Introduction
Numerous studies have previously shown that fruit
and vegetable consumption may reduce the risk of developing cancers
of the oropharynx, oesophagus, lung, stomach and colorectum
(1,2). Furthermore, it may also reduce the
risk of oxidative stress and cell damage (3), cardiovascular diseases and
atherosclerosis (4). Due to the
safety, low toxicity, reduced side effects and general
availability, phytochemicals and dietary compounds have been used
for the treatment of human cancer (5). White blood cells interact with each
other to produce an immune response against specific antigens
(6). It has been well documented
that increasing the immune response will improve the defense
against various diseases, microbial infections and leukemia
(7). Therefore, research has
focused on the identification of novel compounds from plants, which
may promote the immune response.
Polygonum cuspidatum is widely distributed in
southern China and Japan. The root of Polygonum cuspidatum
has previously been used to treat inflammation, infection and
hyperlipidemia (8). Emodin is
isolated from Polygonum cuspidatum and has numerous
biological effects. Emodin has previously been shown to inhibit
Coxsakievirus B4 in vitro and in vivo (9), and numerous studies have reported
that emodin possesses an anticancer function (10–12).
However, there is currently no available information on the effects
of Polygonum cuspidatum on the immune responses of normal
mice in vivo.
The present study aimed to investigate the effects
of the crude extract of Polygonum cuspidatum (CEPC) on the
immune responses of normal BALB/c mice in vivo.
Materials and methods
Materials and reagents
Dimethyl sulfoxide (DMSO) was obtained from
Sigma-Aldrich (St. Louis, MO, USA). RPMI-1640 medium, fetal bovine
serum, L-glutamine and penicillin-streptomycin were obtained from
Gibco Life Technologies (Carlsbad, CA, USA). CEPC, provided by Dr
Fu-Shin Chueh (Department of Health and Nutrition Biotechnology,
Asia University, Taichung, Taiwan), was dissolved in DMSO at 1% and
stored at −20°C, in a 50 ml tube covered with aluminum, until
further use.
Male BALB/c mice
A total of 50 male BALB/c mice, 8 weeks old and
weighing 22–25 g, were obtained from the National Laboratory Animal
Center (Taipei, Taiwan). The mice were maintained in specified
pathogen-free conditions in the animal center of the China Medical
University (Taichung, Taiwan). The mice were monitored and received
a normal diet. The use of mice in the present study was approved by
the Institutional Animal Care and Use Committee of the China
Medical University (Taichung, Taiwan), as previously described
(13).
In vivo treatment of animals with
CEPC
A total of 50 male BALB/c mice were randomly divided
into five groups (10 mice/group): Group I mice were treated with a
normal diet and served as a control group; group II mice were
treated with 25 mg/kg CEPC; group III mice were treated with 50
mg/kg CEPC; group IV mice were treated with 100 mg/kg CEPC; and
group V mice were treated with 200 mg/kg CEPC. The CEPC was mixed
with olive oil and was administered daily by oral gavage, at the
indicated doses, for 27 days. At the end of the treatment, all of
the mice were weighed and sacrificed by euthanasia, performed by
delivering increasing concentrations of CO2, as
previously described (14).
Immunofluorescence staining of the
surface markers of immune cells from each mouse
The mice were weighed following 27 days of CEPC
treatment. Blood samples were then collected by cardiac puncture,
and the spleens were harvested. The splenocytes were isolated to
measure natural killer (NK) cell activity. To determine the number
of leukocyte cells, 1 ml blood was collected from the mice and
lysed using 1× Pharm Lyse™ lysing buffer (BD Biosciences, Franklin
Lakes, NJ, USA). The samples were centrifuged at 1,500 × g, for 15
min at 4°C, in order to collect the white blood cells. The
leukocytes were stained with phycoerythrin (PE)-labeled anti-mouse
CD3 (1:100; catalog number, 553062; clone, 145-2C11), PE-labeled
anti-mouse CD19 (1:100; catalog number, 561740; clone, 1D3),
fluorescein isothiocyanate (FITC)-labeled anti-mouse CD11b (1:100;
catalog number, 57396; clone, M1/70) and Mac-3 (1:100; catalog
number, 553324; clone, M3/84) antibodies (BD Pharmingen, San Diego,
CA, USA), for 30 min at 4°C and were then washed with
phosphate-buffered saline (PBS). The cells were then stained with a
secondary antibody and analyzed by flow cytometry to determine the
percentage of cell markers, as described by previous methods
(14).
Quantification of macrophage phagocytic
activity
Macrophages were isolated from the peripheral blood
mononuclear cells (PBMC) and the peritoneum of the mice. The
isolated macrophages were placed in a fluorescence-activated cell
sorting tube and 50 μl E. coli-FITC was added, according to
the manufacturer’s instructions of the PHAGOTEST® kit
(ORPEGEN Peptide Chemicals GmbH, Heidelberg, Germany), and as
previously described (14) The
samples were analyzed using flow cytometery and quantified using
CellQuest software (BD Biosciences), as previously described
(14,15).
Quantification of NK cell cytotoxic
activity
The isolated splenocytes (1×105 cells)
were placed in each well of a 96-well plate in 50 μl RPMI-1640
medium. YAC-1 mouse lymphoma cells (2.5×107 cells;
Bioresource Collection and Research Center, Hsinchu, Taiwan) in
serum-free RPMI 1640 medium and the PKH-67/Dil.C buffer
(Sigma-Aldrich) were then added to the cells and mixed thoroughly,
for 2 min at 25°C. A total of 50 μl PBS was added to each well for
1 min, followed by 100 μl medium and incubated for 10 min. The
cells were then centrifuged at 1,200 × g, for 2 min at 25°C. NK
cell cytotoxic activity was determined by flow cytometry, as
previously described (14,15).
Determination of T- and B-cell
proliferation
The isolated splenocytes (1×105
cells/well) were placed in a 96-well plate. A total of 100 μl
RPMI-1640 medium was added to each well, and the cells were
stimulated with concanavalin A (Con A, 5 μg/ml) for three days to
initiate T-cell proliferation, and with lipopolysaccharide (LPS, 5
μg/ml), for 5 days to initiate B-cell proliferation. All of the
samples were measured using the CellTiter 96 AQueous One Solution
Cell Proliferation Assay kit (Promega Corporation, Madison, WI,
USA), as previously described (13,15).
Statistical analysis
All of the experiments in the present study were
repeated at least three times. The data were expressed as the means
± standard deviation. Comparisons between the control and
CEPC-treated groups were analyzed by student’s t-test. A P<0.05
was considered to indicate a statistically significant
difference.
Results
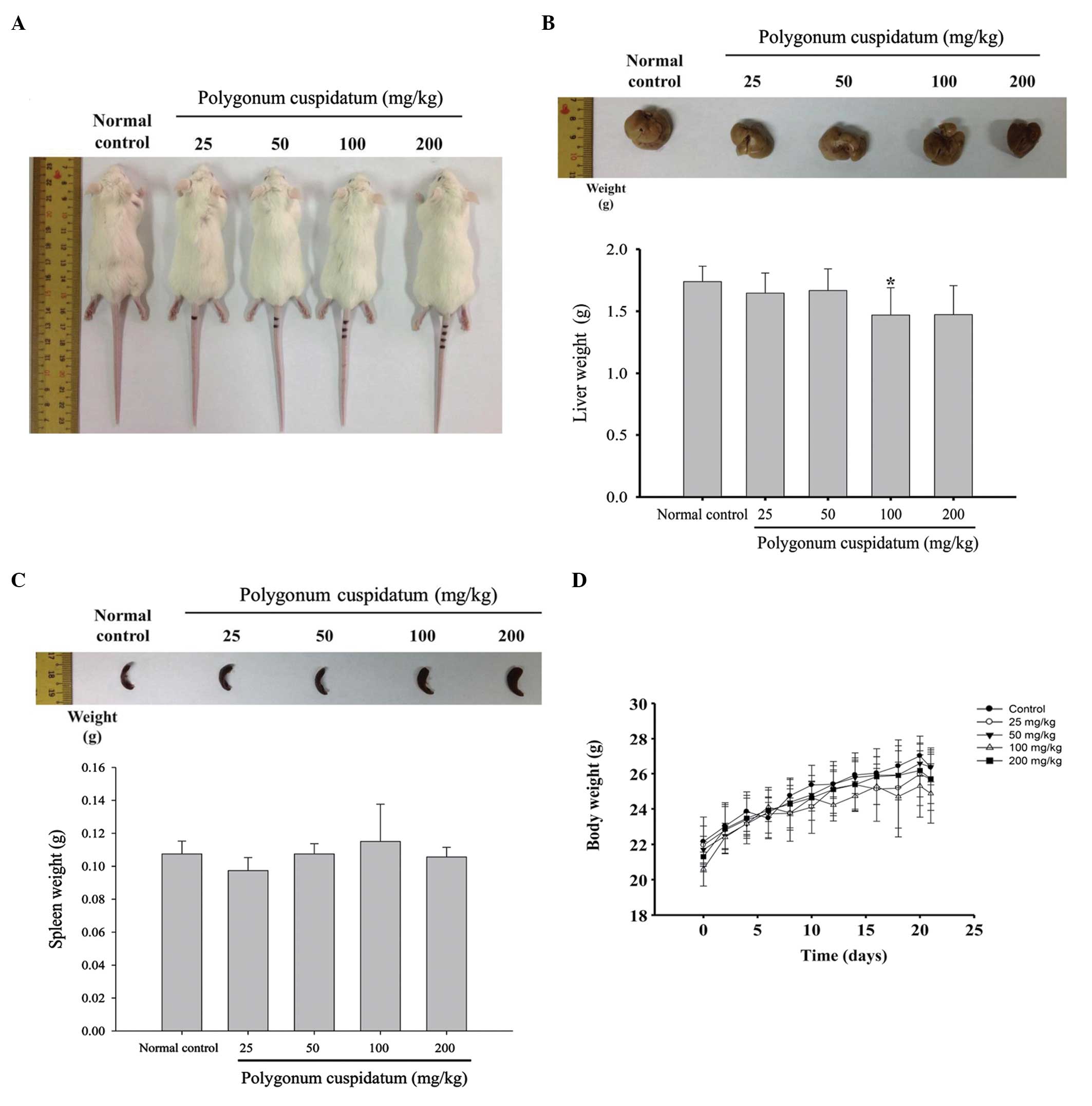
Effects of CEPC on the body and organ
weights of BALB/c mice
The mice were administered CEPC (25, 50, 100, 200
mg/kg), or normal control, for 27 days. Every three days the mice
were weighed, and the murine tissues were weighed at the end of the
CEPC treatment (Fig. 1A–D). CEPC
administration, at any of the four doses, did not significantly
alter body, liver or spleen weight, as compared with the control
mice.
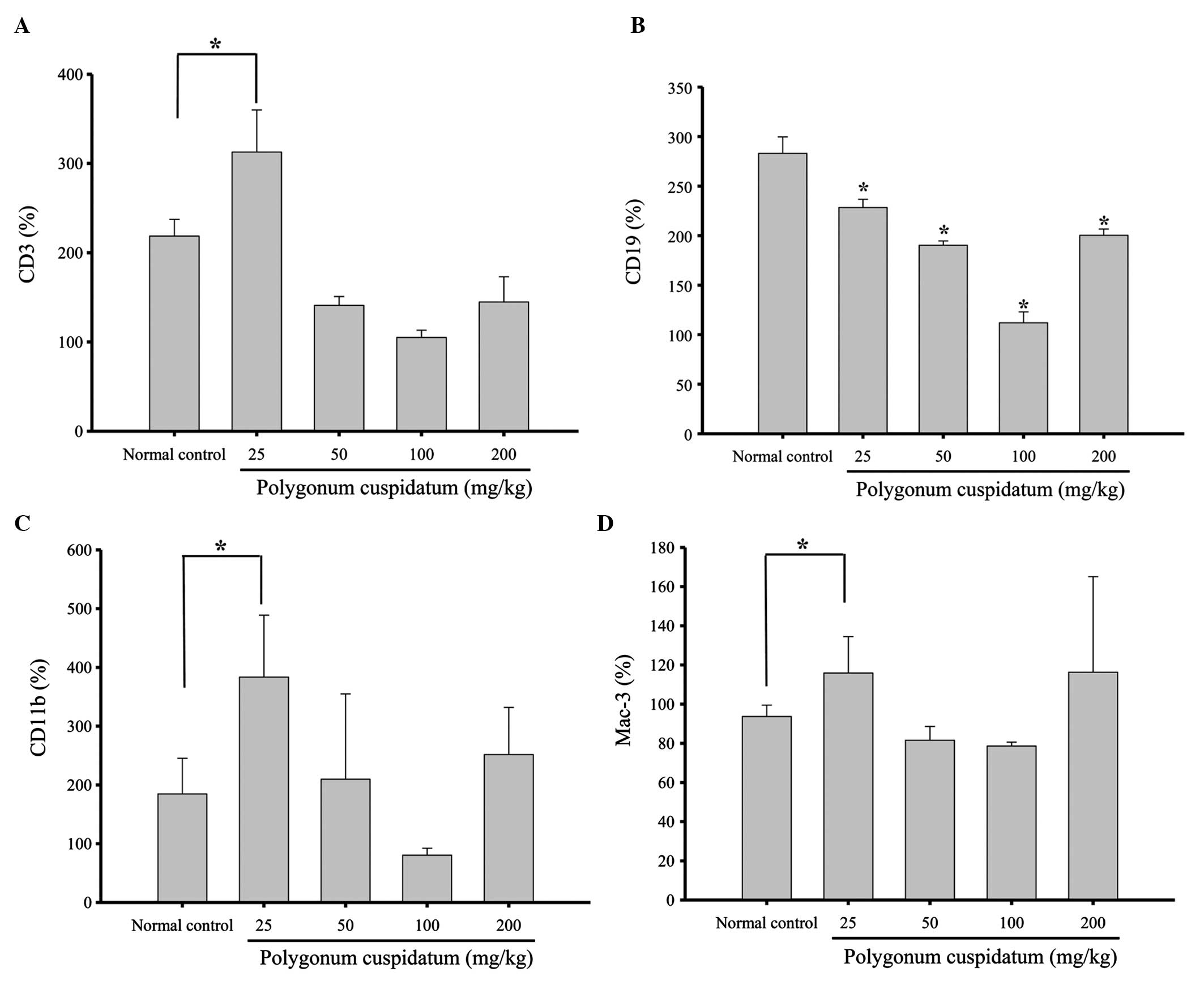
Effects of CEPC on leukocyte cell markers
in BALB/c mice
Flow cytometry was performed to measure the levels
of cell markers CD3, CD19, CD11b and Mac-3, in the CEPC-treated and
control mice. CEPC treatment (25 mg/kg) increased the levels of CD3
(Fig. 2A), CD11 (Fig. 2C) and Mac-3 (Fig. 2D); however, the levels of CD19 were
decreased (Fig. 2C) in response to
25, 50, 100 and 200 mg/kg CEPC treatment, as compared with the
control group. These results demonstrate that CEPC significantly
affects the white blood cell proliferation of normal mice in
vivo.
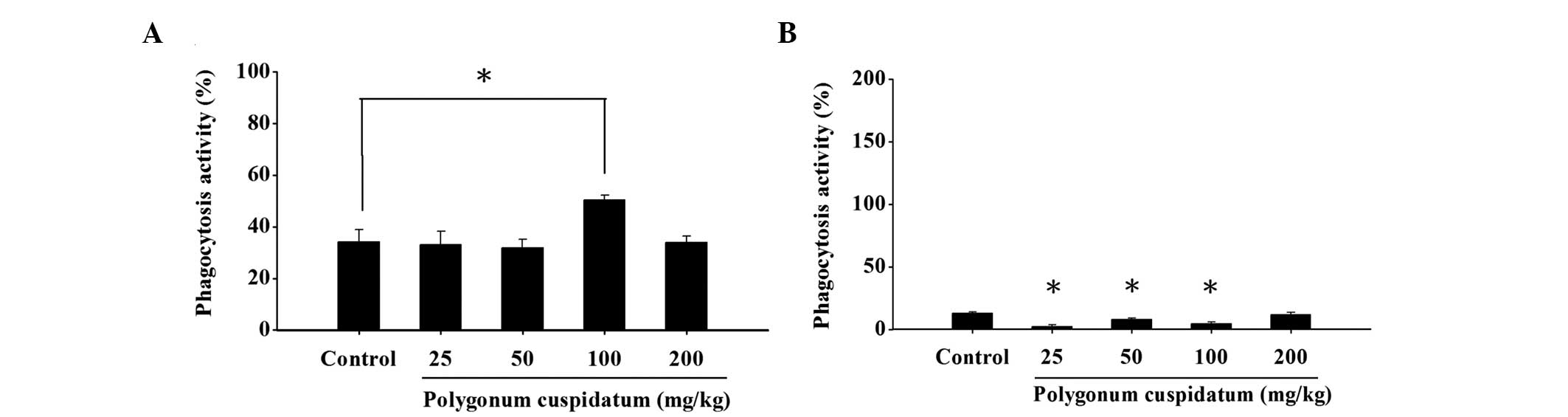
Effects of CEPC on macrophage phagocytic
activity from the PBMC and peritoneal cavity of BALB/c mice
The macrophages were isolated from the PBMC and
peritoneal cavity, and the levels of phagocytosis were analyzed by
flow cytometry. Treatment with CEPC, at all four doses,
significantly reduced macrophage phagocytosis from the PBMC
(Fig. 3A). Conversely, the
macrophage phagocytotic activity was not significantly stimulated
in the cells from the peritoneal cavity at a CEPC dose of 25, 50 or
100 mg/kg, as compared with the control mice (Fig. 3B).
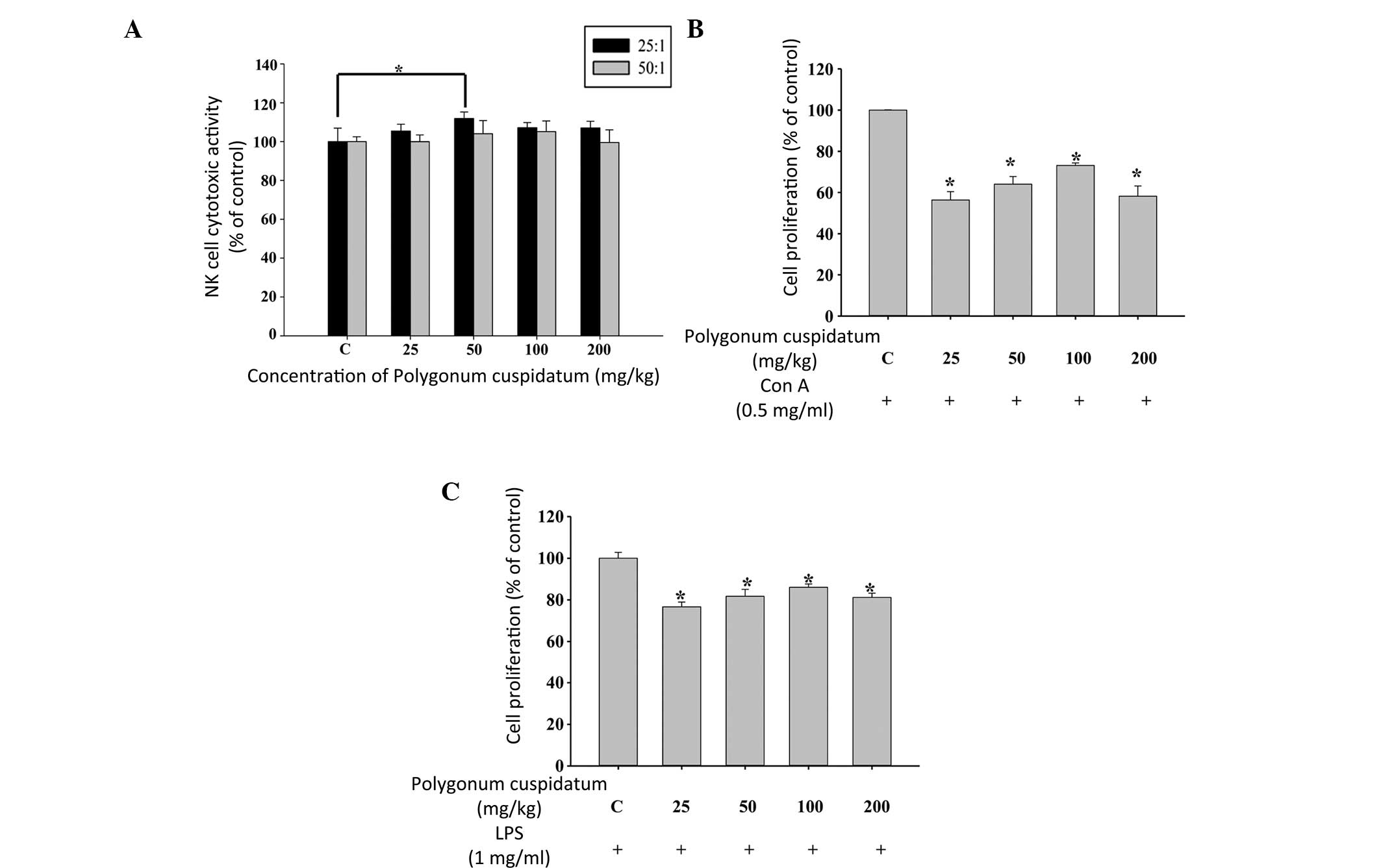
Effects of CEPC on the cytotoxic activity
of NK cells and B- and T-cell proliferation in BALB/c mice
The YAC-1 target cells were destroyed by the NK
cells, which were isolated from the splenocytes of mice treated
with 50 mg/kg CEPC (Fig. 4A).
However, the other CEPC doses did not alter the NK activity.
Treatment with 25 mg/kg CEPC increased both B- (Fig. 4B) and T-cell (Fig. 4C) proliferation. However, CEPC
doses of 100 and 150 mg/kg did not significantly alter the
proliferation of B- and T-cells.
Discussion
There are currently few reports on the biological
effects of CEPC, including its antiviral, antimicrobial, and
cardioprotective activities (16),
and no studies have examined the effects of CEPC on immune
responses in vivo. The present study examined the effects of
CEPC on immune responses in BALB/c mice in vivo. The mice
were treated with or without CEPC at various doses (50, 100, 150
and 200 mg/kg). CEPC treatment did not alter the body weight of the
mice, as compared with the control mice, and liver and spleen
weights were not altered by CEPC treatment. CEPC, at the cellular
level, altered immune responses, including increased proliferation
of T- and B-cells, and increased the levels of monocyte and
macrophage markers. CEPC was also shown to promote the phagocytic
activities of macrophages, and enhance the cytotoxic effects of NK
cells. Furthermore, 25 mg/kg CEPC treatment promoted and enhanced
the populations of CD3, CD11b and Mac-3-positive cells; however, no
significant effects were observed in response to the higher doses
of CEPC (50, 100 and 200 mg/kg). Conversely, all of the doses of
CEPC (25, 50, 100 and 200 mg/kg) significantly decreased the
population of CD19-positive cells, CD19 is an activated B-cell
surface marker (17,18).
Treatment with CEPC increased the number of cells
positive for the T-cell marker CD3. T-cells are involved in
cell-mediated immune responses (14,19).
Deletion of T-cells in animals has been shown to result in the loss
of cellular and humoral immune responses (20). This is the case in patients with
acquired immunodeficiency syndrome, where the loss of T-cells is
due to the destruction of T helper cells by the human
immunodeficiency virus (19).
CD11b and Mac-3 are markers of monocytes and macrophages,
respectively. Both of these cell markers were increased in response
to CEPC (50 mg/kg), which may be indicative of increased phagocytic
activity of the macrophages.
Previous studies have demonstrated that antigens
induce macrophage activity, including phagocytosis and stimulation
of T-cell functions, including cytotoxic and helper T-cells.
Activated T-cells release cytokines which also promote macrophage
function (21,22). Macrophages can suppress
intracellular bacterial growth and lead to a reduction in infection
(23).
Treatment with 100 mg/kg CEPC promoted the
phagocytic activity of macrophages isolated from the PBMC; however,
the other doses of treatment did not result in any significantly
promoted activities. A treatment with 50 mg/kg CEPC promoted NK
cell activities from the spleen samples, but other doses of CEPC
treatment did not show any significant promoted activities of the
NK cells.
Furthermore, CEPC treatment at all of the indicated
doses resulted in a decrease in T- and B-cell proliferation,
following Con A and LPS stimulation, respectively. However, further
investigations are required. The results of the present study
demonstrated that CEPC may enhance the population of Mac-3-positive
cells and promote the phagocytic activity of macrophages.
In conclusion, it may be suggested that CEPC may
stimulate proliferation of monocytes (CD11b) and enhance macrophage
(Mac-3) function, including phagocytosis, in vivo.
Furthermore, CEPC promoted NK cell activities, which may be
associated with the increased levels of T-, monocyte and macrophage
cell surface markers in normal BALB/c mice in vivo.
Acknowledgements
This study was supported by a grant from the China
Medical University, Taichung, Taiwan (no. CMU102-ASIA-20).
References
|
1
|
Soerjomataram I, Oomen D, Lemmens V, et
al: Increased consumption of fruit and vegetables and future cancer
incidence in selected European countries. Eur J Cancer.
46:2563–2580. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Tsai CW, Chen HW, Sheen LY and Lii CK:
Garlic: Health benefits and actions. BioMedicine. 2:17–29. 2012.
View Article : Google Scholar
|
|
3
|
Guizani N, Waly MI, Singh V and Rahman MS:
Nabag (Zizyphus spina-christi) extract prevents aberrant
crypt foci development in colons of azoxymethane-treated rats by
abrogating oxidative stress and inducing apoptosis. Asian Pac J
Cancer Prev. 14:5031–5035. 2013.PubMed/NCBI
|
|
4
|
Toh JY, Tan VM, Lim PC, Lim ST and Chong
MF: Flavonoids from fruit and vegetables: a focus on cardiovascular
risk factors. Curr Atheroscler Rep. 15:3682013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Pratheeshkumar P, Sreekala C, Zhang Z, et
al: Cancer prevention with promising natural products: mechanisms
of action and molecular targets. Anticancer Agents Med Chem.
12:1159–1184. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Yu FS, Yang JS, Yu CS, et al: Safrole
suppresses murine myelomonocytic leukemia WEHI-3 cells in vivo, and
stimulates macrophage phagocytosis and natural killer cell
cytotoxicity in leukemic mice. Environ Toxicol. 28:601–608. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Paul DJ, Laure NB, Guru SK, et al:
Antiproliferative and antimicrobial activities of alkylbenzoquinone
derivatives from Ardisia kivuensis. Pharm Biol. 52:392–397.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Peng W, Qin R, Li X and Zhou H: Botany,
phytochemistry, pharmacology, and potential application of
Polygonum cuspidatum Sieb.et Zucc.: a review. J
Ethnopharmacol. 148:729–745. 2013. View Article : Google Scholar
|
|
9
|
Liu Z, Wei F, Chen LJ, et al: In vitro and
in vivo studies of the inhibitory effects of emodin isolated from
Polygonum cuspidatum on Coxsakievirus B4.
Molecules. 18:11842–11858. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wei WT, Lin SZ, Liu DL and Wang ZH: The
distinct mechanisms of the antitumor activity of emodin in
different types of cancer (Review). Oncol Rep. 30:2555–2562.
2013.PubMed/NCBI
|
|
11
|
Ma YS, Weng SW, Lin MW, et al: Antitumor
effects of emodin on LS1034 human colon cancer cells in vitro and
in vivo: roles of apoptotic cell death and LS1034 tumor xenografts
model. Food Chem Toxicol. 50:1271–1278. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Chang YC, Lai TY, Yu CS, et al: Emodin
induces spoptotic death in murine myelomonocytic leukemia WEHI-3
cells in vitro and enhances phagocytosis in leukemia mice in vivo.
Evid Based Complement Alternat Med. 2011:5235962011.PubMed/NCBI
|
|
13
|
Tan TW, Lin YT, Yang JS, et al: A.
cantoniensis inhibits the proliferation of murine leukemia
WEHI-3 cells in vivo and promotes immunoresponses in vivo. In Vivo.
23:561–566. 2009.
|
|
14
|
Lin CC, Yu CS, Yang JS, et al: Chrysin, a
natural and biologically active flavonoid, influences a murine
leukemia model in vivo through enhancing populations of T- and
B-cells, and promoting macrophage phagocytosis and NK cell
cytotoxicity. In Vivo. 26:665–670. 2012.
|
|
15
|
Tsou MF, Tien N, Lu CC, et al: Phenethyl
isothiocyanate promotes immune responses in normal BALB/c mice,
inhibits murine leukemia WEHI-3 cells, and stimulates
immunomodulations in vivo. Environ Toxicol. 28:127–136. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhang H, Li C, Kwok ST, Zhang QW and Chan
SW: A review of the pharmacological effects of the dried root of
Polygonum cuspidatum (Hu Zhang) and its constituents. Evid
Based Complement Alternat Med. 2013:2083492013.PubMed/NCBI
|
|
17
|
Asano N, Fujimoto M, Yazawa N, et al: B
Lymphocyte signaling established by the CD19/CD22 loop regulates
autoimmunity in the tight-skin mouse. Am J Pathol. 165:641–650.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Jarasch-Althof N, Wiesener N, Schmidtke M,
Wutzler P and Henke A: Antibody-dependent enhancement of
coxsackievirus B3 infection of primary CD19+ B lymphocytes. Viral
Immunol. 23:369–376. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Bixler SL and Mattapallil JJ: Loss and
dysregulation of Th17 cells during HIV infection. Clin Dev Immunol.
2013:8524182013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Porichis F, Hart MG, Zupkosky J, Barblu L
and Kaufmann DE: In vitro assay to evaluate the impact of
immunoregulatory pathways on HIV-specific CD4 T cell effector
function. J Vis Exp. 15:e508212013.PubMed/NCBI
|
|
21
|
Bhardwaj N, Nash TW and Horwitz MA:
Interferon gamma-activated human monocytes inhibit the
intracellular multiplication of Legionella pneumophila. J
Immunol. 137:2662–2669. 1986.PubMed/NCBI
|
|
22
|
Nash TW, Libby DM and Horwitz MA:
IFN-gamma-activated human alveolar macrophages inhibit the
intracellular multiplication of Legionella pneumophila. J
Immunol. 140:3978–3981. 1988.PubMed/NCBI
|
|
23
|
Horwitz MA: Cell-mediated immunity in
Legionnaires’ disease. J Clin Invest. 71:1686–1697. 1983.
|