Introduction
The inflammatory reaction induced by
ischemia/reperfusion (I/R) is an important process in the
development of myocardial ischemia-reperfusion (MI/R) injury
(1). During inflammation, various
cytokines are released, including tumor necrosis factor-α (TNF-α),
interleukin (IL)-6 and IL-8 (2).
TNF-α triggers an inflammatory reaction in response to MI/R. In
addition, vascular endothelial cell injury and inflammatory cells,
such as neutrophils, which are activated by cytokines and adhesion
molecules, are known to be involved in inflammation. Thus, levels
of TNF-α and the degree of neutrophil infiltration may be
considered to be indicators of an inflammatory reaction.
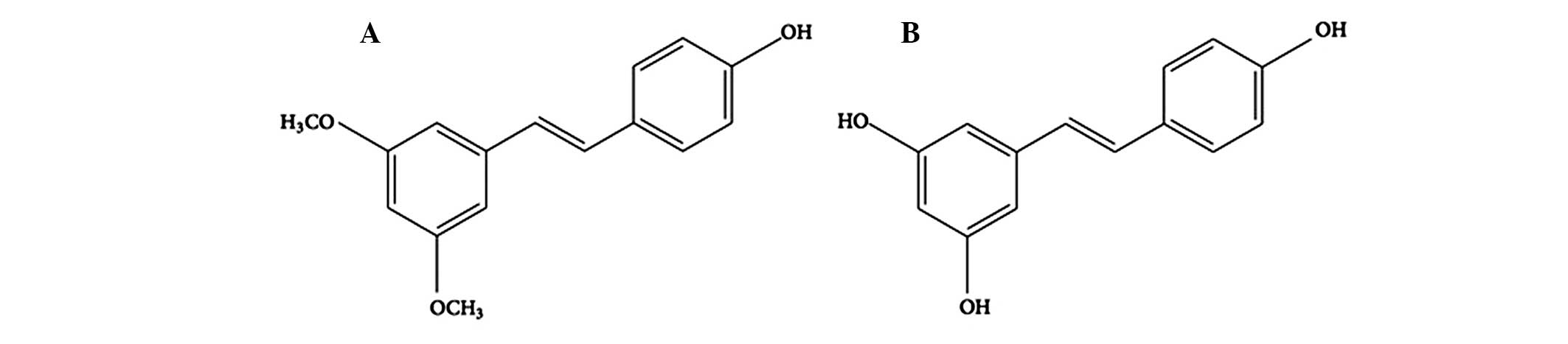
Pterostilbene
(trans-3,5-dimethoxy-4-hydroxystilbene; Pte) is a naturally-derived
compound found primarily in blueberries and Pterocarpus
marsupium heartwood (3,4). It
is structurally similar to resveratrol, a compound found in red
wine that has comparable antioxidant, anti-inflammatory and
anticarcinogenic properties. However, Pte exhibits increased
bioavailability compared with resveratrol, due to the presence of
two methoxy groups, which confer properties of increased lipophilic
and oral absorption (Fig. 1)
(5–7). Increasing evidence suggests that Pte
may have numerous preventive and therapeutic properties in a range
of human diseases, including neurological, metabolic and
hematologic disorders (8). Further
benefits of Pte have been reported in preclinical trials, in which
Pte was shown to be a potent anticancer agent in a number of
malignancies (9). However, to the
best of our knowledge, the role of Pte in inflammation induced by
MI/R has not yet been reported. The present study therefore aimed
to investigate the effect of Pte on neutrophil infiltration and
TNF-α production in a rat model of MI/R and its underlying
mechanisms.
Materials and methods
Reagents
Pte was obtained from Sigma (St. Louis, MO, USA).
The myeloperoxidase (MPO) assay kits, creatine kinase (CK) test
kits and lactate dehydrogenase (LDH) assay kits were purchased from
JianCheng Bioengineering Institute (Nanjing, China). TNF-α
enzyme-linked immunosorbent assay kits were purchased from R&D
Systems, Inc. (Minneapolis, MN, USA). L-NAME and methylene blue
(MB) were purchased from Sigma-Aldrich (St. Louis, MO, USA). A
bicinchoninic acid (BCA) protein quantification kit was purchased
from Bio-Rad Laboratories (Hercules, CA, USA).
Animals
Fifty adult male Sprague-Dawley rats (250–300 g)
were purchased from the Center of Experimental Animal in Jilin
University (Changchun, China). All animals used in this study were
cared for in accordance with the Guidance for the Care and Use of
Laboratory Animals published by the United States National
Institute of Health (NIH; publication no. 85-23, revised 1996), and
all procedures were approved by the Committee of Experimental
Animals of Jilin University.
MI/R model and experimental protocol
Male Sprague-Dawley rats (weight, 250–300 g) were
anesthetized intraperitoneally with 40 mg/kg sodium pentobarbital
(Sigma Aldrich). Myocardial ischemia was induced by exteriorizing
the heart with a left thoracic incision followed by a slipknot (5-0
silk; Johnson & Johnson China, Ltd., Shanghai, China) around
the left anterior descending (LAD) coronary artery. Following 30
min of ischemia, slipknots were released and animals received 120
min of reperfusion.
Rats were randomly assigned to five experimental
groups and there were ten rats in each group. The groups were as
follows: Sham group, silk was fed under the LAD coronary artery but
the LAD coronary artery was not ligated; MI/R group, the LAD
coronary artery was ligated for 30 min and then allowed 120 min
reperfusion and was treated with vehicle [0.9% NaCl intravenously
(i.v.)]; MI/R + Pte group, 100 μmol/l Pte i.v. was administered 5
min prior to reperfusion; MI/R + Pte + L-NAME group, 1 mmol/l
L-NAME i.v., a nitric oxide (NO) synthase inhibitor, was
administered 20 min prior to reperfusion. At 15 min
post-administration of L-NAME, 100 μmol/l Pte, i.v. was
administered; and MI/R + Pte + MB group, 50 μmol/l methylene blue
(MB) i.v., a cyclic guanosine monophosphate (cGMP) inhibitor, was
administered 20 min prior to reperfusion. At 15 min following
treatment with MB, 100 μmol/l Pte, i.v. was administered.
Assay of myocardial infarct area
Following reperfusion, myocardial infarct size was
determined by means of a double-staining technique and a digital
imaging system (Adobe Systems Incorporated, San Jose, CA, USA)
(10). Following reperfusion, the
coronary blood flow was again disrupted and 4 ml of 2% Evans blue
was injected into the right ventricle in order to ensure rapid
distribution around the body. Hearts were taken to a −20°C
refrigerator for cryopreservation. Hearts were cut into 1-mm
slices, placed in 1% 2,3,5-triphenyltetrazolium chloride (TTC)
solution, incubated for 15 min, and maintained in 4% formaldehyde
solution overnight. Evans blue-stained areas (blue staining,
non-ischemic areas), TTC-stained areas (red staining, ischemic
areas) and non-TTC-stained areas (white, infarct areas) were
analyzed by computer using a digital imaging system. The percentage
of myocardial infarct was then calculated using the following
formula: Infarct area/area at risk (INF/AAR) × 100.
Determination of MPO levels
Following reperfusion, mocardial tissue was
maintained at −70°C for preservation. An MPO test kit was employed
to detect the level of MPO in the myocardial tissue, according to
the manufacturer’s instructions.
Detection of CK activity
Following reperfusion, blood was taken from the
carotid artery and kept at room temperature for 30 min at 4°C.
Serum was separated by centrifugation at 3,000 × g for 20 min and
maintained at −70°C for preservation. A CK test kit was utilized
according to the manufacturer’s instructions in order to measure
serum CK activity.
Determination of LDH levels
Following reperfusion, blood was taken from the
carotid artery and kept at room temperature for 30 min. Serum was
separated by centrifugation at 3,000 × g for 20 min at 4°C and
maintained at −70°C for preservation. The extent of cell injury was
monitored by measuring leakage of LDH. An LDH test kit was utilized
according to the manufacturer’s instructions in order to measure
serum LDH levels.
Detection of TNF-α levels
Following reperfusion, the levels of TNF-α in
myocardial tissue homogenate and serum were measured according to
the manufacturer’s instructions. A BCA kit was used to detect the
protein quantization.
Statistical analysis
Data are presented as the mean ± standard deviation.
The significance of the differences among groups was evaluated by
Student’s t-test for unpaired data or Dunnett’s t-test for multiple
comparisons, preceded by one-way analysis of variance. SPSS version
13.0 was used for analysis (SPSS, Inc., Chicago, IL, USA) P<0.05
was considered to indicate a statistically significant
difference.
Results
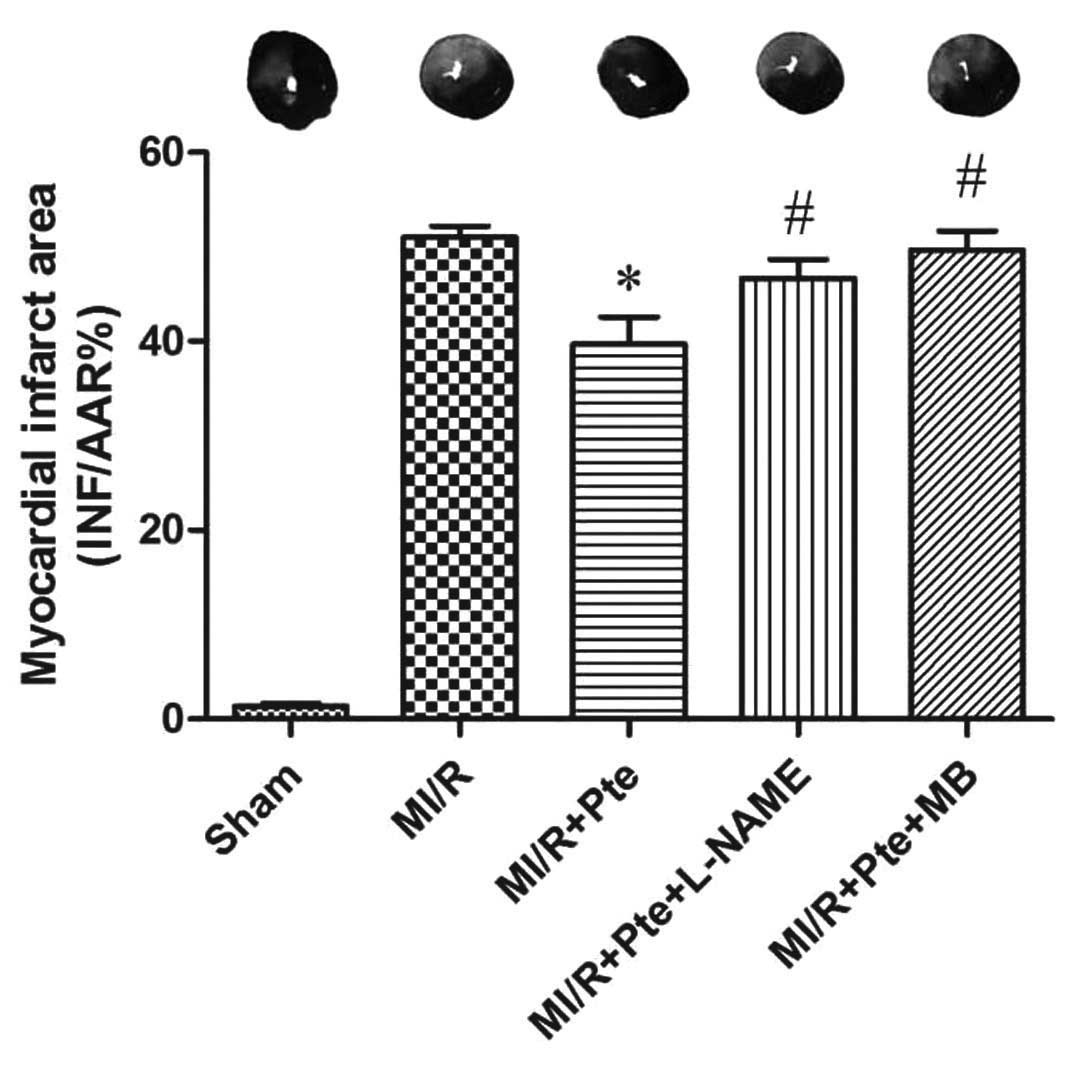
Pte reduces the myocardial infarction
area induced by MI/R
MI/R induced an area of infarction in the
myocardium. Compared with the MI/R group, Pte reduced the infarcted
area in the myocardium significantly. This effect of Pte was
eliminated by the administration of L-NAME, a NO synthase
inhibitor. In addition, the effect of Pte was significantly
attenuated by administration of MB, a cGMP inhibitor (Fig. 2).
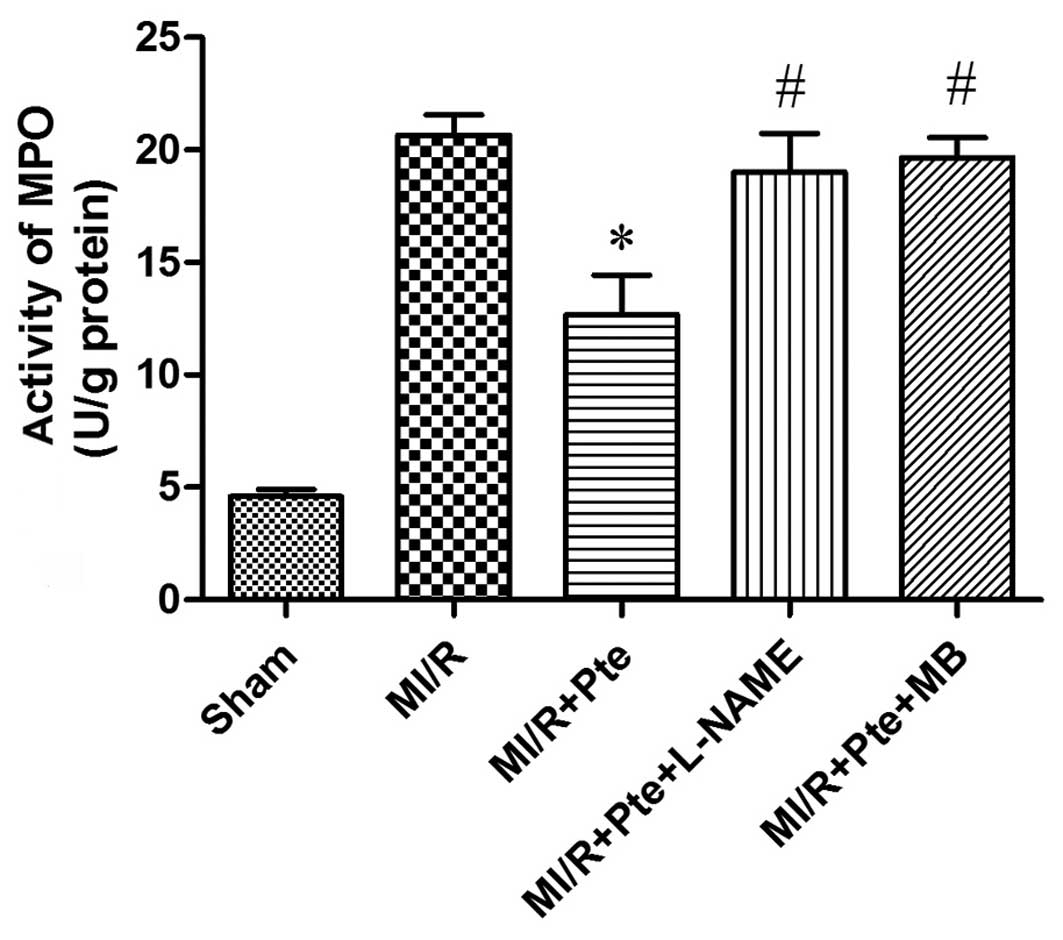
Pte inhibits neutrophil infiltration in
MI/R tissue
Neutrophils contain a certain quantity of MPO, which
accounts for ~5% of dry cell weight. Thus, the activity of MPO in
the myocardium may be considered as an indication of neutrophil
infiltration. As shown in Fig. 3,
the activity in the sham group was relatively low, whereas, by
comparison, the MPO activity in the MI/R group was significantly
increased. Pte significantly decreased myocardial MPO activity
compared with the MI/R group, whilst the administration of L-NAME
and MB attenuated this effect of Pte.
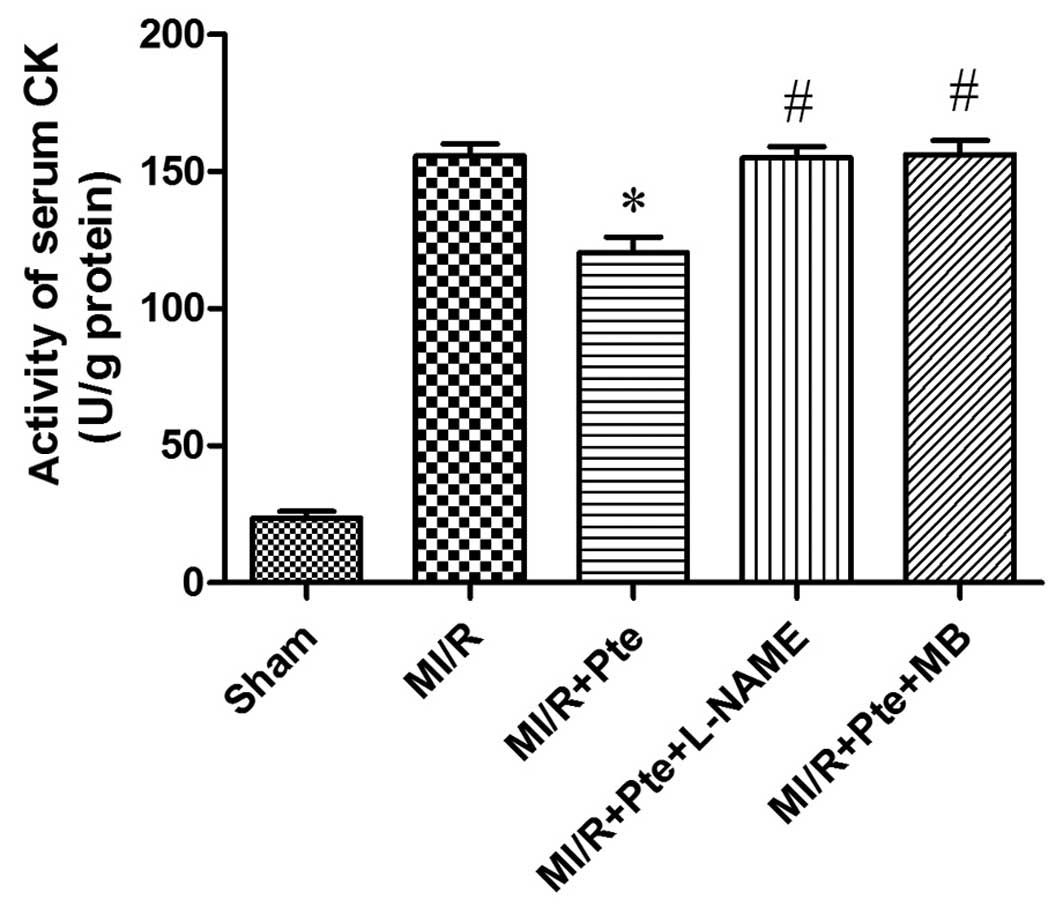
Pte reduces the activity of serum CK in
MI/R rats
As shown in Fig. 4,
the activity of CK increased significantly in the MI/R group
compared with the sham group. CK activity was significantly reduced
in the MI/R + Pte group compared with the MI/R group. This effect
of Pte was eliminated by the administration of L-NAME and MB.
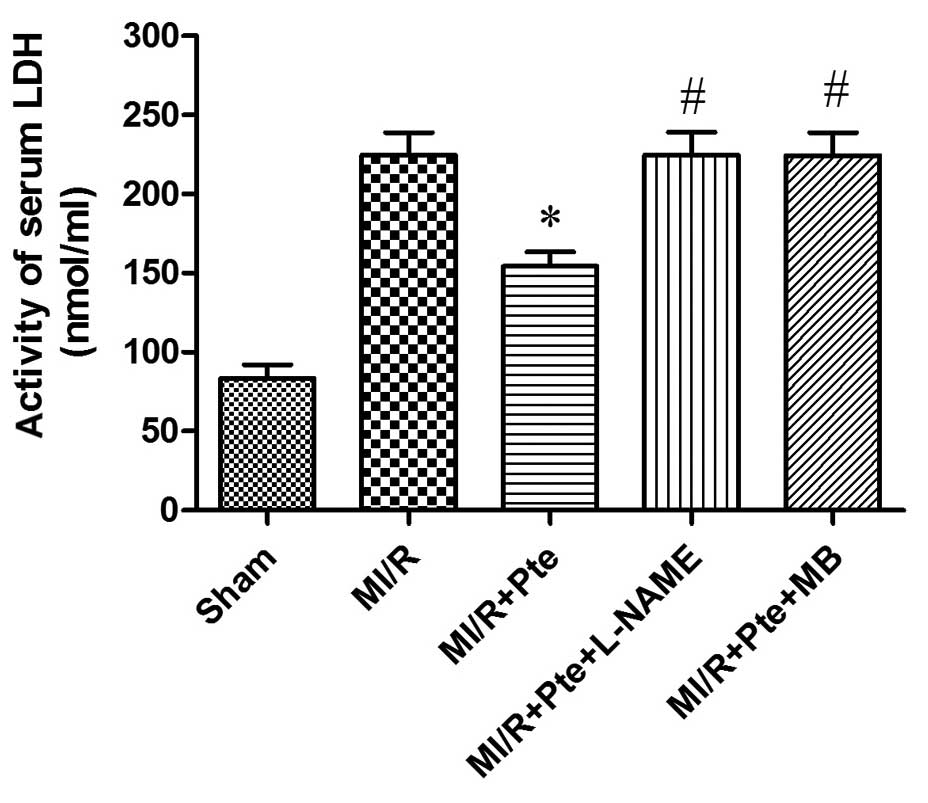
Pte reduces LDH activity in MI/R
rats
As shown in Fig. 5,
the LDH activity increased significantly in the MI/R group compared
with the sham group. LDH activity was significantly decreased in
the MI/R + Pte group compared with the MI/R group. This effect of
Pte was eradicated by L-NAME and MB administration.
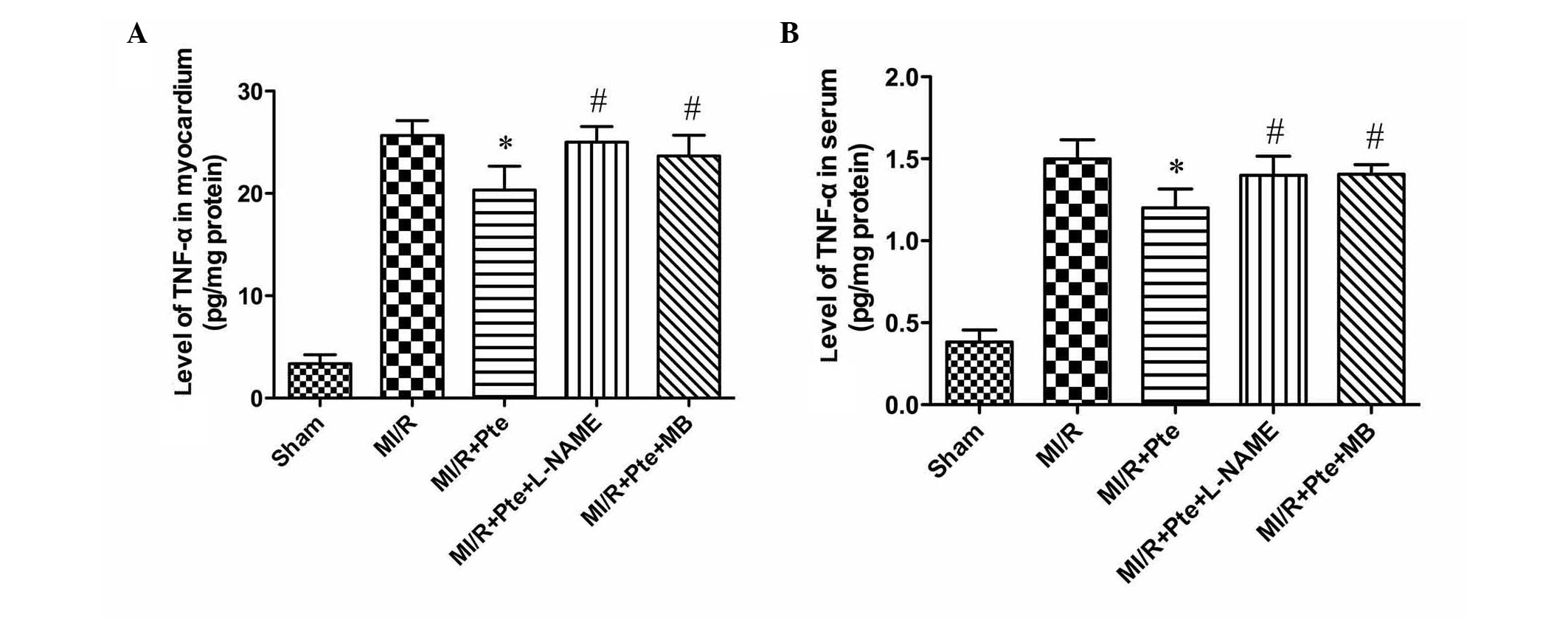
Pte reduces TNF-α levels in the serum and
MI/R tissue
MI/R injury is known to result in the production of
increased levels of TNF-α. Thus, myocardial and serum TNF-α levels
were examined. As shown in Fig. 6,
compared with the MI/R group, Pte significantly reduced the levels
of TNF-α in myocardium and serum. This effect was eliminated by
L-NAME and MB administration.
Discussion
The current study found that Pte reduces the
inflammatory reaction induced by I/R injury by inhibiting
neutrophil infiltration and TNF-α production. In addition, it
demonstrated that NO and cGMP may be important mediators of the
protective effects of Pte.
The inflammatory reaction is known to be involved in
MI/R injury (1). The release of
inflammatory cytokines and the aggregation and infiltration of
inflammatory cells are key steps in inflammation (11).
TNF-α is secreted predominantly by macrophages. It
promotes an inflammatory cascade by increasing the release of other
proinflammatory cytokines and influencing neutrophil recruitment
(12). TNF-α is an important
cytokine in the process of inflammation, and is involved in the
initiation of the inflammation induced by MI/R (13). TNF-α induces the release of other
inflammatory mediators, increases the expression of cell adhesion
factors and promotes neutrophil adhesion to endothelial cells. In
addition, TNF-α has a negative inotropic effect, which inhibits
myocardial contractility and lowers blood pressure. TNF-α also
induces cardiomyocyte apoptosis and participates in ventricular
remodeling (14). Previous studies
have suggested that the level of TNF-α increases significantly
following MI/R (15), whilst the
administration of a TNF-α monoclonal antibody attenuated edema and
aided recovery of cardiac function (16).
MI/R injury appears to be induced in part by
neutrophil activation. There are a number of mechanisms underlying
this effect. Cell damage, caused by the release of oxygen free
radicals, proteolytic enzymes and cytotoxic substances leads to
increased neutrophil activation. In addition, inflammatory
mediators cause vascular endothelial cell damage, increased
vascular permeability and edema. Further activation of inflammatory
cells leads to further increases in the inflammatory response
(17). Finally, neutrophil
adhesion to the vascular endothelium and the occlusion of small
blood vessels result in a no-reflow phenomenon.
Previous studies have demonstrated an association
between neutrophil and MI/R injury. The removal of neutrophils or
drug-induced inhibition of neutrophil activity has been shown to
reduce MI/R injury (18,19). The present study found that
neutrophil accumulation and TNF-α production increased
significantly in the MI/R group compared with the sham control
group. Pte was shown to reduce neutrophil accumulation and TNF-α
production, indicating that Pte inhibits neutrophil accumulation
and TNF-α production and thereby attenuates neutrophil-mediated I/R
injury.
It is hypothesized that NO production is associated
MI/R-induced inflammation (20).
Endothelial-derived NO inhibits cell adhesion factors, including
P-selectin and ICAM-1 levels, thereby inhibiting leukocyte adhesion
and inward membrane migration (21). Endothelial-derived NO also inhibits
expression of TNF-α and other pro-inflammatory factors. In
addition, it increases levels of IL-10 and other anti-inflammatory
factors and indirectly inhibits inflammatory cells from aggregating
in areas of local inflammation, thereby reducing the inflammatory
response (22). In the present
study, following addition of L-NAME, a NO synthase inhibitor, the
protective effect of Pte was eliminated. This suggested that NO is
pivotal in mediating the protective effects of Pte. Similarly,
following addition of MB, a cGMP inhibitor, the protective effects
of Pte were eradicated, indicating that the cGMP pathway is also
involved in the protective actions of Pte.
In conclusion, the present study demonstrated that
Pte attenuates inflammation induced by MI/R injury. The protective
effects of Pte are associated with inhibition of neutrophil
infiltration and TNF-α production, increases in the levels of NO
and possible upregulation of the cGMP signaling pathway. The
present study provides new insights into the mechanisms involved in
the cardioprotective effect of pterostilbene against myocardial
ischemia/reperfusion injury, which may be a new clinical therapy
for myocardial ischemia/reperfusion injury.
References
|
1
|
Xiong J, Xue FS, Yuan YJ, Wang Q, Liao X
and Wang WL: Cholinergic anti-inflammatory pathway: a possible
approach to protect against myocardial ischemia reperfusion injury.
Chin Med J (Engl). 123:2720–2726. 2010.PubMed/NCBI
|
|
2
|
Naidu BV, Farivar AS, Woolley SM, Grainger
D, Verrier ED and Mulligan MS: Novel broad-spectrum chemokine
inhibitor protects against lung ischemia-reperfusion injury. J
Heart Lung Transplant. 23:128–134. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Roupe KA, Remsberg CM, Yáñez JA and Davies
NM: Pharmacometrics of stilbenes: seguing towards the clinic. Curr
Clin Pharmacol. 1:81–101. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lin HS, Yue BD and Ho PC: Determination of
pterostilbene in rat plasma by a simple HPLC-UV method and its
application in pre-clinical pharmacokinetic study. Biomed
Chromatogr. 23:1308–1315. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kapetanovic IM, Muzzio M, Huang Z,
Thompson TN and McCormick DL: Pharmacokinetics, oral
bioavailability, and metabolic profile of resveratrol and its
dimethylether analog, pterostilbene, in rats. Cancer Chemother
Pharmacol. 68:593–601. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Perecko T, Drabikova K, Rackova L, et al:
Molecular targets of the natural antioxidant pterostilbene: effect
on protein kinase C, caspase-3 and apoptosis in human neutrophils
in vitro. Neuro Endocrinol Lett. 31(Suppl 2): 84–90.
2010.PubMed/NCBI
|
|
7
|
Athar M, Back JH, Tang X, et al:
Resveratrol: a review of preclinical studies for human cancer
prevention. Toxicol Appl Pharmacol. 224:274–283. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
McCormack D and McFadden D: A review of
pterostilbene antioxidant activity and disease modification. Oxid
Med Cell Longev. 2013:5754822013.PubMed/NCBI
|
|
9
|
McCormack D and McFadden D: Pterostilbene
and cancer: current review. J Surg Res. 173:e53–e61. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Black SC and Rodger IW: Methods for
studying experimental myocardial ischemic and reperfusion injury. J
Pharmacol Toxicol Methods. 35:179–190. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Speyer CL and Ward PA: Role of endothelial
chemokines and their receptors during inflammation. J Invest Surg.
24:18–27. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Khimenko PL, Bagby GJ, Fuseler J and
Taylor AE: Tumor necrosis factor-alpha in ischemia and reperfusion
injury in rat lungs. J Appl Physiol (1985). 85:2005–2011.
1998.PubMed/NCBI
|
|
13
|
Batista ML Jr, Rosa JC, Lopes RD, et al:
Exercise training changes IL-10/TNF-alpha ratio in the skeletal
muscle of post-MI rats. Cytokine. 49:102–108. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhu J, Liu M, Kennedy RH and Liu SJ:
TNF-alpha-induced impairment of mitochondrial integrity and
apoptosis mediated by caspase-8 in adult ventricular myocytes.
Cytokine. 34:96–105. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Meldrum DR, Cleveland JC Jr, Cain BS, Meng
X and Harken AH: Increased myocardial tumor necrosis factor-alpha
in a crystalloid-perfused model of cardiac ischemia-reperfusion
injury. Ann Thorac Surg. 65:439–443. 1998. View Article : Google Scholar
|
|
16
|
Gurevitch J, Frolkis I, Yuhas Y, et al:
Anti-tumor necrosis factor-alpha improves myocardial recovery after
ischemia and reperfusion. J Am Coll Cardiol. 30:1554–1561. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lefer AM, Ma XL, Weyrich A and Lefer DJ:
Endothelial dysfunction and neutrophil adherence as critical events
in the development of reperfusion injury. Agents Actions Suppl.
41:127–135. 1993.PubMed/NCBI
|
|
18
|
Ma XL, Lefer DJ, Lefer AM and Rothlein R:
Coronary endothelial and cardiac protective effects of a monoclonal
antibody to intercellular adhesion molecule-1 in myocardial
ischemia and reperfusion. Circulation. 86:937–946. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Chandrasekar B, Smith JB and Freeman GL:
Ischemia-reperfusion of rat myocardium activates nuclear
factor-KappaB and induces neutrophil infiltration via
lipopolysaccharide-induced CXC chemokine. Circulation.
103:2296–2302. 2001. View Article : Google Scholar
|
|
20
|
Liu P, Hock CE, Nagele R and Wong PY:
Formation of nitric oxide, superoxide, and peroxynitrite in
myocardial ischemia-reperfusion injury in rats. Am J Physiol.
272:H2327–H2336. 1997.PubMed/NCBI
|
|
21
|
Li J, Wu F, Zhang H, et al: Insulin
inhibits leukocyte-endothelium adherence via an Akt-NO-dependent
mechanism in myocardial ischemia/reperfusion. J Mol Cell Cardiol.
47:512–519. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Li J, Zhang H, Wu F, et al: Insulin
inhibits tumor necrosis factor-alpha induction in myocardial
ischemia/reperfusion: role of Akt and endothelial nitric oxide
synthase phosphorylation. Crit Care Med. 36:1551–1558. 2008.
View Article : Google Scholar
|