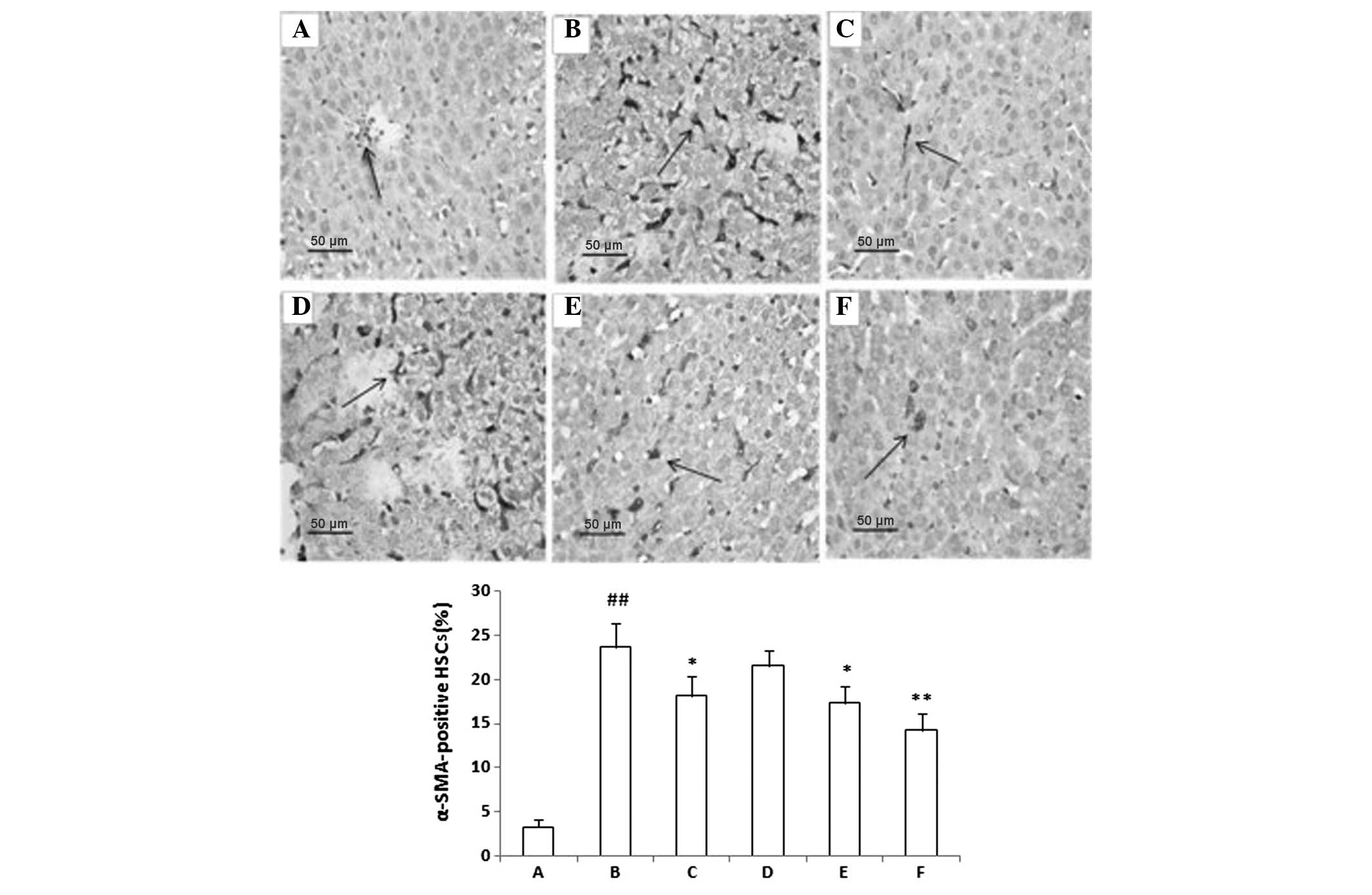
Immunohistochemical expression of
a-SMA
The immunohistochemical expression of α-SMA is shown
in Fig. 2. Few cells were detected
to be positively stained for α-SMA in the control group (Fig. 2A). The percentage of α-SMA-positive
cells was significantly increased in the PS-treated group as
compared with that in the control group (P<0.001), with most of
the α-SMA-positive cells detected around the periportal fibrotic
band areas, central vein and fibrous septa (Fig. 2B). AMM (350 and 700 mg/kg)
significantly decreased the percentage of α-SMA-positive cells as
compared with the PS-treated group (P<0.01 and P<0.001,
respectively), with a similar efficacy to that of colchicine (0.1
mg/kg) (Fig. 2E and F).
Expression of Smad2/3 phosphorylation and
Smad7
A higher expression of p-Smad2/3 was observed in the
PS-treated group as compared with the control group (P<0.001)
(Fig. 3A and B). Conversely, the
expression of Smad7 was significantly decreased in the PS-treated
group (P<0.001) (Fig. 3C). AMM
(350 and 700 mg/kg) and colchicine (0.1 mg/kg) significantly
decreased the expression of p-Smad2 and p-Smad3 and increased the
expression of Smad7 (P<0.05 and P<0.01)(Fig. 3A–C). These results demonstrated
that AMM (350 and 700 mg/kg) may act to prevent hepatic fibrosis by
blocking Smad2/3 phosphorylation and enhancing expression of the
inhibitor Smad7 in the TGF-β/Smad signaling pathway.
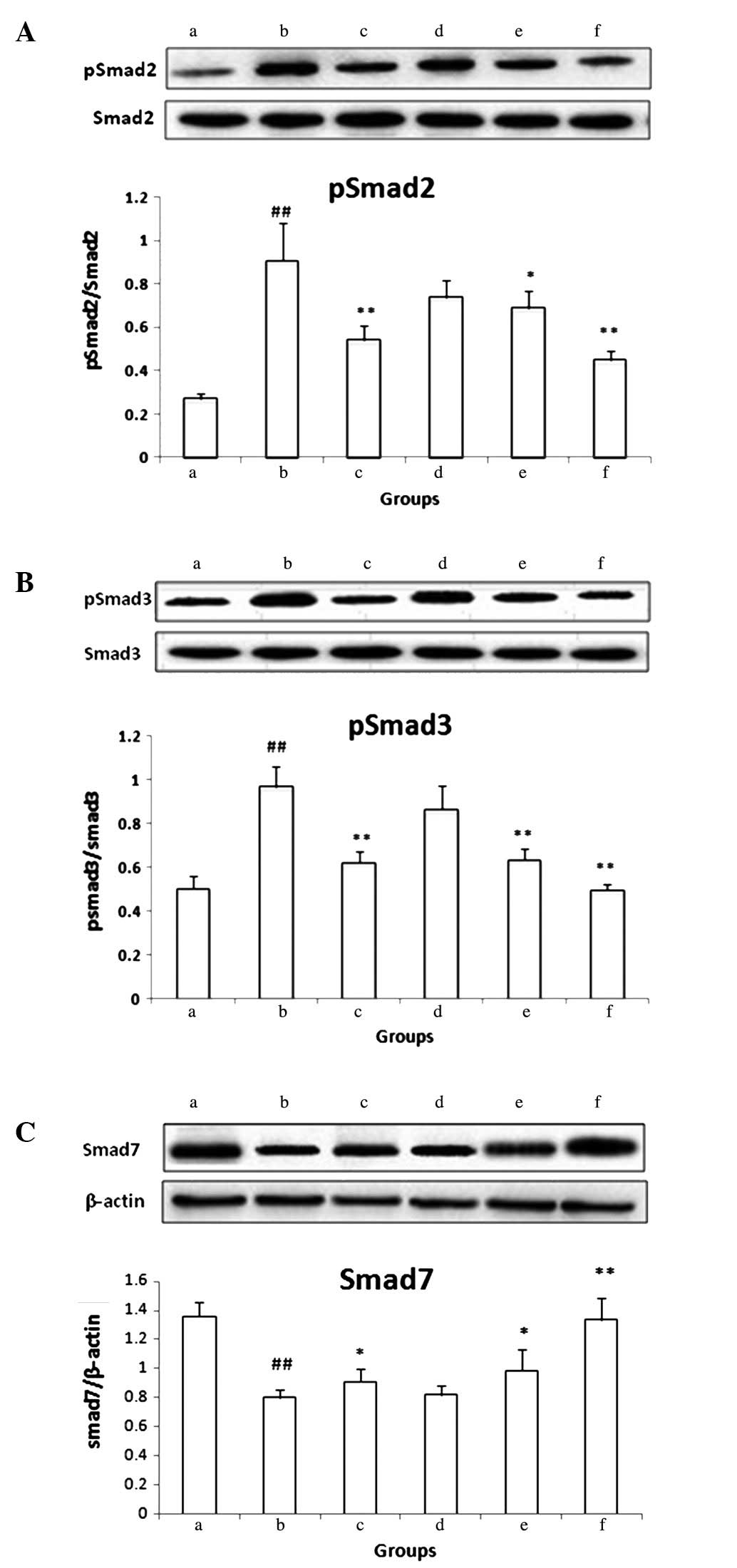 | Figure 3Western blot analysis of (A) p-Smad2,
(B) p-Smad3 and (C) Smad7. (a) Control group; (b) PS-treated group;
(c) PS and colchicine-treated group (0.1 mg/kg colchicine); (d–f)
PS and AMM-treated groups (175, 350 and 700 mg/kg AMM,
respectively). The intensities of p-Smad2, p-Smad3 and Smad7 were
normalized to those of total Smad2, Smad3 and β-actin,
respectively, in the corresponding treatment groups. The presented
data are based on at least five independent experiments. Error bars
represent the mean ± standard deviation for five rats in each
group. ##P<0.001 vs. the control group;
*P<0.05 and **P<0.01 vs. the PS-treated
group. AMM, Acremoniumterricola milleretal mycelium; PS, porcine
serum; p-Smad, phosphorylated-Smad. |
Discussion
Hepatic fibrosis is a common consequence of almost
all causes of chronic liver disease. HSCs, the predominant
ECM-producing cells, are activated by fibrogenic cytokines, such as
TGF-β1, angiotensin II and leptin. It was originally believed that
hepatic fibrosis was an irreversible process due to disruption of
the normal liver architecture (20); however, hepatic fibrosis is more
recently considered to be a reversible wound-healing response to
chronic liver injury (21). The
development of antifibrotic drugs to restrain fibrogenesis is of
particular importance. The present study showed by H&E staining
that low numbers of inflammatory cells were present around the
portal area and central vein without notable hepatocyte necrosis,
in the PS-treated (model) group. This was consistent with the data
showing no significant elevation in ALT and AST levels. Sirius Red
staining can be used to detect varying degrees of hepatic fibrosis
and to score this fibrosis (22).
The results showed that AMM (350 and 700 mg/kg) markedly attenuated
the pathology grading, which was consistent with the findings for
Hyp content. SOD and GSH-Px are two primary enzymes of the
antioxidant defense system (23).
The results presented in this study indicated that AMM could
significantly enhance the levels of SOD and GSH-Px. The analysis of
Hyp content was used to directly determine the quantity of collagen
in the tissue and can be used as a measure to reflect the degree of
hepatic fibrosis (24).
Additionally, HA, LN and PCIII in serum are considered to be
important biomarkers of hepatic fibrogenesis (25). In this study, AMM (350 and 700
mg/kg) significantly decreased the Hyp content and the levels of
HA, LN and PCIII in PS-induced hepatic fibrotic rats, suggesting
that this may be the mechanism underlying its hepatoprotective
effects.
HSCs have a key function in the pathogenesis of
fibrosis (26,27). Upon the activation of HSCs, levels
of fibrillar collagen, particularly types I and II, become markedly
increased. α-SMA is an indicator of activated HSCs (28). Therefore, the expression level of
α-SMA was assessed among the groups. Few α-SMA-positive HSCs were
detected in the AMM (350 and 700 mg/kg) treatment groups. Thus, AMM
could inhibit the expression of α-SMA-positive HSCs and
subsequently prevent hepatic fibrosis.
TGF-β1 activates HSCs and promotes ECM production,
which regulates the fibrogenic process. TGF-β1 protein is stored in
an inactive form. Once activated, TGF-β1 signals via its cognate
receptors to Smad proteins (29).
The TGFβ1/Smad signaling pathway has a central role in hepatic
fibrosis. Through this pathway, TGFβ1 predominantly activates HSCs,
which can result in hepatic fibrosis (30,31).
Activated TGF-β1 binds to the constitutively active TGF-β Type II
(TβRII) receptor in the cell membrane, which leads to the
recruitment of the TβRI receptor. A heterotetrameric complex is
formed and results in the phosphorylation and activation of the
TβRI receptor. Activated TβRI in turn phosphorylates Smad2 and
Smad3, known as receptor-activated Smads (R-Smads), which form a
hetero-oligomeric complex with a common mediator, Smad4 (Co-Smad).
This complex translocates to the nucleus, where it can regulate the
transcription of target genes (32). Smad7 is an inhibitory Smad (I-Smad)
that acts differently to the signal-transducing R-Smads and
Co-Smads. Smad7 competitively associates with TβRI to prevent the
phosphorylation of Smad2 and Smad3 and therefore inhibit the signal
transduction of TGFβ1. A previous study showed that Smad7
overexpression inhibited TGFβ/Smad signaling in rat liver
fibroblasts (33). The present
study results showed that AMM (350 and 700 mg/kg) treatment
markedly suppressed the expression of p-Smad2, and p-Smad3 and
increased that of Smad7, indicating that AMM had a significant
effect on TGFβ/Smad signaling.
In conclusion, the present findings indicate that
AMM plays an important role in the inhibition of PS-induced
immunological hepatic fibrosis. The molecular mechanism of this
therapeutic effect could be due to a decrease in oxidative stress,
a reduction in the accumulation of collagens and the inhibition of
TGFβ/Smad signaling.
Acknowledgements
This study was supported by grants from the Nature
Science foundation of Anhui Province (nos. KJ2009A031 and
KJ2010A164) and The PhD Programs Foundation of Anhui Medical
University (no. XJ200821).
References
|
1
|
Dai WJ and Jiang HC: Advances in gene
therapy of liver cirrhosis: a review. World J Gastroenterol. 7:1–8.
2001.PubMed/NCBI
|
|
2
|
Mormone E, George J and Nieto N: Molecular
pathogenesis of hepatic fibrosis and current therapeutic
approaches. Chem Biol Interact. 193:225–231. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Pinzani M and Rombouts K: Liver fibrosis:
from the bench to clinical targets. Dig Liver Dis. 36:231–242.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ochi T, Kawakita T and Nomoto K: Effects
of Hochu-ekki-to and Ninjin-youei-to, traditional Japanese
medicines, on porcine serum-induced liver fibrosis in rats.
Immunopharmacol Immunotoxicol. 26:285–298. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Shiga A, Shirota K, Ikeda T and Nomura Y:
Morphological and immunohistochemical studies on porcine
serum-induced rat liver fibrosis. J Vet Med Sci. 59:159–167. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Baba Y and Doi K: MHC class II-related
genes expression in porcine-serum-induced rat hepatic fibrosis. Exp
Mol Pathol. 77:214–221. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Seki E, Brenner DA and Karin M: A liver
full of JNK: signaling in regulation of cell function and disease
pathogenesis, and clinical approaches. Gastroenterology.
143:307–320. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Canbay A, Friedman S and Gores GJ:
Apoptosis: the nexus of liver injury and fibrosis. Hepatology.
39:273–278. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Domitrović R and Jakovac H: Effects of
standardized bilberry fruit extract (Mirtoselect®) on
resolution of CCl4-induced liver fibrosis in mice. Food Chem
Toxicol. 49:848–854. 2011.PubMed/NCBI
|
|
10
|
Friedman SL: Stellate cells: a moving
target in hepatic fibrogenesis. Hepatology. 40:1041–1043. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wang BE: Treatment of chronic liver
diseases with traditional Chinese medicine. J Gastroenterol
Hepatol. 15(Suppl): E67–E70. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Liu Z, Li P, Zhao D, Tang H and Guo J:
Protective effect of extract of Cordyceps sinensis in middle
cerebral artery occlusion-induced focal cerebral ischemia in rats.
Behav Brain Funct. 6:612010.PubMed/NCBI
|
|
13
|
Li J, Tian XP, Zhu TJ, Yang LL and Li WJ:
Streptomyces fildesensis sp nov, a novel streptomycete
isolated from Antarctic soil. Antonie Van Leeuwenhoek. 100:537–543.
2011. View Article : Google Scholar
|
|
14
|
Wu CS, Piao XX, Piao DM, Jin YR and Li CH:
Treatment of pig serum-induced rat liver fibrosis with
Boschniakia rossica, oxymatrine and interferon-alpha. World
J Gastroenterol. 11:122–126. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Andrade RG, Gotardo BM, Assis BC, Mengel J
and Andrade ZA: Immunological tolerance to pig-serum partially
inhibits the formation of septal fibrosis of the liver in
Capillaria hepatica-infected rats. Mem Inst Oswaldo Cruz.
99:703–707. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Jamall IS, Finelli VN and Que Hee SS: A
simple method to determine nanogram levels of 4-hydroxyproline in
biological tissues. Anal Biochem. 112:70–75. 1981. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Tsai JH, Liu JY, Wu TT, Ho PC, Huang CY,
Shyu JC, Hsieh YS, Tsai CC and Liu YC: Effects of silymarin on the
resolution of liver fibrosis induced by carbon tetrachloride in
rats. J Viral Hepat. 15:508–514. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kawai K, Uetsuka K, Doi K and Nakayama H:
The activity of matrix metalloproteinases (MMPS) and tissue
inhibitors of metalloproteinases (TIMPs) in mammary tumors of dogs
and rats. J Vet Med Sci. 68:105–111. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Yamada G: Histopathological
characteristics and clinical significance of New Inuyama
Classification in chronic hepatitis B. Nihon Rinsho.
62(Suppl)8:290–292. 2004.(In Japanese).
|
|
20
|
Popper H and Uenfriend S: Hepatic
fibrosis. Correlation of biochemical and morphologic
investigations. Am J Med. 49:707–721. 1970.PubMed/NCBI
|
|
21
|
Toda K, Kumagai N, Kaneko F, Tsunematsu S,
Tsuchimoto K, Saito H and Hibi T: Pentoxifylline prevents pig
serum-induced rat liver fibrosis by inhibiting interleukin-6
production. J Gastroenterol Hepatol. 24:860–865. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Hsu YC, Chiu YT, Lee CY, Lin YL and Huang
YT: Increases in fibrosis-related gene transcripts in livers of
dimethylnitrosamine-intoxicated rats. J Biomed Sci. 11:408–417.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Polavarapu R, Spitz DR, Sim JE, Follansbee
MH, Oberley LW, Rahemtulla A and Nanji AA: Increased lipid
peroxidation and impaired antioxidant enzyme function is associated
with pathological liver injury in experimental alcoholic liver
disease in rats fed diets high in corn oil and fish oil.
Hepatology. 27:1317–1323. 1998. View Article : Google Scholar
|
|
24
|
Dang SS, Wang BF, Cheng YA, Song P, Liu ZG
and Li ZF: Inhibitory effects of saikosaponin-d on CCl4-induced
hepatic fibrogenesis in rats. World J Gastroenterol. 13:557–563.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kaneda H, Hashimoto E, Yatsuji S,
Tokushige K and Shiratori K: Hyaluronic acid levels can predict
severe fibrosis and platelet counts can predict cirrhosis in
patients with nonalcoholic fatty liver disease. J Gastroenterol
Hepatol. 21:1459–1465. 2006.
|
|
26
|
Choi JH, Hwang YP, Choi CY, Chung YC and
Jeong HG: Anti-fibrotic effects of the anthocyanins isolated from
the purple-fleshed sweet potato on hepatic fibrosis induced by
dimethylnitrosamine administration in rats. Food Chem Toxicol.
48:3137–3143. 2010. View Article : Google Scholar
|
|
27
|
Otogawa K, Ogawa T, Shiga R, Ikeda K and
Kawada N: Induction of tropomyosin during hepatic stellate cell
activation and the progression of liver fibrosis. Hepatol Int.
3:378–383. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Carpino G, Morini S, Ginanni Corradini S,
et al: Alpha-SMA expression in hepatic stellate cells and
quantitative analysis of hepatic fibrosis in cirrhosis and in
recurrent chronic hepatitis after liver transplantation. Dig Liver
Dis. 37:349–356. 2005. View Article : Google Scholar
|
|
29
|
Gressner AM and Weiskirchen R: Modern
pathogenetic concepts of liver fibrosis suggest stellate cells and
TGF-beta as major players and therapeutic targets. J Cell Mol Med.
10:76–99. 2006. View Article : Google Scholar
|
|
30
|
Tsukada S, Westwick JK, Ikejima K, Sato N
and Rippe RA: SMAD and p38 MAPK signaling pathways independently
regulate alpha1(I) collagen gene expression in unstimulated and
transforming growth factor-beta-stimulated hepatic stellate cells.
J Biol Chem. 280:10055–10064. 2005. View Article : Google Scholar
|
|
31
|
Inagaki Y and Okazaki I: Emerging insights
into Transforming growth factor beta Smad signal in hepatic
fibrogenesis. Gut. 56:284–292. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Massagué J, Seoane J and Wotton D: Smad
transcription factors. Genes Dev. 19:2783–2810. 2005.PubMed/NCBI
|
|
33
|
Kopp J, Preis E, Said H, Hafemann B,
Wickert L, Gressner AM, Pallua N and Dooley S: Abrogation of
transforming growth factor-beta signaling by SMAD7 inhibits
collagen gel contraction of human dermal fibroblasts. J Biol Chem.
280:21570–21576. 2005. View Article : Google Scholar : PubMed/NCBI
|