Introduction
As one of the most common altitude illnesses, acute
mountain sickness (AMS) causes lung and brain injury, with symptoms
including headache, loss of appetite, dizziness and insomnia
(1). The decreased barometric
pressure and subsequent reduction in available oxygen are the
primary causal factors of AMS (2).
Long-term hypoxia causes irreversible damage and ultimately leads
to organ failure. Currently, the normal vasodilating agents,
nifedipine and acetazolamide, are used as prophylactic agents to
reduce the incidence and severity of AMS at high altitudes
(3). However, serious adverse
effects, including headache and cardiopalmus have been observed
during the clinical treatment (4),
creating a demand for the identification of alternative agents.
Cordyceps militaris, which belongs to the
class Ascomycetes and the Dong Chong Xia Cao group of
Chinese herbs, possesses potential antioxidant (5), immunomodulatory (6), antitumor and anti-inflammatory
properties (7). In Western
countries, Cordyceps militaris is considered to be a Chinese
herb with anti-aging and anticancer effects (8). Several studies have investigated the
polysaccharides of Cordyceps, which are a rich and important
activity group. One Cordyceps militaris
polysaccharide-enriched fraction produced hypoglycemic activity
(9) and reduced plasma glucose in
normal Wistar-Kyoto rats (10). In
our previous study, Cordyceps militaris polysaccharides
exhibited significant anti-diabetic and anti-nephropathic
activities (11). As reported
previously, the bioactivities of polysaccharides are associated
with their chemical composition, glycosidic linkages, conformation,
molecular weight and degree of branching (12). Previous studies have examined
different polysaccharides extracted from the Cordyceps
militaris sporocarp and these are comprised mainly of mannose,
rhamnose, galactose and glucose and have an average molecular
weight of 30 kDa (13–15). However, few studies have
investigated the purification and bioactivities of polysaccharides
separated from Cordyceps militaris mycelium, which is
cultured by submerged fermentation (16,17).
Submerged fermentation presents the advantage of simultaneously
enabling a decrease in disposal costs and the production of
value-added products and, thus, has been widely used.
In the present study, polysaccharides extracted from
Cordyceps militaris mycelium were purified and
characterized. Furthermore, the anti-hypoxic effects of the
purified Cordyceps polysaccharides were detected in
vivo.
Materials and methods
Strain and reagents
The study was approved by the ethics committee of
the Lab Animal Center of Jilin University; ref no. 2009–0011).
Cordyceps militaris was purchased from the National
Biological Resource Center (Chiba, Japan; cat no. NBRC9787). Sodium
nitrite, glucose, peptone, yeast extract powder,
H2SO4, NaIO4, acetonitrile, glycol
(analytical grade), NaOH, potassium hydrogen phthalate, acetic
acid, potassium borohydride, BaCO3,
KH2PO4, MgSO4·7H2O,
(NH4)2SO4, ZnCl2, Vitamin B1 and
dextran standards were all obtained from Sigma-Aldrich (St. Louis,
MO, USA).
Submerged incubation and
fermentation
Cordyceps militaris was cultured in a rotary
shaker incubator (10 L, Biostat B; Sartorius AG, Goettingen,
Germany) at 150 rpm for 5 days at 26°C. The cultured medium
contained 20 g/l glucose, 10 g/l peptone, 18 g/l yeast extract
powder, 3 g/l KH2PO4, 3 g/l MgSO4
7H2O, 10 g/l (NH4)2SO4, 0.01 g/l
ZnCl2 and 0.24 g/l vitamin B1. The mycelia were then
harvested and lyophilized for further use.
Crude extract preparation and preliminary
identification
As described previously (18), the aqueous extract from
Cordyceps militaris was prepared by extracting 100 g mycelia
powder twice using hot water at 80°C for 3 h. Following
centrifugation at 4,500 × g for 10 min, the supernatant was
sequentially concentrated and freeze-dried to produce the solid
aqueous extract of Cordyceps militaris (CM (indicating the
Cordyceps militaris extract)). According to the previously
described method (19), different
chromogenic reactions were used to determine the constituents of
CM.
Purification of the CM
Using Sevag reagent [V (n-butanol): V
(chloroform)=1:4, 50 ml, Sigma-Aldrich, St. Louis, MO, USA], the
proteins present in the CM were removed (20). Ethanol (4-fold) was added to the
supernatant, which was placed at 4°C overnight. The precipitation
was then dissolved in double distilled (DD) water and placed in a
2.6 cm × 35 cm DEAE-52 cellulose anion exchange column (21). The column was eluted with DD water,
followed by 0.1 and 0.3 mol/l NaCl, respectively, at a flow rate of
1 ml/min. The polysaccharide fraction was collected and detected
using an anthrone acid method (19). The gel permeation chromatography
system, Sepharose G-100 (General Electric Co.Salt Lake City, Utah,
USA) was used for further purification. The column was eluted with
DD water at a flow rate of 0.4 ml/min. The fractions (10 ml each)
were then collected (13) and
freeze-dried.
Fourier transform infrared spectroscopy
(FTIR) determination
The purified polysaccharides (4 mg) were ground
thoroughly using 150 mg KBr (Sigma-Aldrich). The average
transmission spectra (n=50) were recorded using an IRPrestige-21
FTIR spectrometer (Shimadzu Corporation, Tokyo, Japan) between 400
and 4,000 cm−1 and the absorbance was determined.
Homogeneity and molecular weight
determination
The homogeneity and molecular weight were analyzed
using a high performance liquid chromatography (HPLC)/evaporative
light scattering detector (ELSD) system (22). An LC-10ATvp HPLC system (Shimadzu
Corporation) equipped with a TSKgel G4000PWXL column (Tosoh, Tokyo,
Japan) and an Alltech 2000ES ELSD (Shimadzu Corporation) was used.
Briefly, DD water served as the mobile phase, which was driven by a
double pump (Waters 150; Millipore, Billerica, MA, USA) at a flow
rate of 0.45 ml/min. The aerosol level was 60%, the drift tube
temperature was 120°C and the nebulizing nitrogen pressure was 25
psi. Dextran standards were used to create a calibration curve, as
previously described (23).
Monosaccharides analysis
The analysis of monosaccharides was investigated
according to the previous method (24). The polysaccharide (20 mg) was
hydrolyzed with 1 M H2SO4 (1 ml) for 6 h at
105°C in a sealed glass tube and the pH was adjusted to 7.0 using
BaCO3. The solution was then centrifuged at 3,200 × g
for 10 min to separate the hydrolysates, which were further
analyzed using the HPLC/ELSD system. The chromatograph was fitted
with a Prevail™ ES carbohydrate analysis column (Alltech
Associates, Inc., Deerfield, IL, USA), which was eluted with 75%
acetonitrile (Sigma-Aldrich) at a flow rate of 1.0 ml/min. The
results were compared with the following monosaccharide standards:
D-glucose, L-rhamnose (Rha), D-xylose, D-galactose (Gal), D-mannose
(Man) and L-arabinose (Ara), trehalose (Sigma-Aldrich) (25).
Periodate oxidation-Smith degradation
reaction of polysaccharides
The polysaccharide (20 mg) was dissolved in 15 mM
NaIO4 (25 ml; pH 4) in darkness at 4°C. Subsequently,
100 μl was withdrawn at 6 h intervals, diluted with distilled water
and measured spectrophotometrically at 223 nm until a stable
absorbance was reached (26). The
consumption of HIO4 was calculated and the production of
formic acid was determined by titration with 0.005 M NaOH and
glycol (2 ml) was added to terminate the oxidation reaction. The
remaining periodate product was then fully dialyzed against DD
water for 48 h. This dialysate was concentrated and reduced with
potassium borohydride (70 mg) overnight at room temperature and the
pH was adjusted using acetic acid (Sigma-Aldrich) to pH 7.0. The
solution was dialyzed against DD water for 24 h and 3 ml sample was
further analyzed using the HPLC/ELSD system. The remaining product
was hydrolyzed using 1 M H2SO4 at 25°C for 40
h and was adjusted to pH 7.0 using BaCO3. Following
centrifugation at 3,200 × g for 10 min, the hydrolysates were
analyzed using HPLC/ELSD under the same conditions that were used
for the monosaccharide composition analysis.
In vivo experiments in an animal model of
hypoxia
The experimental animal procedure was approved by
the Lab Animal Centre of Jilin University [Changchun, China;
License no. SCXK-(JI) 2006–0001)]. Mice weighing 18–22 g were
maintained under a constant 12 h light/dark cycle at 23±1°C, with a
humidity of 60±2% with water and food available ad libitum.
All mice were fed with standard laboratory feed in an animal room
for 3 days prior to the experiments. To produce experimental models
of hypoxia, 180 mice (90 males and 90 females) were randomly
divided into three groups for the normobarie hypoxia test, sodium
nitrite toxicosis test and acute cerebral ischemic/hypoxic test
(27), respectively. The
normobarie hypoxia test was performed in a sealed wide-mouth bottle
containing soda lime (Sigma-Aldrich). The sodium nitrite toxicosis
test was performed by injection of 2 g/ml sodium nitrite. The acute
cerebral ischemic-hypoxic test was performed by decapitation of the
mice. In all three tests, mice received either physiological saline
or 1.5 ml/kg/day rhodiola oral liquid, which served as the normal
control (NC) group or the positive control (PC) group. The
remaining mice were divided into three groups (n=12) and treated
orally with CMN1 (0.0 g/kg, 0.2 g/kg or 0.5 g/kg) once a day. After
24 days, the survival rate of hypoxia under normal pressure and
following sodium nitrite injection were determined and the
persistence of gasping following decapitation was examined.
Statistical analysis
All data are expressed as the mean ± standard
deviation. Data were evaluated using one-way analysis of variance
to detect statistical significance, followed by post-hoc multiple
comparisons (Dunn’s test). P≤0.05 was considered to indicate a
statistically significant difference.
Results
Phytochemical assessment
The constituents of the Cordyceps militaris
extract, including proteins, organic acid and sugars were detected
(Table I). At room temperature,
organic acid was unstable, which suggested that the major effective
components in the Cordyceps militaris extract were protein
and polysaccharides. In addition, the content of the total
polysaccharides was 163 mg/g in the cultured Cordyceps
militaris mycelium.
 | Table ISummary of the preliminary chemical
analysis of crude extracts. |
Table I
Summary of the preliminary chemical
analysis of crude extracts.
| Test | CM |
|---|
| Amino
acid/polypeptide/protein | + |
| Soluble reducing
sugar | + |
| Sugars | + |
|
Phenolics/tannin | − |
| Alkaloids | − |
| Sterols | − |
| Terpenes | − |
| Organic acid | + |
| Essential
oil/oil | − |
| Anthraquinone | − |
| Flavonoids | − |
|
Coumarin/lactone | − |
Purification and characterization of
polysaccharides
The neutral or acidic polysaccharides in
Cordyceps militaris water extracts were separated using
anion exchange chromatography in a DEAE-cellulose column. Two
fractions, CM1 and CM2, were eluted using DD water and 0.1 M NaCl,
respectively and the yields of CM1 and CM2 were 0.475 and 0.025
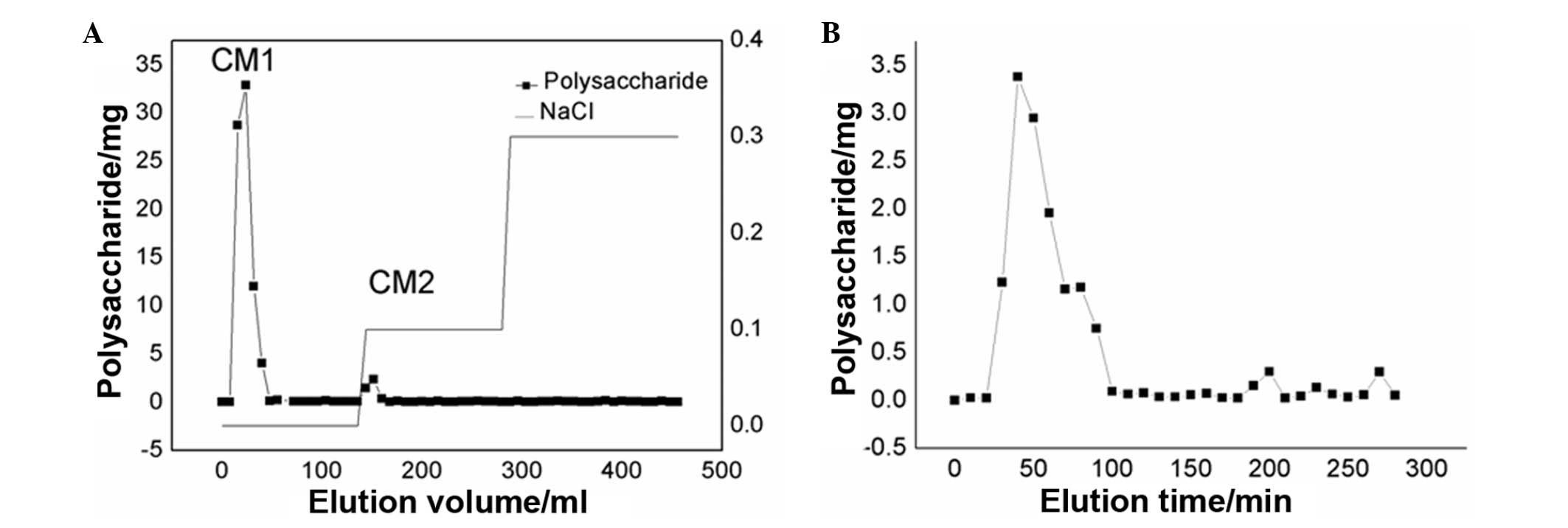
g/g, respectively (Fig. 1A). When
examining the rate of production, only CM1 was further purified
using the gel permeation chromatography system Sepharose G-100. A
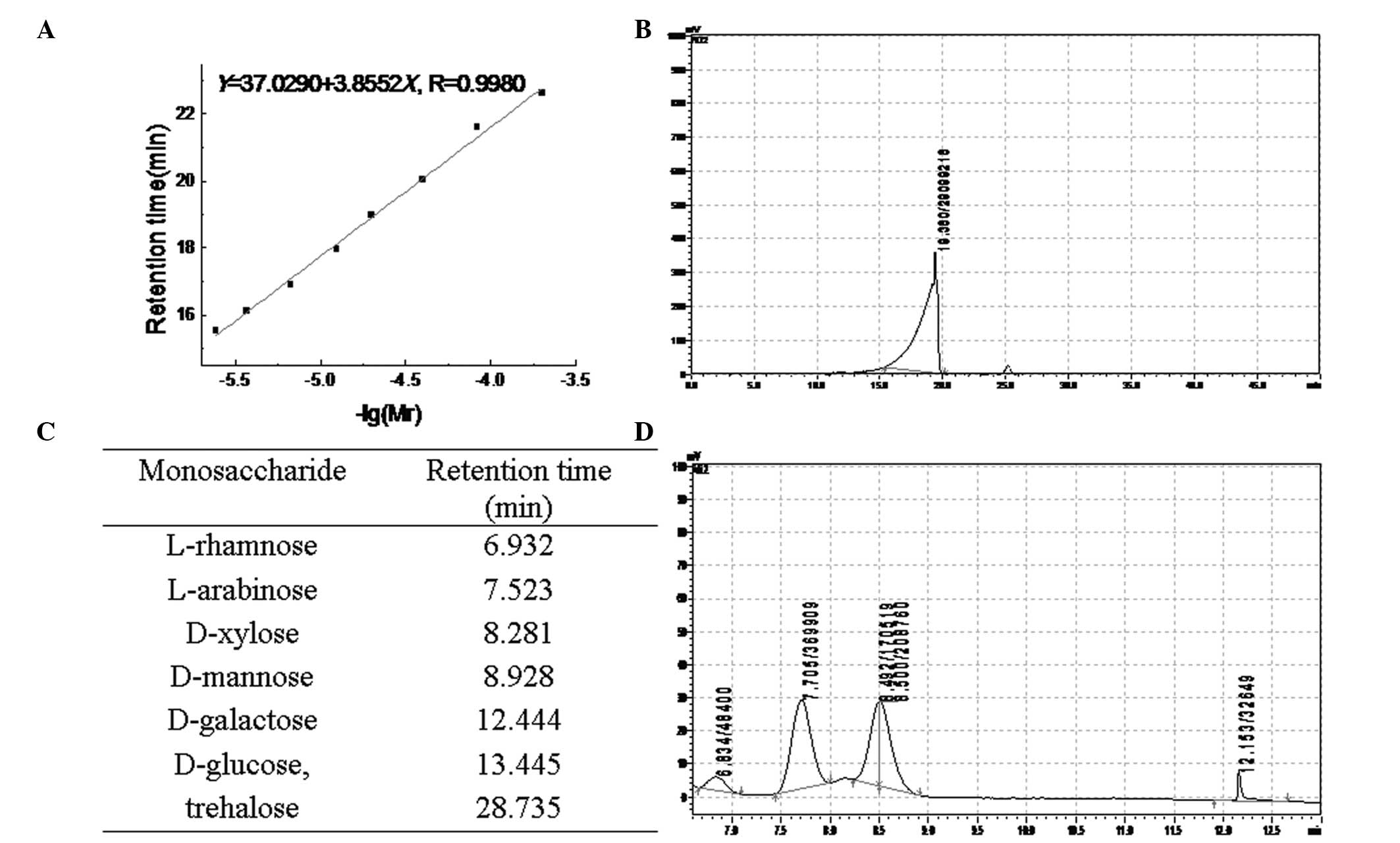
single elution peak (CMN1) appeared at 60 min (Fig. 1B) with a molecular mass of 37,842
Da (Fig. 2A and B). Rha, Ara, Man
and Gal were present in CMN1, according to the retention time in
the HPLC figure print (Fig. 2C and
D). The molar ratio of Rha, Ara, Man and Gal was
1.48:11.34:11.62:1.00.
Furthermore, the characteristic structure of CMN1
was analyzed using FTIR and periodate oxidation-Smith degradation.
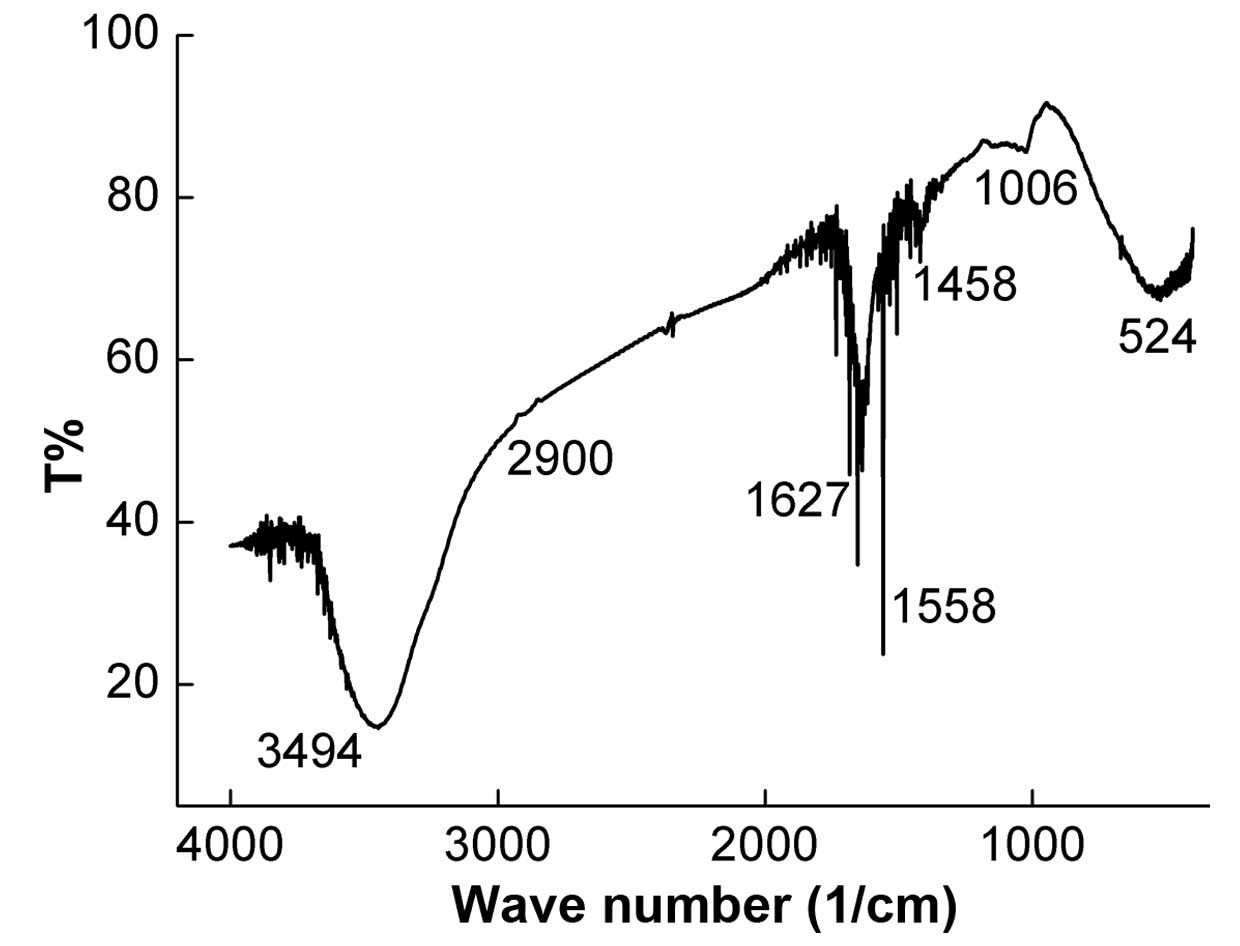
In the FTIR spectra (Fig. 3),
peaks at 3,494, 2,900, 1,627, 1,558, 1,458, 1,006 and 524
cm−1 were noted.
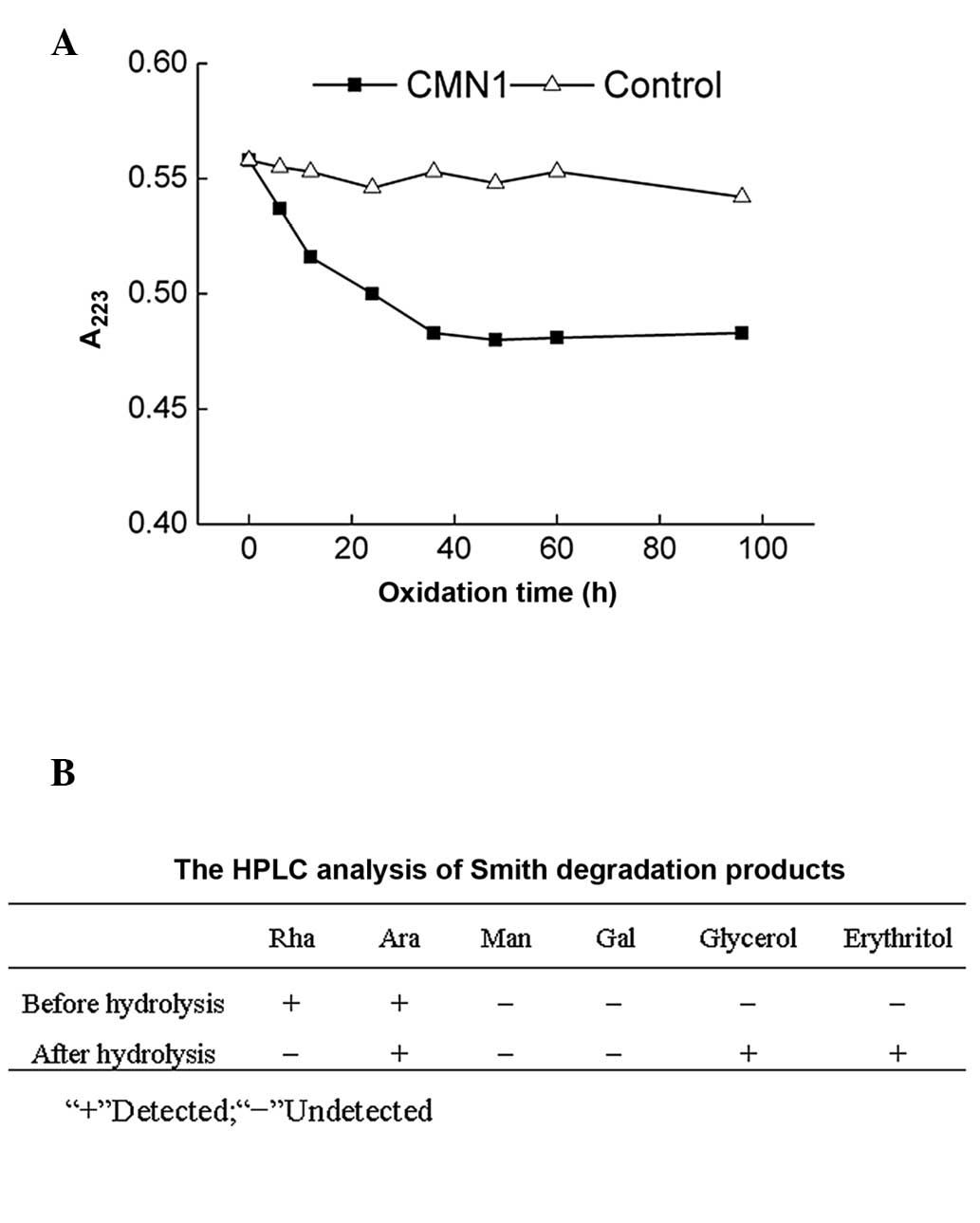
Periodate oxidation-Smith degradation was performed
to confirm the linkage mode of the glucose present in CMN1
(Fig. 4) and the HPLC method was
performed to analyze the products following Smith degradation. Rha
and Ara were noted prior to hydrolysis, however, Ara, glycerol and
erythritol were observed following hydrolysis (Fig. 4B).
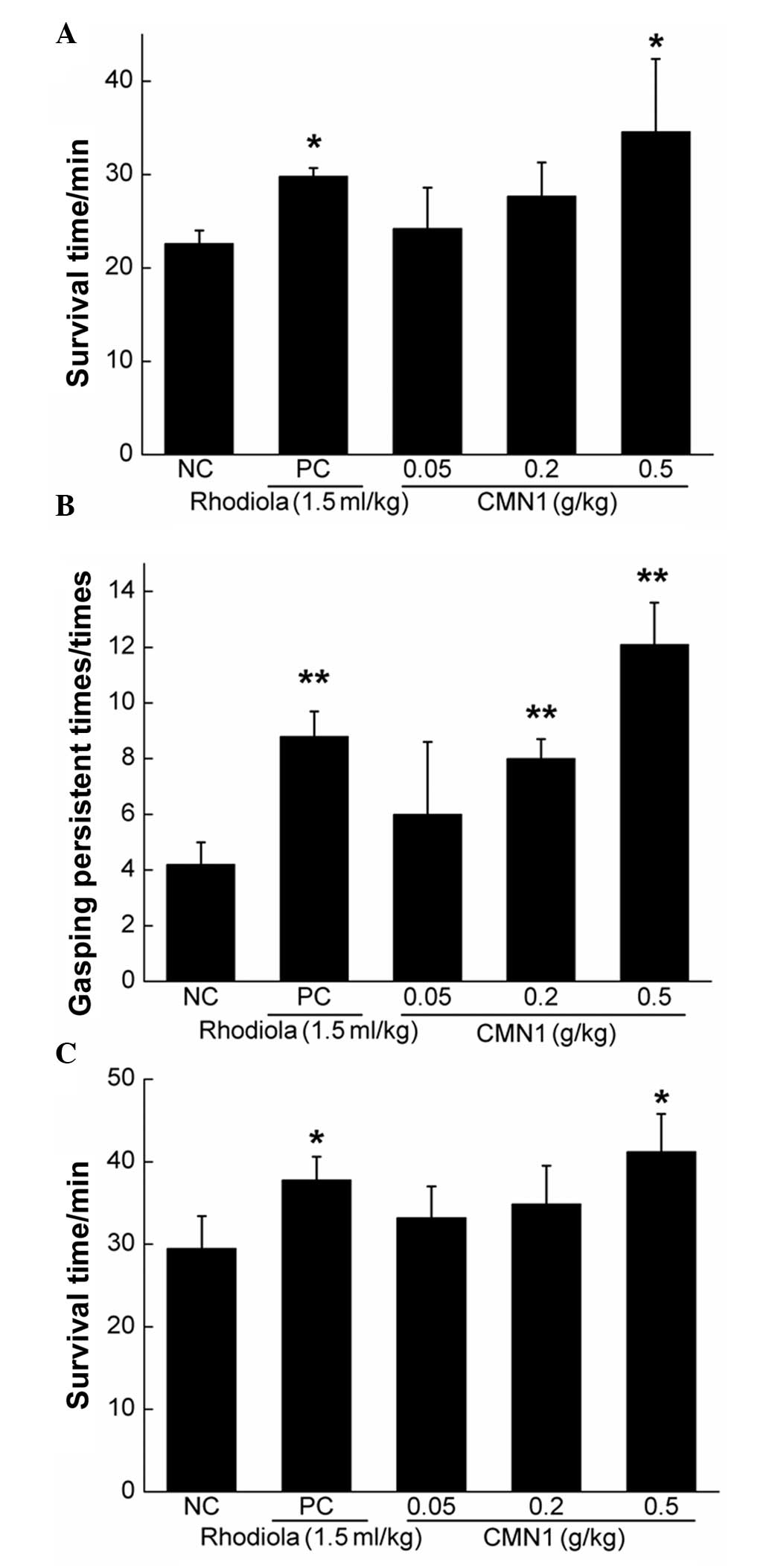
Anti-hypoxic effects of CMN1
In the normobarie hypoxia test and acute cerebral
ischemic-hypoxic test, mice were sacrificed by suffocation. In
sodium nitrite-induced acute acquired methemoglobinemia, less
oxygen is carried by hemoglobin, which leads to tissue death
(25). Compared with the NC group,
0.5 g/kg CMN1 improved survival rate by almost 53% (P<0.05) in
the sodium nitrite intoxication survival test and by 39% in the
hypoxia-resistance test (P<0.05), respectively (Fig. 5A and C). Furthermore, treatment
with 0.5 g/kg CMN1 resulted in a 1.88-fold increase in gasping
persistence (P<0.01; Fig. 5B).
No significant differences were observed between the CMN1-treated
and PC groups indicating that CMN1 possesses an anti-hypoxic effect
similar to that of rhodiola oral liquid.
Discussion
AMS, which produces a large number of oxygen
radicals, leads to irreversible tissue damage (29). In previous clinical trials,
Sipunculus nudus L. (27),
Brassica rapa L. (28),
Rhodobryum giganteum Par. (30) and rhodiola were used to alleviate
the symptoms of AMS. In the present study, Cordyceps
polysaccharides were purified and characterized and their
anti-hypoxic effects were detected in vivo.
In the FTIR spectra, a broad stretching peak was
observed at ~3,494 cm−1 and a weak peak at ~2,900
cm−1. These are characteristic absorption peaks of
saccharides and were produced by marked hydroxyl group vibration
(31) and by C-H bending vibration
of the -CH2 groups (32), respectively. The bands at 1,627 and
1,558 cm−1 suggested the existence of a C=O bond and an
N-H bond, possibly from the amidogen in Gal. The weak peak at 1,458
cm−1 was attributed to vibrations of CH3 and
an absorption peak at 1,006 and 524 cm−1, may have been
contributed to by the ether linkage (C-O-C) and the hydroxyl in the
pyranose ring.
Analysis of the results obtained in the periodate
oxidation-Smith degradation suggested the possible linkage of
monosaccharides within CMN1. The consumption of <1 mol periodate
(0.82 mol) in the present study indicated the existence of a 1→3
linkage, which does not consume periodate during oxidation.
Following periodate oxidation, the presence of Rha and Ala revealed
that CMN1 contained a section of (1→3)-linked-Rha and
(1→3)-linked-Ala. The monosaccharide ratio suggested that the
majority of (1→3)-linked-Ala is located in the backbone and the
(1→3)-linked-Rha may be located in the backbone or the side chains.
Formic acid production during periodate oxidation confirmed the
existence of a 1→6 linkage. The low formic acid yield (0.06
mol/sugar residue, less than 0.1 mol/sugar residue) suggested that
the 1→6 linkage in CMN1 was unimportant. In addition, the 1→2
linkages may have been the main chain linkage of CMN1, which was
indicated by the high-yield glycerol production. A trace quantity
of erythritol revealed the low content of 1→4 linkages in the main
chain or branch. Collectively, the backbone of CMN1 was found to be
composed of (1→2) linkages and (1→3) linkages with branched (1→6)
linkages and (1→4) linkages.
The normobarie hypoxia, sodium nitrite toxicosis and
acute cerebral ischemic-hypoxic tests (24) were used to detect the anti-hypoxic
activity of CMN1. Compared with the NC group, CMN1 markedly
improved the survival rate in the sodium nitrite toxicosis and
normobarie hypoxia tests. Enhanced gasping persistence was also
observed in the acute cerebral ischemic-hypoxic models.
Additionally, water extracts from Cordyceps sinensis have
been found to exhibit a scavenging effect on reactive oxygen
species, superoxide anions and hydroxyl radicals by inhibiting
malondialdehyde formation (33).
Overall, the polysaccharide extracted from Cordyceps
militaris was also effective against hypoxia.
Furthermore, the anti-hypoxic activity of CMN1 may
be associated with its characteristic structure. CMN1 was observed
to have a molecular weight of 37,842 Da and its monosaccharide
composition consisted of Rha, Ara, Man and Gal. Compared with
previous studies, CMN1 exhibits a similar primary structure
(14), however, additional studies
are required to identify the pharmacological efficacy and
characteristic structures of the polysaccharides.
The present study was limited to one
polysaccharide-enriched fraction separated from Cordyceps
militaris. Whether other components exhibit significant roles
in hypoxia remains to be elucidated. Therefore, further
investigations focusing on Cordyceps militaris are
required.
Acknowledgements
This study was supported by the twelfth Five-Year
National key Technology R&D Program (grant no.
2012BAI29B00).
References
|
1
|
Cueto-Martin B, De La Cruz-Marquez J and
Garcia-Torres L: Effect of altitude in the blood pressure
regulation system (renin-angiotensin-aldosterone) in team sports.
Case study: Female volleyball. Med Sport. 52:261–269. 1999.(In
Italian).
|
|
2
|
Fiore D, Hall S and Shoja P: Altitude
illness: risk factors, prevention, presentation, and treatment. Am
Fam Physician. 82:1103–1110. 2010.PubMed/NCBI
|
|
3
|
Wang J, Ke T, Zhang X, et al: Effects of
acetazolamide on cognitive performance during high-altitude
exposure. Neurotoxicol Teratol. 35:28–33. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Fagenholz PJ, Gutman JA, Murray AF and
Harris NS: Treatment of high altitude pulmonary edema at 4240 m in
Nepal. High Alt Med Biol. 8:139–146. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Li X, Mitchell J, Wood S, Coen C, Lightman
S and O’Byrne K: The effect of oestradiol and progesterone on
hypoglycaemic stress-induced suppression of pulsatile luteinizing
hormone release and on corticotropin-releasing hormone mRNA
expression in the rat. J Neuroendocrinol. 15:468–476. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kim CS, Lee SY, Cho SH, et al: Cordyceps
militaris induces the IL-18 expression via its promoter activation
for IFN-gamma production. J Ethnopharmacol. 120:366–371. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Rao YK, Fang SH, Wu WS and Tzeng YM:
Constituents isolated from Cordyceps militaris suppress enhanced
inflammatory mediator’s production and human cancer cell
proliferation. J Ethnopharmacol. 131:363–367. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Paterson RR: Cordyceps: a traditional
Chinese medicine and another fungal therapeutic biofactory?
Phytochemistry. 69:1469–1495. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhang G, Huang Y, Bian Y, Wong JH, Ng TB
and Wang H: Hypoglycemic activity of the fungi Cordyceps militaris,
Cordyceps sinensis, Tricholoma mongolicum, and Omphalia lapidescens
in streptozotocin-induced diabetic rats. Appl Microbiol Biotechnol.
72:1152–1156. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Cheng YW, Chen YI, Tzeng CY, et al:
Extracts of Cordyceps militaris lower blood glucose via the
stimulation of cholinergic activation and insulin secretion in
normal rats. Phytother Res. 26:1173–1177. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Dong Y, Jing T, Meng Q, Liu C, Hu S, Ma Y,
Liu Y, Lu J, Cheng Y, Wang D and Teng LR: Studies on the
anti-diabetic activities of Cordyceps militaris extract in
diet-streptozotocin-induced diabetic Sprague-Dawley rats. Biomed
Res Int. 2014.160980:2104(Epub ahead of print).
|
|
12
|
Methacanon P, Madla S, Kirtikara K and
Prasitsil M: Structural elucidation of bioactive fungi-derived
polymers. Carbohydr Polym. 60:199–203. 2005. View Article : Google Scholar
|
|
13
|
Wu F, Yan H, Ma X, et al: Comparison of
the structural characterization and biological activity of acidic
polysaccharides from Cordyceps militaris cultured with different
media. World J Microbiol Biotechnol. 28:2029–2038. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Yu RM, Yang W, Song LY, Yan CY, Zhang Z
and Zhao Y: Structural characterization and antioxidant activity of
a polysaccharide from the fruiting bodies of cultured Cordyceps
militaris. Carbohydr Polym. 70:430–436. 2007. View Article : Google Scholar
|
|
15
|
Lee JS, Kwon JS, Won DP, et al: Study of
macrophage activation and structural characteristics of purified
polysaccharide from the fruiting body of Cordyceps militaris. J
Microbiol Biotechnol. 20:1053–1060. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Castilho LR, Mirchel DA and Freire DM:
Production of polyhydroxyalkanoates (PHAs) from waste materials and
by-products by submerged and solid-state fermentation. Bioresour
Technol. 100:5996–6009. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Shih IL, Tsai KL and Hsieh C: Effects of
culture conditions on the mycelial growth and bioactive metabolite
production in submerged culture of Cordyceps militaris. Biochem Eng
J. 33:193–201. 2007. View Article : Google Scholar
|
|
18
|
Du L, Song J, Wang H, et al: Optimization
of the fermentation medium for Paecilomyces tenuipes N45 using
statistical approach. Afr J Microbiol Res. 6:6130–6141. 2012.
View Article : Google Scholar
|
|
19
|
Dhanabal S, Kokate C, Ramanathan M, Kumar
E and Suresh B: Hypoglycaemic activity of Pterocarpus marsupium
Roxb. Phytother Res. 20:4–8. 2006. View
Article : Google Scholar : PubMed/NCBI
|
|
20
|
Yan H, Zhu D, Xu D, Wu J and Bian X: A
study on Cordyceps militaris polysaccharide purification,
composition and activity analysis. Afr J Biotechnol. 7:4004–4009.
2008.
|
|
21
|
Zhang Al, Lu JH, Zhang N, Zheng D, Zhang
GR and Teng LR: Extraction, purification and anti-tumor activity of
polysaccharide from mycelium of mutant Cordyceps militaris. Chem
Res Chin Univ. 26:798–802. 2010.(In Chinese).
|
|
22
|
Zhang N, Liu Y, Lu J, et al: Isolation,
purification and bioactivities of polysaccharides from Irpex
lacteus. Chem Res Chin Univ. 28:249–254. 2012.(In Chinese).
|
|
23
|
Cui H, Chen Y, Wang S, Kai G and Fang Y:
Isolation, partial characterisation and immunomodulatory activities
of polysaccharide from Morchella esculenta. J Sci Food Agric.
91:2180–2185. 2011.PubMed/NCBI
|
|
24
|
Chambers RE and Clamp JR: An assessment of
methanolysis and other factors used in the analysis of
carbohydrate-containing materials. Biochem J. 125:1009–1018.
1971.PubMed/NCBI
|
|
25
|
Xie J, Xie M, Nie S, Shen M, Wang Y and Li
C: Isolation, chemical composition and antioxidant activities of a
water-soluble polysaccharide from Cyclocarya paliurus (Batal.)
Iljinskaja. Food Chem. 119:1626–1632. 2010. View Article : Google Scholar
|
|
26
|
Linker A, Evans L and Impallomeni G: The
structure of a polysaccharide from infectious strains of
Burkholderia cepacia. Carbohydr Res. 335:45–54. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zhang CX and Dai ZR: Anti-hypoxia activity
of a polysaccharide extracted from the Sipunculus nudus L. Int J
Biol Macromol. 49:523–526. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Xie Y, Jiang SP, Su DH, Pi NN, Ma C and
Gao P: Composition analysis and anti-hypoxia activity of
polysaccharide from Brassica rapa L. Int J Biol Macromol.
47:528–533. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Hu D, Chen F, Guan C, Yang F and Qu Y:
Anti-hypoxia effect of adenovirus-mediated expression of heat shock
protein 70 (HSP70) on primary cultured neurons. J Neurosci Res.
91:1174–1182. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Cai Y, Lu Y, Chen R, Wei Q and Lu X:
Anti-hypoxia activity and related components of Rhodobryum
giganteum Par. Phytomedicine. 18:224–229. 2011. View Article : Google Scholar
|
|
31
|
Fang X, Jiang B and Wang X: Purification
and partial characterization of an acidic polysaccharide with
complement fixing ability from the stems of Avicennia marina. J
Biochem Mol Biol. 39:546–555. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Santhiya D, Subramanian S and Natarajan K:
Surface chemical studies on sphalerite and galena using
extracellular polysaccharides isolated from Bacillus polymyxa. J
Colloid Interface Sci. 256:237–248. 2002. View Article : Google Scholar
|
|
33
|
Duh PD: Rebuttal on comparison of
protective effects between cultured Cordyceps militaris and natural
Cordyceps sinensis against oxidative damage. J Agric Food Chem.
55:7215–7216. 2007. View Article : Google Scholar
|