Introduction
Melanogenesis is a physiological process, which is
involved in the production of melanin. Melanin is synthesized
within membrane-bound organelles, termed melanosomes, in
melanocytes and is transferred to keratinocytes where it forms
melanin caps above keratinocyte nuclei (1). Keratinocytes and melanosomes provide
strong protection against ultraviolet radiation (UVR)-induced
photodamage by scattering incoming light and absorbing diverse free
radicals in cells (2).
Furthermore, reduced or defective melanin pigmentation is
associated with an increased risk of skin cancer and various other
pathological conditions (3–4).
In melanocytes and melanoma cells, melanin synthesis
is primarily controlled through an enzymatic cascade that is
regulated by tyrosinase, tyrosinase-related protein 1 and
tyrosinase-related protein 2 (5).
Tyrosinase is the rate-limiting enzyme, which is critical in the
regulation of melanin production. Tyrosinase catalyzes the
hydroxylation of tyrosine to 3,4-dihydroxyphenylalanine (DOPA) and
the oxidation of DOPA to dopaquinone (6). In addition, one of the most important
transcription factors involved in the regulation of tyrosinase is
microphthalmia-associated transcription factor (MITF), which has
been reported to bind to the M-box within the tyrosinase promoter,
thus enhancing tyrosinase gene expression (7).
Cyclic adenosine monophosphate (cAMP) is involved in
various signal transduction pathways and has been reported to have
a role in the regulation of melanogenesis (8). The mechanism by which cAMP regulates
melanogenesis involves the activation of protein kinase A (PKA).
Activated PKA translocates to the nucleus where it phosphorylates
the cAMP responsive element-binding protein (CREB). Phosphorylated
CREB subsequently binds to the CRE site on the MITF promoter and
interacts with the CREB binding protein to increase the expression
of MITF, resulting in melanogenesis (9–10).
Various factors lead to an increase in intracellular cAMP,
including α-melanocyte stimulating hormone, forskolin and
isobutyryl xanthine, and are capable of inducing melanogenesis in
melanocytes and melanoma cells (11). Thus, chemicals or plant extracts,
which modulate intracellular cAMP levels, are considered to be
capable of regulating melanogenesis in human and mouse melanocytes
(12–13).
The extracellular signal-regulated kinase (ERK) and
phosphatidylinositol 3-kinase (PI3K)/Akt signaling pathways have
been shown to negatively regulate melanogenesis in melanocytes and
melanoma cells (14–15). Furthermore, numerous agents have
been identified that upregulate melanogenesis in B16F10 cells via
inhibition of the ERK and/or Akt signaling pathways. For example,
lupenone and fluvastatin were reported to increase melanin
synthesis by inhibiting the activation of ERK and Akt, respectively
(16–17).
Stimulation of melanin synthesis has been proposed
as a defense mechanism to prevent UVR-induced DNA damage in human
skin and the stimulation of melanin synthesis is used to treat
various diseases, which are characterized by a lack of skin
pigmentation, including vitiligo (18). Due to the increasing demand to
overcome the UVR-associated increase in skin cancer risk, as well
as hypopigmentation diseases, including vitiligo, the development
of novel, natural plant extract-derived tanning cosmetics has
attracted much research attention. Extracts from certain herbs,
including mangosteen (13),
Erica multiflora (16),
Pyrostegia venusta (19)
and Daphne gnidium (20)
have been shown to increase melanogenesis in B16 melanoma cells,
thus these extracts may have potential for use in tanning
cosmetics.
Ardisia crenata (AC) is a species of
flowering plant from the Ardisia genus and the Myrsinaceae
family, which is native to East Asia and commonly used as an
ornamental plant. The root extracts of AC have been used in
traditional Chinese medicine for the treatment of certain diseases,
including tonsillitis, respiratory tract infections and menstrual
disorders (21–22). Furthermore, various constituents of
AC were reported to have significant anti-metastatic effects in
tumors (23), as well as
vasorelaxant effects on the aortic artery of rats (24). However, despite numerous studies in
various fields, the effect of AC on melanogenesis has yet to be
elucidated. The present study aimed to investigate the effect of a
methanol extract of AC on melanin synthesis in B16F10 cells as well
as the underlying molecular mechanisms involved.
Materials and methods
Materials
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl
tetrazolium bromide (MTT), forskolin and L-DOPA, were purchased
from Sigma-Aldrich (St. Louis, MO, USA). Antibodies specific to
phosphorylated (P)-ERK1/2 (Thr202/Tyr204; catalog no. 9101S), total
(T) ERK1/2 (catalog no. 9102), P-Akt (Ser473; catalog no. 9271) and
T-Akt (catalog no. 9272) were purchased from Cell Signaling
Technology Inc. (Beverly, MA, USA). Antibodies against tyrosinase
and β-actin were purchased from Santa Cruz Biotechnology, Inc.
(Santa Cruz, CA, USA) and anti-MITF antibodies were purchased from
NeoMarkers Inc. (Fremont, CA, USA). B16F10 mouse melanoma cells
were obtained from the Korean Cell Line Bank (Seoul, Korea).
Preparation of AC extract
Leaves and small branches from AC plants, which were
more than three-years old and present in all areas of Jeju Island
(Korea) were harvested, dried in the shade at room temperature, and
stored in a dark and cold room until required. The dried plant
material was extracted twice using methanol (20-times the mass of
the dried material) for 72 h at 25°C. The methanol AC extract was
subsequently passed through 0.45-μm filter paper and evaporated at
60°C. The viscous residue was lyophilized to yield the product.
Dimethyl sulfoxide (DMSO) was used to dissolve the product in order
to produce the stock solution.
Cell culture
B16F10 mouse melanoma cells were cultured in phenol
red-free Dulbecco’s modified Eagle’s medium (DMEM) supplemented
with glutamine (2 mmol/l), penicillin (400 U/ml), streptomycin (50
g/l) and 10% fetal bovine serum (FBS) at 37°C in a humidified
atmosphere containing 5% CO2.
Cell viability assay
Cell viability was assessed using an MTT-based
assay. B16F10 cells were incubated overnight with DMEM (phenol
red-free) containing 10% FBS. Cells were subsequently treated with
various concentrations of AC extract for 48 h. Following treatment,
0.5 g/l MTT dissolved in phosphate-buffered saline (PBS) was added
and cells were incubated for an additional 3 h. The supernatant was
removed and DMSO was added to dissolve the formazan crystals.
Absorbance was measured at 570 nm using a microplate reader
(VersaMax; Molecular Devices, LLC., Sunnyvale, CA, USA).
Analysis of melanin content
Melanin content was measured as described
previously, with slight modifications (25). B16F10 cells were incubated
overnight with DMEM (phenol red-free) containing 10% FBS. Cells
were treated with various concentrations of AC extract for 48 h.
Following treatment, 100 μl aliquots of the media were placed in
96-well plates and the optical density (OD) was read at 405 nm
using a microplate reader. Cells were scraped from the dishes,
lysed in cell lysis buffer and protein concentration was determined
using the Bradford assay (Bio-Rad, Hercules, CA, USA). Relative
melanin production was calculated by normalizing the OD values with
the protein concentrations (absorbance/μg protein).
Analysis of tyrosinase activity
B16F10 cells were incubated with various
concentrations of AC extract for 48 h, washed with ice-cold PBS and
lysed with PBS containing 1% Triton X-100. Following centrifugation
(5424R; Eppendorf, Hamburg, Germany) at 15,000 × g for 10 min, the
supernatants were collected. The quantity of each cell lysate was
adjusted using lysis buffer to generate equal protein
concentrations. A total of 90 μl each lysate and 10 μl L-DOPA (10
mmol/l) was combined in the well of a 96-well plate. Control wells
contained 90 μl lysis buffer and 10 μl L-DOPA (10 mmol/l).
Absorbance was measured at 475 nm using a microplate reader
(VersaMax; Molecular Devices, LLC) subsequent to incubation at 37°C
for 30 min.
Western blot analysis
For the analysis of MITF and tyrosinase proteins,
B16F10 cells were treated with AC extract for 24 and 48 h,
respectively. For P- or T-ERK1/2 and P- or T-Akt protein expression
analysis, cells were treated with AC extract, and harvested at 1/3,
2, 4 and 8 h. After the B16F10 cells were harvested, the cells were
lysed in cell lysis buffer [20 mmol/l Tris-HCl (pH 7.5), 150 mmol/l
NaCl, 1 mmol/l EDTA, 1 mmol/l ethylene glycol tetraacetic acid, 1%
Triton X-100, 2.5 mmol/l sodium pyrophosphate, 1 mmol/l
β-glycerophosphate, 1 mmol/l Na3VO4, 1 mmol/l dithiothreitol, 0.01
g/l leupeptin and 1 mmol/l phenylmethylsulfonyl fluoride]. Equal
quantities of total protein were loaded onto 8% SDS-polyacrylamide
gels. Separated proteins were transferred onto polyvinylidene
difluoride membranes (Roche Diagnostics GmbH, Mannheim, Germany),
which were washed with 5% dry milk in Tris-buffered saline
containing 0.4% Tween 20. The membranes were incubated with primary
antibodies, followed by further incubation with horseradish
peroxidase-conjugated secondary antibodies. Antibody-bound proteins
were visualized using enhanced chemiluminescence (Amersham
Biosciences UK Ltd., Little Chalfont, UK).
Statistical analysis
Statistical significance was determined using
Student’s t-test. Data are presented as the mean ± standard
deviation. P<0.05 was considered to indicate a statistically
significant difference.
Results
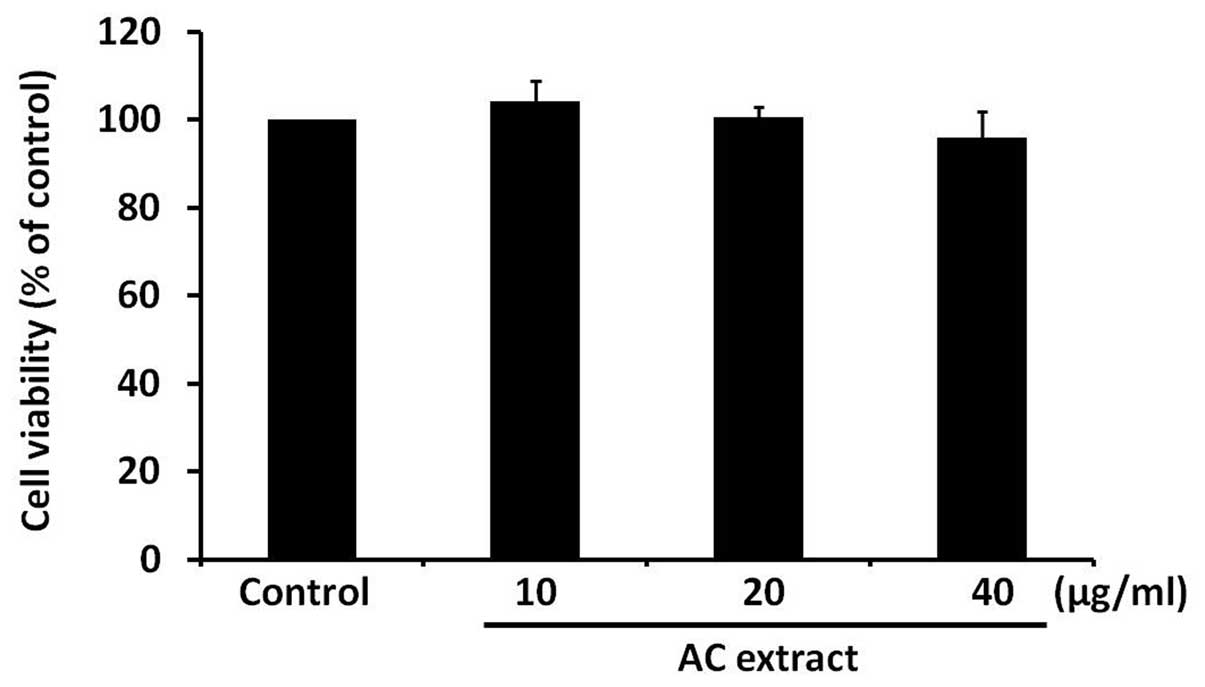
AC extract treatment does not induce
cytotoxicity in B16F10 cells
To determine whether AC extract has a cytotoxic
effect on B16F10 cells, B16F10 cells were treated with AC extract
for 48 h at various concentrations, ranging between 10 and 40
μg/ml. Cell viability was assessed using an MTT-based assay. AC
extract was observed to have no significant effect on cell
viability (Fig. 1). This finding
indicates that AC extract is not cytotoxic to B16F10 cells at the
concentrations used in the present study.
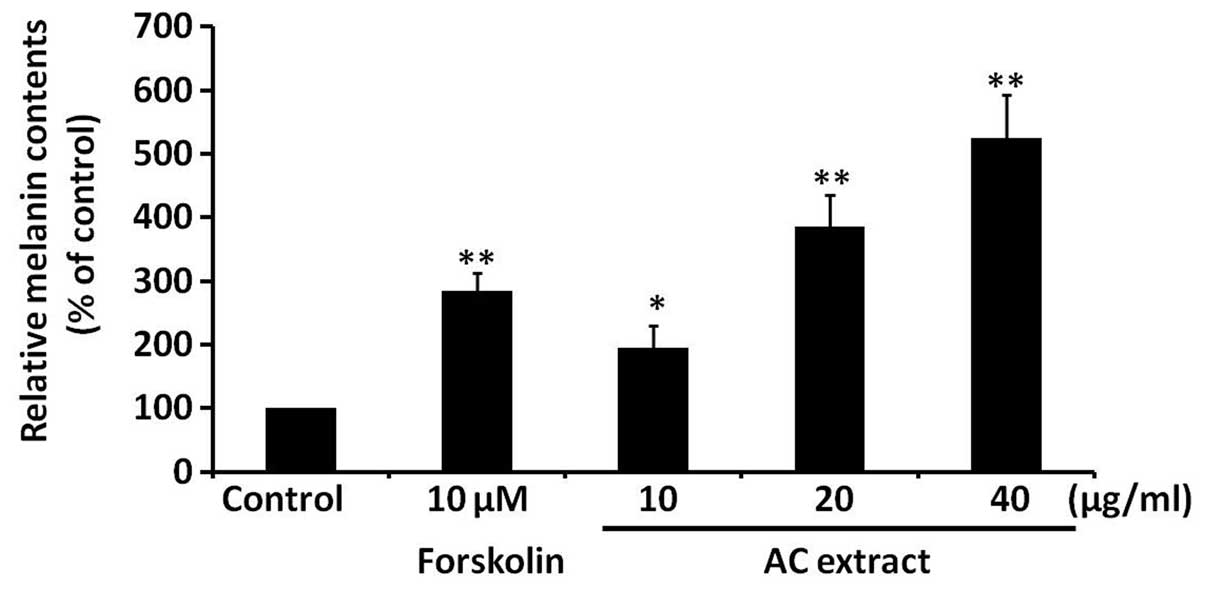
AC extract induces melanogenesis in
B16F10 cells
To assess the effect of AC extract on melanogenesis,
melanin levels were analyzed in B16F10 cells treated with AC
extract at concentrations between 10 and 40 μg/ml. Melanin levels
were found to be significantly increased in a dose-dependent manner
by AC extract treatment (P<0.05; Fig. 2). Forskolin, a well-established
melanogenesis inducer, served as a positive control. These findings
indicate that AC extract induces melanogenesis in B16F10 cells.
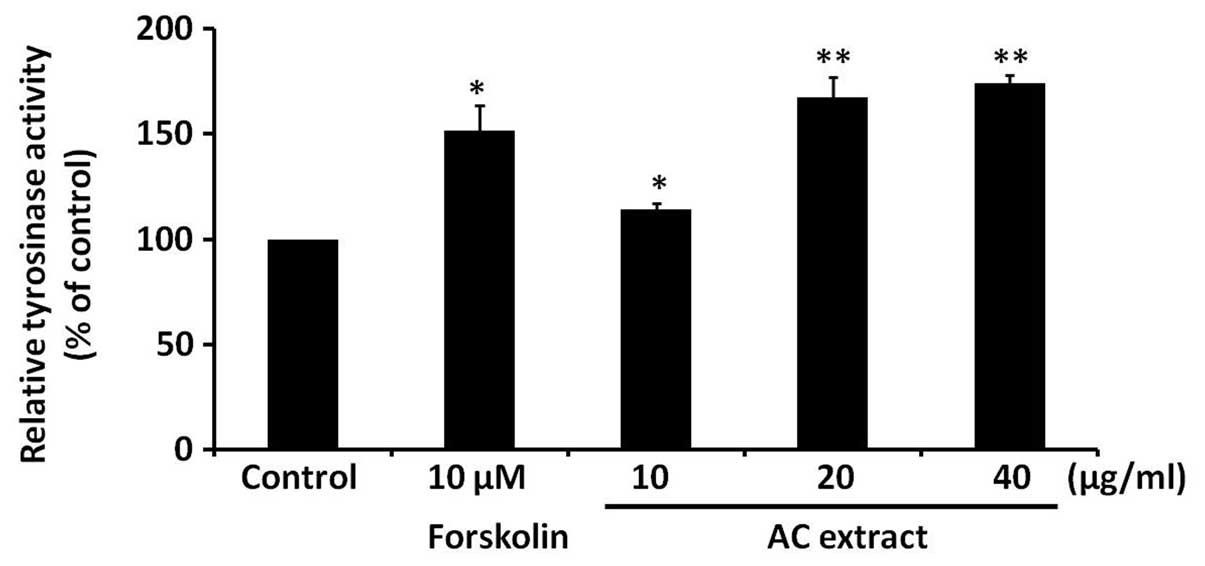
AC extract induces tyrosinase activity in
B16F10 cells
To investigate the possible mechanisms responsible
for the AC extract-induced increase in melanogenesis in B16F10
cells, the effect of AC extract on tyrosinase activity was
assessed. Tyrosinase is a rate-limiting enzyme in melanin
synthesis. B16F10 cells were treated with AC extract at the
indicated concentrations for 48 h and intracellular tyrosinase
activity was analyzed. Forskolin served as a positive control.
Fig. 3 demonstrates that AC
extract significantly induced intracellular tyrosinase activity in
a dose-dependent manner (P<0.05).
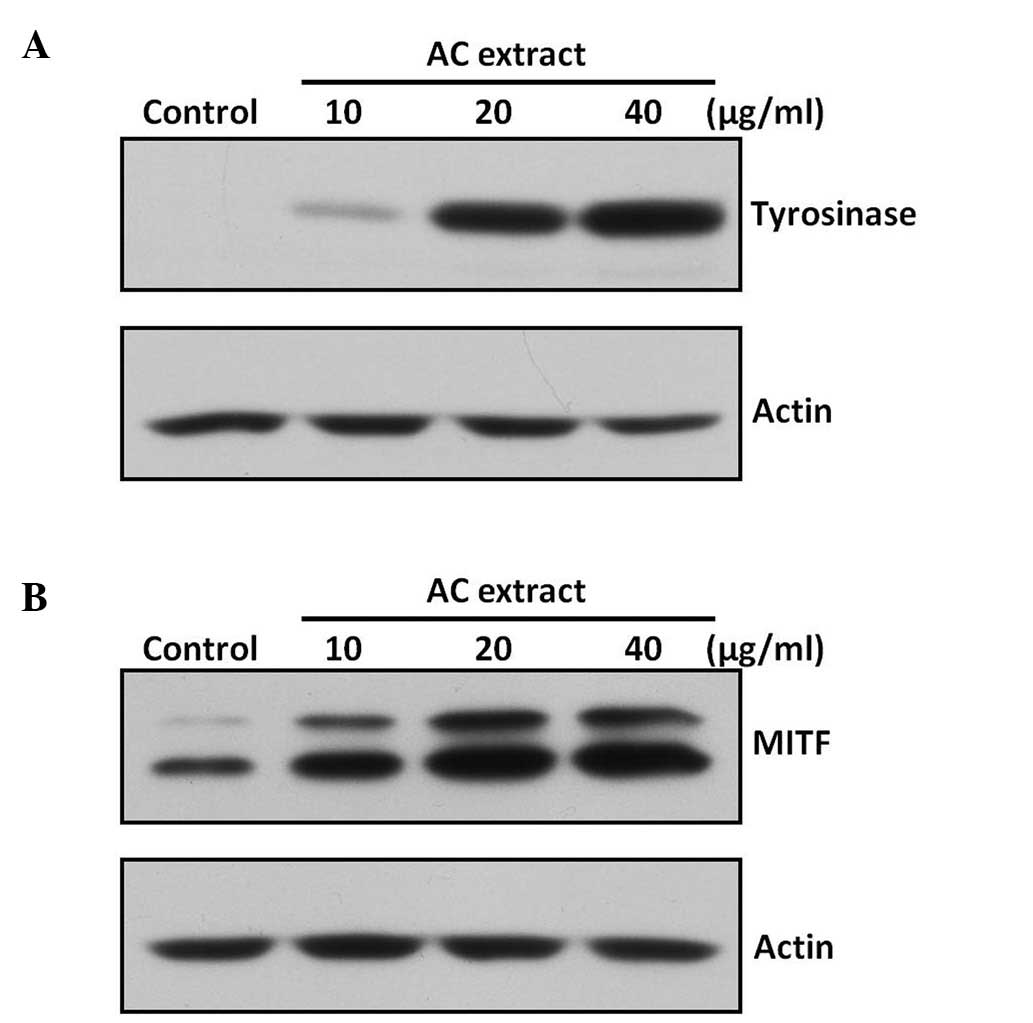
AC extract increases expression of
tyrosinase and MITF in B16F10 cells
In order to clarify the mechanism underlying AC
extract-induced tyrosinase activation, tyrosinase protein
expression was assessed in B16F10 cells using western blot
analysis. AC extract was observed to markedly increase tyrosinase
protein expression (Fig. 4A). MITF
is a major transcription factor for tyrosinase expression (7), therefore, the effect of AC extract on
the expression of MITF was also investigated. As shown in Fig. 4B, AC extract was found to increase
MITF protein expression.
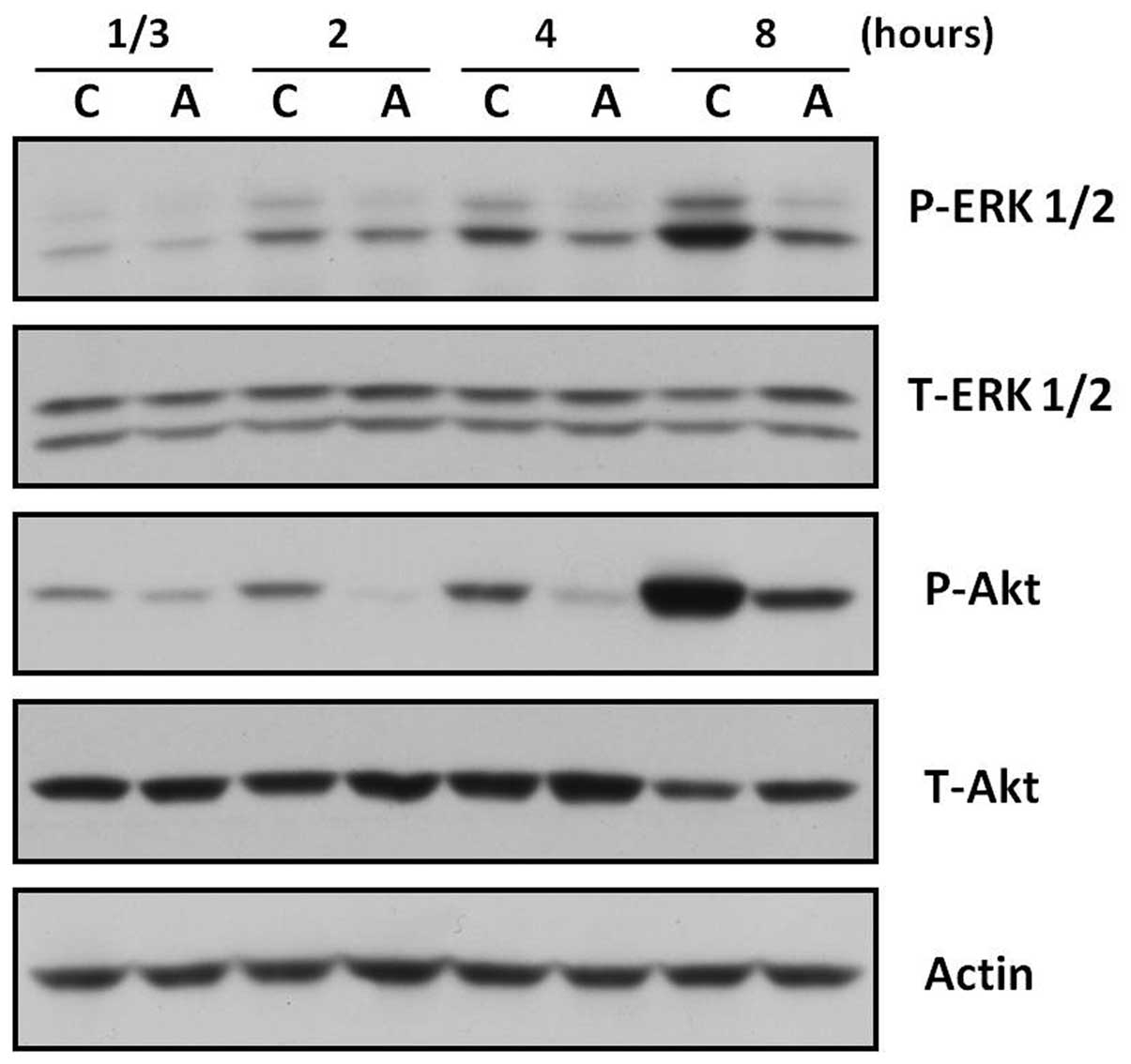
AC extract suppresses the activation of
ERK and Akt in B16F10 cells
The ERK and the Akt signaling pathways have been
shown to negatively regulate melanogenesis in melanocytes and
melanoma cells (14–15). Furthermore, inhibition of ERK and
PI3K/Akt has been reported to stimulate melanogenesis (26–27).
Thus, the affect of AC extract on the ERK and Akt pathways in
B16F10 cells was analyzed. B16F10 cells were treated with AC
extract (40 μg/ml) for the indicated durations. Activation of the
ERK or Akt signaling pathways was determined using western blot
analysis with specific antibodies against phosphorylated forms of
ERK and Akt. As shown in Fig. 5,
ERK and Akt phosphorylation was inhibited by AC treatment at all
time points.
Discussion
In the present study, the effect of AC extract on
melanogenesis was investigated using a melanin content assay,
intracellular tyrosinase activity assay and western blot analysis.
AC extract was found to upregulate melanin synthesis in a
concentration-dependent manner (10–40 μg/ml) without inducing
cytotoxicity in B16F10 cells (Fig.
1 and 2). Tyrosinase is a key
enzyme involved in melanogenesis (6), therefore, the effect of AC extract on
tyrosinase activity and expression was also analyzed. AC extract
was observed to increase tyrosinase activity and expression
(Figs. 3 and 4A). Furthermore, as shown in Fig. 4B, AC extract was found to increase
the expression of MITF, a transcription factor which controls
pigmentation through regulating the expression of melanogenic
enzymes, including tyrosinase (7).
It is well established that the ERK signaling
pathway is involved in cell proliferation and differentiation
(28–29). Furthermore, the ERK signaling
pathway has been identified as a negative regulator of melanin
synthesis and activation of the ERK signaling pathway has been
reported to lead to MITF protein degradation, thus reducing
melanogenesis (30). Stimulation
of c-kit signaling has also been reported to induce MITF protein
degradation, which is prevented by the MAPK/ERK signaling pathway
inhibitor, PD98059 (30).
Moreover, inhibition of the ERK signaling pathway by PD98059 has
been found to induce melanogenesis in B16F10 cells (25). It is well established that the
PI3K/Akt signaling pathway is important in the regulation of
various cellular processes, including cell growth and apoptosis
(31–32). The PI3K/Akt signaling pathway has
also been shown to be involved in melanogenesis, with inhibition of
the PI3K/Akt signaling pathway observed to stimulate melanogenesis
in G361 melanoma and B16F10 cells (26,33).
Furthermore, a previous study demonstrated that the PI3K/Akt
signaling pathway inhibitor, LY294002 upregulated MITF protein
expression, which increased tyrosinase expression, resulting in
increased melanogenesis in B16F10 cells (33).
In order to identify the mechanisms underlying the
AC extract-induced increase in melanogenesis in B16F10 cells, the
effect of AC extract on the ERK and Akt signaling pathways was
assessed. As shown in Fig. 5, AC
extract inhibited ERK and Akt activation as early as 20 min after
AC extract treatment; this inhibition was sustained for ≥8 h. These
findings indicate that the inhibition of ERK and/or Akt by AC
extract may contribute to AC extract-induced melanogenesis through
upregulating the protein expression of MITF and tyrosinase.
In conclusion, the present study has demonstrated
the melanogenic effect of a methanol extract of AC in B16F10 cells
and the underlying mechanisms involved. The findings indicate that
AC extract may be useful for the treatment of hypopigmentation
disorders and the development of self-tanning cosmetic
products.
Acknowledgements
The authors would like to thank Bioland Co. Ltd
(Cheonan, Korea) for preparation of the reagents. The present study
was partially supported by grants from the National Research
Foundation of Korea (the Korean government; grant no. 2011-0029819)
and the Korean Health Technology R&D Project (grant no.
A121851).
Abbreviations:
|
α-MSH
|
α-melanocyte stimulating hormone
|
|
MITF
|
microphthalmia-associated
transcription factor
|
|
AC
|
Ardisia crenata
|
|
MTT
|
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide
|
|
PKA
|
protein kinase A
|
|
CREB
|
cyclic adenosine monophosphate
response element binding protein
|
|
L-DOPA
|
L-3,4-dihydroxyphenylalanine
|
|
TRP
|
tyrosinase-related protein
|
|
ERK
|
extracellular signal-regulated
kinases
|
References
|
1
|
Hearing VJ: Biogenesis of pigment
granules: a sensitive way to regulate melanocyte function. J
Dermatol Sci. 37:3–14. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Brenner M and Hearing VJ: The protective
role of melanin against UV damage in human skin. Photochem
Photobiol. 84:539–549. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Slominski A, Tobin DJ, Shibahara S and
Wortsman J: Melanin pigmentation in mammalian skin and its hormonal
regulation. Physiol Rev. 84:1155–1228. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Slominski A: Neuroendocrine activity of
the melanocyte. Exp Dermatol. 18:760–763. 2009. View Article : Google Scholar
|
|
5
|
del Marmol V and Beermann F: Tyrosinase
and related proteins in mammalian pigmentation. FEBS Lett.
381:165–168. 1996.PubMed/NCBI
|
|
6
|
Hearing VJ and Jiménez M: Mammalian
tyrosinase - the critical regulatory control point in melanocyte
pigmentation. Int J Biochem. 19:1141–1147. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Bentley NJ, Eisen T and Goding CR:
Melanocyte-specific expression of the human tyrosinase promoter:
activation by the microphthalmia gene product and role of the
initiator. Mol Cell Biol. 14:7996–8006. 1994.PubMed/NCBI
|
|
8
|
Buscà R and Ballotti R: Cyclic AMP a key
messenger in the regulation of skin pigmentation. Pigment Cell Res.
13:60–69. 2000.PubMed/NCBI
|
|
9
|
Saito H, Yasumoto K, Takeda K, Takahashi
K, Yamamoto H and Shibahara S: Microphthalmia-associated
transcription factor in the Wnt signaling pathway. Pigment Cell
Res. 16:261–265. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Widlund HR and Fisher DE:
Microphthalamia-associated transcription factor: a critical
regulator of pigment cell development and survival. Oncogene.
22:3035–3041. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Koo JH, Kim HT, Yoon HY, et al: Effect of
xanthohumol on melanogenesis in B16 melanoma cells. Exp Mol Med.
40:313–319. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Jiang Z, Li S, Liu Y, Deng P, Huang J and
He G: Sesamin induces melanogenesis by microphthalmia-associated
transcription factor and tyrosinase up-regulation via cAMP
signaling pathway. Acta Biochim Biophys Sin (Shanghai). 43:763–770.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Hamid MA, Sarmidi MR and Park CS:
Mangosteen leaf extract increases melanogenesis in B16F1 melanoma
cells by stimulating tyrosinase activity in vitro and by
up-regulating tyrosinase gene expression. Int J Mol Med.
29:209–217. 2012.PubMed/NCBI
|
|
14
|
Oka M, Nagai H, Ando H, et al: Regulation
of melanogenesis through phosphatidylinositol 3-kinase-Akt pathway
in human G361 melanoma cells. J Invest Dermatol. 115:699–703. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kim DS, Kim SY, Chung JH, Kim KH, Eun HC
and Park KC: Delayed ERK activation by ceramide reduces melanin
synthesis in human melanocytes. Cell Signal. 14:779–785. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Villareal MO, Han J, Matsuyama K, et al:
Lupenone from Erica multiflora leaf extract stimulates
melanogenesis in B16 murine melanoma cells through the inhibition
of ERK1/2 activation. Planta Med. 79:236–243. 2013.
|
|
17
|
Galus R, Niderla J, Sladowski D, Sajjad E,
Włodarski K and Jóźwiak J: Fluvastatin increases tyrosinase
synthesis induced by alpha-melanocyte-stimulating hormone in B16F10
melanoma cells. Pharmacol Rep. 62:164–169. 2010. View Article : Google Scholar
|
|
18
|
Grimes PE: New insights and new therapies
in vitiligo. JAMA. 293:730–735. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Moreira CG, Horinouchi CD, Souza-Filho CS,
et al: Hyperpigmentant activity of leaves and flowers extracts of
Pyrostegia venusta on murine B16F10 melanoma. J
Ethnopharmacol. 141:1005–1011. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Chaabane F, Pinon A, Simon A, Ghedira K
and Chekir-Ghedira L: Phytochemical potential of Daphne
gnidium in inhibiting growth of melanoma cells and enhancing
melanogenesis of B16-F0 melanoma. Cell Biochem Funct. 31:460–467.
2013. View
Article : Google Scholar
|
|
21
|
Maotian W, Xiongtai G, Xiuwen H and
Shanhai H: A new triterpenoid saponin from Ardisia crenata.
Planta Med. 58:205–207. 1992. View Article : Google Scholar
|
|
22
|
Jia Z, Koike K, Ohmoto T and Ni M:
Triterpenoid saponins from Ardisia crenata. Phytochemistry.
37:1389–1396. 1994. View Article : Google Scholar
|
|
23
|
Wang X, Tang S, Zhai H and Duan H: Studies
on anti-tumor metastatic constituents from Ardisia crenata.
Zhongguo Zhong Yao Za Zhi. 36:881–885. 2011.(In Chinese).
|
|
24
|
Zaima K, Deguchi J, Matsuno Y, Kaneda T,
Hirasawa Y and Morita H: Vasorelaxant effect of FR900359 from
Ardisia crenata on rat aortic artery. J Nat Med. 67:196–201.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Yao C, Oh JH, Oh IG, Park CH and Chung JH:
[6]-Shogaol inhibits melanogenesis in B16 mouse melanoma cells
through activation of the ERK pathway. Acta Pharmacol Sin.
34:289–294. 2013.
|
|
26
|
Buscà R, Bertolotto C, Ortonne JP and
Ballotti R: Inhibition of the phosphatidylinositol
3-kinase/p70(S6)-kinase pathway induces B16 melanoma cell
differentiation. J Biol Chem. 271:31824–31830. 1996.PubMed/NCBI
|
|
27
|
Englaro W, Bertolotto C, Buscà R, et al:
Inhibition of the mitogen-activated protein kinase pathway triggers
B16 melanoma cell differentiation. J Biol Chem. 273:9966–9970.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Cowley S, Paterson H, Kemp P and Marshall
CJ: Activation of MAP kinase kinase is necessary and sufficient for
PC12 differentiation and for transformation of NIH 3T3 cells. Cell.
77:841–852. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Sale EM, Atkinson PG and Sale GJ:
Requirement of MAP kinase for differentiation of fibroblasts to
adipocytes, for insulin activation of p90 S6 kinase and for insulin
or serum stimulation of DNA synthesis. EMBO J. 14:674–684.
1995.PubMed/NCBI
|
|
30
|
Wu M, Hemesath TJ, Takemoto CM, et al:
c-Kit triggers dual phosphorylations, which couple activation and
degradation of the essential melanocyte factor Mi. Genes Dev.
14:301–312. 2000.PubMed/NCBI
|
|
31
|
Ahmad S, Singh N and Glazer RI: Role of
AKT1 in 17beta-estradiol- and insulin-like growth factor I
(IGF-I)-dependent proliferation and prevention of apoptosis in
MCF-7 breast carcinoma cells. Biochem Pharmacol. 58:425–430. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Tang Y, Zhou H, Chen A, Pittman RN and
Field J: The Akt proto-oncogene links Ras to Pak and cell survival
signals. J Biol Chem. 275:9106–9109. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Khaled M, Larribere L, Bille K, Ortonne
JP, Ballotti R and Bertolotto C: Microphthalmia associated
transcription factor is a target of the
phosphatidylinositol-3-kinase pathway. J Invest Dermatol.
121:831–836. 2003. View Article : Google Scholar : PubMed/NCBI
|