Introduction
The ocular disorder of glaucoma, characterized by
the progressive loss of retinal ganglion cells and their axons,
accompanied by gradual loss of the visual field, is a leading cause
of blindness, estimated to affect 79.6 million people worldwide by
2020 (1). Dysregulation of the
blood flow with subsequent hypoxia has been suggested to be a
mechanism of retinal damage in glaucoma (2). Hypoxia-inducible factor 1 (HIF-1) is
a transcriptional activator that functions as a master regulator of
oxygen homeostasis and plays an essential role in mammalian
development, physiology and disease pathogenesis (3). HIF-1 is a heterodimer composed of the
inducible expressed HIF-1α subunit and the constitutively expressed
HIF-1β subunit (3,4). HIF-1α expression is induced in
hypoxic cells with an exponential increase in expression as cells
are exposed to an oxygen concentration of <6% (5). Previous studies have reported that
the activation of the hypoxia-responsive transcription factor
HIF-1α is involved in the pathophysiology of glaucoma (6,7).
Moreover, a number of HIF-1α targeted genes have also been
investigated in retinas with glaucoma, including EPO,
FLT-1, HSP-27, PAI-1, VEGF-A,
ET-1, IGF2 and TGFβ3 (7,8).
Our previous study investigated the expression
levels of HIF-1α and inducible nitric oxide synthase (iNOS), and
the effect of erythropoietin (EPO) on the HIF-1\iNOS signal
transduction pathway in the retina of chronic ocular hypertension
rats (9). EPO is capable of
protecting retinal ganglion cells from degeneration induced by
acute ischemia-reperfusion injury, axotomy injury and light- and
genetic-induced degeneration, and promoting the survival of retinal
ganglion cells in DBA/2J glaucoma mice (10). Significant advances have been made
in understanding the molecular pathology of glaucoma, particularly
through the development of rat models of experimental glaucoma and
the characterization of a spontaneous secondary form of glaucoma in
DBA/2 substrains of inbred mice (2). However, the correlation between
HIF-1α and its targeted genes remains unclear and an investigation
into EPO in glaucoma is warranted.
Cyclooxygenase-2 (COX-2) has been identified in the
cornea, iris, ciliary body and various cell types within the
neurosensory retina. The expression of the COX-2 enzyme in the
ocular tissues plays a functional role for its prostanoid products
(11). It has been reported that
HIF-1 is an essential regulator of COX-2 induction in hypoxic
monkey choroidal retinal endothelial cells (12). HIF-1α has been reported to promote
apoptosis, since caspase-9 promoter contained HIF-1-binding
elements and caspase-9 transcription could be induced by a hypoxic
episode (13,14). Activation of caspase-9 has been
identified in a rat model of experimental glaucoma (15). In the present study, chronic ocular
hypertension rats were induced with episcleral vein cauterization
(EVC). The mRNA and protein expression levels of HIF-1α, iNOS,
COX-2 and caspase-9 were investigated in normal, EVC-treated, and
EVC combined with EPO (EVC+EPO)-treated rats. In addition, the
correlation of HIF-1α with intraocular pressure (IOP),
electroretinogram b-wave (ERG-b), iNOS, COX-2 and caspase-9 was
also studied.
Subjects and methods
Animals
A total of 120 male Wistar rats (age, 40–50 days;
weight, 200–250 g) without eye disease were obtained from the
Experimental Animal Center of China Medical University, Shenyang,
China. All rats were housed in a specific pathogen-free facility.
The rats were randomly divided into 12 groups: One blank group; one
group with rats injected with EPO and without ocular hypertension;
five groups with rats under ocular hypertension for 3, 7, 14, 21
and 28 days, respectively; and five groups with rats injected with
EPO and under ocular hypertension for 3, 7, 14, 21 and 28 days,
respectively. The right eyes of rats in each group were selected as
the experimental eyes to receive treatments and the left eyes were
used as controls. The study was approved by the Animal Ethics
Committee of Shengjing Hospital Affiliated to China Medical
University.
Model establishment and sample
preparation
Chronic ocular hypertension rats were induced with
EVC as described previously (9).
Two or three of the episcleral veins were coagulated by heat cure
hemostat in the experimental group, while nothing was done to the
veins in the blank group. A Tono-Pen II tonometer (Mentor
Opthalmics, Inc., Norwell, MA, USA) was used to measure the IOP of
the Wistar rats prior to surgery, half an hour after surgery and 3,
7, 14, 21 and 28 days after surgery. The postoperative IOP was 40%
higher than that prior to surgery, indicating that the chronic
ocular hypertension model was established successfully. This model
closely mimics the natural disease process in certain forms of
secondary glaucoma, and is one of the most commonly used models in
rats (16). The rats were
sacrificed in each group following the correct treatment. The
entire retina was separated and stored as described previously for
subsequent experiments (9).
Drug administration and ERG-b
detection
Recombinant human EPO was purchased from Shenyang
Sunshine Pharmaceutical Co., Ltd. (China). Wistar rats in the drug
group were intraperitoneally injected with EPO (5,000 U/kg) 1 day
before surgery, and 2, 6, 13, 20 and 27 days after surgery. The
change in ERG-b was detected by VETS-3 electrophysiolography as
described previously (9).
Measurement of mRNA expression levels by
semi-quantitative RT-PCR
First, total RNA from normal and glaucomatous whole
retinas from each group was reverse transcribed into cDNA using
random primers and SuperScriptTM II RT (Invitrogen,
Carlsbad, CA, USA). The target levels in each retina were
normalized with the housekeeping gene glyceraldehyde-3-phosphate
dehydrogenase (GAPDH) or β-actin. The sequences of the PCR primers
and the product length of target genes (HIF-1α, iNOS, COX-2 and
caspase-9) are listed in Table I.
The PCR conditions and the electrophoresis and gel imaging
processes were as described in our previous study (9). Briefly, PCR was performed in a 25 μl
reaction volume: 2.5 μl cDNA, 2.5 μl 10× PCR buffer (Takara Shuzo,
Shiga, Japan), 2.0 μl dNTP (2.5 mM; Takara Shuzo), 1.0 μl of each
primer (100 pm/μl), 1.0 μl of each primer (50 pm/μl) and 0.3 μl Taq
DNA polymerase (5 U/μl, Takara Shuzon). The amplification was
performed as follows: 95°C for 5 min followed by 32 cycles of 94°C
for 40 sec, 60°C for 40 sec and 72°C for 60 sec, with a final
extension step of 10 min at 72°C. mRNA expression levels were
recorded by calculating the absorbance ratio of the target gene
strap and GAPDH/β-actin strap.
 | Table ISequences of PCR primers and product
length of genes. |
Table I
Sequences of PCR primers and product
length of genes.
| Gene | Sequence (5′-3′) | Product length
(bp) |
|---|
| F-β-actin |
ACACTGTGCCCATCTACGAGG | 621 |
| R-β-actin |
AGGGGCCGGACTCGTCATACT | |
| F-GAPDH |
ACCACAGTCCATGCCATCAC | 452 |
| R-GAPDH |
TCCACCACCCCTGTTGCTGTA | |
| F-COX-2 |
GCAAATCCTTGCTGTTCCAACCCA | 482 |
| R-COX-2 |
TTGGGGATCCGGGATGAACTCTCT | |
| F-caspase-9 |
TTTAAGCTCTCAGAAGACATG | 363 |
| R-caspase-9 |
TGTTGAAGTACAGACAGTACCCCCA | |
| F-iNOS |
TCCAGGAGGACATGCAGCAC | 260 |
| R-iNOS |
TCTTGACGCCTTCCCGC | |
| F-HIF-1α |
CTGATTGCATCTCCACCTTCTACC | 343 |
| R-HIF-1α |
TTCCAAGAAAGCGACATAGTAGGG | |
Western blotting
The retinas were dissected and homogenized in lysis
buffer (10 mM Tris, pH 7.4; 150 mM NaCl; 1 mM EDTA; 1 mM EGTA)
supplemented with 10% protease inhibitor cocktail and 1%
phosphatase inhibitor cocktail from Sigma (St. Louis, MO, USA).
Cell debris was removed by centrifugation. The protein
concentration of the supernatant was measured using a Bio-Rad DC
protein assay kit (Bio-Rad, Hercules, CA, USA). A total of 40 μg
protein from each group was subjected to 10% SDS polyacrylamide gel
electrophoresis and transferred onto polyvinylidene fluoride
membranes at 50 V for 2 h. The membranes were blocked with 5%
non-fat dry milk and 2% bovine serum albumin in Tris-buffered
saline containing 0.1% Tween-20 for 1 h. Incubations with primary
polyclonal rabiit anti-rat antibodies of HIF-1α, iNOS, COX-2 and
caspase-9 (1:200; Wuhan Boshide Company, China) in blocking buffer
were performed overnight at 4°C. After washing, the membranes were
incubated with horseradish peroxidase-conjugated secondary
polyclonal anti-rabbit antibody (1:2,000; Wuhan Boshide Company,
China) in blocking buffer for 1 h at room temperature. Bands were
quantified and scanned using Floorchem V2.0 software provided by
the UVI pro gel analysis system (Kodak, Rochester, NY, USA).
β-actin was used as an internal control.
Statistical analysis
The results are expressed as the mean ± standard
deviation. Comparisons among groups were performed by one
way-analysis of variance using SPSS 12.0 (SPSS, Inc., Chicago, IL,
USA). The correlations between the mRNA expression levels of HIF-1α
and IOP and ERG-b, and the mRNA expression levels of iNOS, COX-2
and caspase-9 were analyzed using Pearson’s correlation, Kendall’s
correlation and Spearman’s correlation. The correlation analysis of
protein expression levels of HIF-1α with iNOS, COX-2 and caspase-9
was also performed using these three methods.
Results
Induction of experimental glaucoma
Cauterization of the major episcleral vein trunks
resulted in increased IOP as aqueous outflow was interrupted
(Table II). There was no
significant difference in IOP between the experimental and control
eyes of the 120 rats prior to the EVC procedure (0 days; P>0.05;
Table II). At 0.5 h after the EVC
procedure, the IOP of the experimental eyes was increased to
30.166±6.029 mmHg, which was significantly higher than that of the
control eyes (P<0.01). The peak IOP (33.942±9.208 mmHg) was
obtained 7 days after the EVC procedure, which was 2.66-fold higher
than that at 0 days. The IOP of the experimental eyes 14, 21 and 28
days after surgery remained significantly increased, at >40% of
that of the control eyes (P<0.01). The ERG-b level of the
EVC+EPO group was significantly higher than that of the EVC group
7, 14, 21 and 28 days after surgery (P<0.05, Table III).
 | Table IIIntraocular pressure of eyes in
experimental and control eyes preoperatively and at various times
postoperatively. |
Table II
Intraocular pressure of eyes in
experimental and control eyes preoperatively and at various times
postoperatively.
| Time | Experimental (n=120,
mmHg) | Control (n=120,
mmHg) |
|---|
| 0 days | 12.767±3.176 | 12.846±2.769 |
| 0.5 h | 30.166±6.029b | 12.517±2.429 |
| 3 days | 22.718±4.131a | 12.233±3.711 |
| 7 days | 33.942±9.208b | 13.466±2.270 |
| 14 days | 32.557±7.937b | 12.825±2.791 |
| 21 days | 25.395±2.703b | 12.634±3.109 |
| 28 days | 26.000±3.978b | 12.809±2.693 |
 | Table IIIElectroretinogram b-wave in retina
following EPO injection. |
Table III
Electroretinogram b-wave in retina
following EPO injection.
| Time (days) | EVC group | EVC+EPO group | P-value |
|---|
| 0 | 113.05±9.53 | 119.45±11.43 | 0.480 |
| 3 | 92.71±7.38 | 97.34±8.45 | 0.430 |
| 7 | 72.37±8.15 | 98.21±10.23a | 0.025 |
| 14 | 65.51±0.042 | 95.57±9.29b | 0.009 |
| 21 | 68.65±0.059 | 93.32±6.42a | 0.016 |
| 28 | 69.91±0.037 | 95.32±9.12a | 0.038 |
mRNA expression levels of HIF-1α, iNOS,
COX-2 and caspase-9
The mRNA expression levels of HIF-1α, iNOS, COX-2
and caspase-9 in normal, EVC-treated, and EVC and EPO-treated rat
retinas were investigated (Table
IV and Fig. 1). Compared with
the preoperative rats (0 days), the mRNA expression levels of
HIF-1α, iNOS, COX-2 and caspase-9 in EVC-treated rats were
increased. The peak expression levels of HIF-1α, iNOS, COX-2 and
caspase-9 respectively occurred 7, 7, 7 and 14 days after surgery.
Compared with the EVC-treated rats, the mRNA expression levels of
HIF-1α, iNOS, COX-2 and caspase-9 in the EPO-treated group were
statistically significantly reduced 7, 14, 21 and 28 days after
surgery (P<0.01, Table
IV).
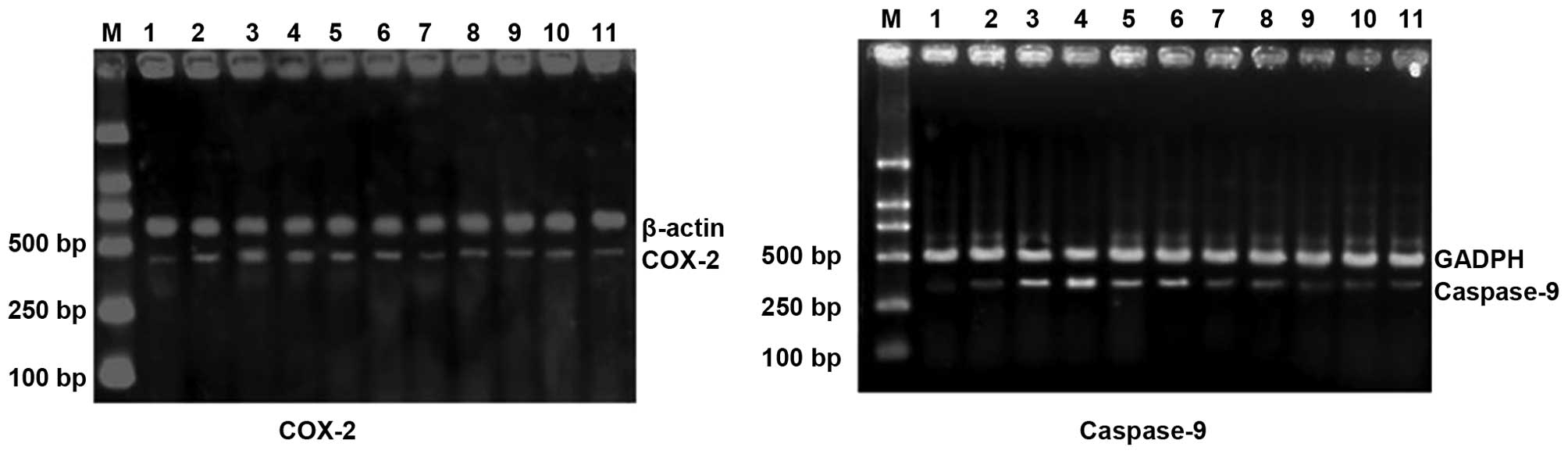 | Figure 1mRNA expression levels of HIF-1α,
iNOS, COX-2 and caspase-9 in experimental glaucoma rats. Lane 1,
control; lane 2, 3 days after EVC treatment; lane 3, 7 days after
EVC treatment; lane 4, 14 days after EVC treatment; lane 5, 21 days
after EVC treatment; lane 6, 28 days after EVC treatment; lane 7, 3
days after EVC and EPO treatment; lane 8, 7 days after EVC and EPO
treatment; lane 9, 14 days after EVC and EPO treatment; lane 10, 21
days after EVC and EPO treatment; lane 11, 28 days after EVC and
EPO treatment; lane M, DNA marker. HIF-1α, hypoxia-inducible
factor-1α; iNOS, inducible nitric oxide synthase; COX-2,
cyclooxygenase-2; EVC, episcleral vein cauterization; EPO,
erythropoietin. |
 | Table IVmRNA expression level of HIF-1α,
iNOS, COX-2 and caspase-9 in retinas following EPO injection. |
Table IV
mRNA expression level of HIF-1α,
iNOS, COX-2 and caspase-9 in retinas following EPO injection.
| Gene | Time (days) | EVC group (μV) | EVC+EPO group
(μV) | P-value |
|---|
| HIF-1α | 0 | 0.248±0.087 | 0.234±0.072 | 0.790 |
| 3 | 0.408±0.029 | 0.240±0.027a | 0.020 |
| 7 | 0.512±0.028 | 0.288±0.031b | 0.000 |
| 14 | 0.406±0.032 | 0.312±0.026a | 0.010 |
| 21 | 0.342±0.044 | 0.226±0.027a | 0.015 |
| 28 | 0.338±0.026 | 0.240±0.020a | 0.030 |
| iNOS | 0 | 0.259±0.053 | 0.284±0.037 | 0.480 |
| 3 | 0.423±0.085 | 0.358±0.026 | 0.088 |
| 7 | 0.676±0.065 |
0.448±0.0441b | 0.005 |
| 14 | 0.526±0.042 | 0.376±0.029b | 0.001 |
| 21 | 0.414±0.059 | 0.336±0.032a | 0.036 |
| 28 | 0.394±0.037 | 0.328±0.026a | 0.037 |
| COX-2 | 0 | 0.218±0.037 | 0.202±0.031 | 0.317 |
| 3 | 0.392±0.033 | 0.380±0.022 | 0.550 |
| 7 | 0.622±0.048 | 0.388±0.029b | 0.001 |
| 14 | 0.552±0.043 | 0.402±0.033a | 0.010 |
| 21 | 0.438±0.038 | 0.374±0.029a | 0.014 |
| 28 | 0.458±0.031 | 0.312±0.032b | 0.000 |
| Caspase-9 | 0 | 0.146±0.044 | 0.154±0.033 | 0.640 |
| 3 | 0.350±0.042 | 0.240±0.032a | 0.018 |
| 7 | 0.530±0.063 | 0.348±0.031b | 0.009 |
| 14 | 0.696±0.045 | 0.232±0.027b | 0.000 |
| 21 | 0.390±0.037 | 0.252±0.024b | 0.002 |
| 28 | 0.412±0.043 | 0.300±0.043a | 0.032 |
Protein expression levels of HIF-1α,
iNOS, COX-2 and caspase-9
The protein expression levels of HIF-1α, iNOS, COX-2
and caspase-9 in normal, EVC-treated, and EVC and EPO-treated rat
retinas were also investigated (Table
V and Fig. 2). Similarly to
mRNA expression levels, the protein expression levels of HIF-1α,
iNOS, COX-2 and caspase-9 in EVC-treated rats were increased
compared with those of preoperative rats. The peak expression of
HIF-1α, iNOS, COX-2 and caspase-9 respectively occurred 7, 7, 7 and
14 days after surgery. Compared with EVC-treated rats, the protein
expression levels of HIF-1α, iNOS, COX-2 and caspase-9 in the
EPO-treated group were notably reduced 7, 14, 21 and 28 days after
surgery (P<0.01, Table V).
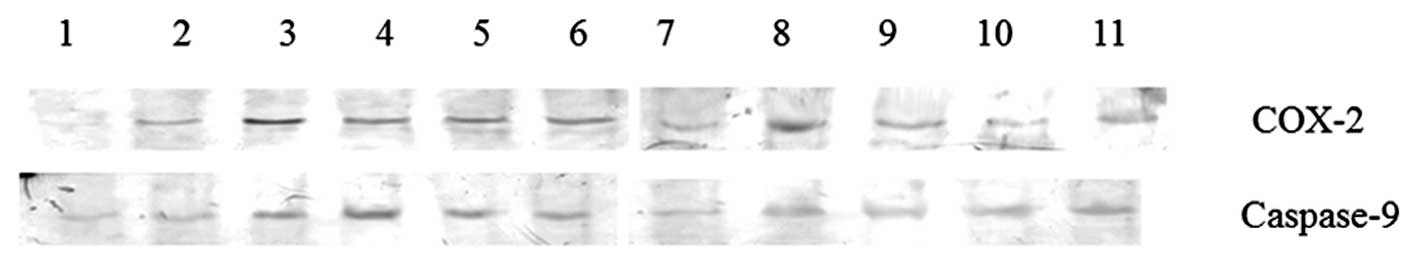 | Figure 2Protein expression levels of COX-2 and
caspase-9 in experimental glaucoma rats. Lane 1, control; lane 2, 3
days after EVC treatment; lane 3, 7 days after EVC treatment; lane
4, 14 days after EVC treatment; lane 5, 21 days after EVC
treatment; lane 6, 28 days after EVC treatment; lane 7, 3 days
after EVC and EPO treatment; lane 8, 7 days after EVC and EPO
treatment; lane 9, 14 days after EVC and EPO treatment; lane 10, 21
days after EVC and EPO treatment; lane 11, 28 days after EVC and
EPO treatment; lane M, DNA marker. COX-2, cyclooxygenase-2; EVC,
episcleral vein cauterization; EPO, erythropoietin. |
 | Table VProtein expression levels of HIF-1α,
iNOS, COX-2 and caspase-9 in retinas following EPO injection. |
Table V
Protein expression levels of HIF-1α,
iNOS, COX-2 and caspase-9 in retinas following EPO injection.
| Gene | Time (days) | EVC group | EVC+EPO group | P-value |
|---|
| HIF-1α | 0 | 0.084±0.021 | 0.118±0.019 | 0.058 |
| 3 | 0.210±0.026 | 0.164±0.011a | 0.045 |
| 7 | 0.624±0.029 | 0.262±0.019b | 0.000 |
| 14 | 0.456±0.032 | 0.216±0.019b | 0.000 |
| 21 | 0.274±0.027 | 0.214±0.027b | 0.009 |
| 28 | 0.242±0.019 | 0.176±0.017b | 0.005 |
| iNOS | 0 | 0.264±0.037 | 0.228±0.029 | 0.0125 |
| 3 | 0.436±0.034 | 0.404±0.019 | 0.210 |
| 7 | 0.860±0.050 | 0.472±0.038b | 0.000 |
| 14 | 0.792±0.063 | 0.414±0.029b | 0.000 |
| 21 | 0.528±0.032 | 0.370±0.027b | 0.000 |
| 28 | 0.378±0.026 | 0.320±0.026a | 0.011 |
| COX-2 | 0 | 0.108±0.024 | 0.096±0.018 | 0.470 |
| 3 | 0.188±0.019 | 0.146±0.032 | 0.058 |
| 7 | 0.462±0.036 | 0.274±0.021b | 0.000 |
| 14 | 0.374±0.024 | 0.252±0.028b | 0.001 |
| 21 | 0.324±0.021 | 0.206±0.021b | 0.000 |
| 28 | 0.340±0.027 | 0.188±0.038b | 0.002 |
| Caspase-9 | 0 | 0.184±0.031 | 0.172±0.029 | 0.640 |
| 3 | 0.222±0.026 | 0.180±0.022 | 0.055 |
| 7 | 0.376±0.021 | 0.252±0.026b | 0.003 |
| 14 | 0.552±0.044 | 0.280±0.040b | 0.000 |
| 21 | 0.318±0.041 | 0.216±0.031a | 0.031 |
| 28 | 0.306±0.021 | 0.224±0.027a | 0.012 |
Correlation analysis
Pearson’s correlation, Kendall’s correlation and
Spearman’s correlation were used for correlation analysis. HIF-1α
was positively correlated with iNOS, COX-2 and caspase-9 at the
mRNA level (Table VI) and protein
level (Table VII). Moreover, the
mRNA expression level of HIF-1α also demonstrated a significant
positive correlation with IOP and ERG-b (Table VI).
 | Table VICorrelation analysis of mRNA
expression level of HIF-1α with IOP, ERG-b and mRNA expression
levels of iNOS, COX-2 and caspase-9 in chronic ocular hypertension
rats. |
Table VI
Correlation analysis of mRNA
expression level of HIF-1α with IOP, ERG-b and mRNA expression
levels of iNOS, COX-2 and caspase-9 in chronic ocular hypertension
rats.
| Pearson’s
correlation | Kendall’s
correlation | Spearman’s
correlation |
|---|
| IOP | 0.751b | 0.476a | 0.598a |
| ERG-b | 0.594b | 0.397a | 0.482a |
| iNOS | 0.821b | 0.572b | 0.733b |
| COX-2 | 0.791b | 0.518a | 0.672b |
| Caspase-9 | 0.599b | 0.345a | 0.516b |
 | Table VIICorrelation analysis of protein
expression levels of HIF-1α with iNOS, COX-2 and caspase-9 in
chronic ocular hypertension rats. |
Table VII
Correlation analysis of protein
expression levels of HIF-1α with iNOS, COX-2 and caspase-9 in
chronic ocular hypertension rats.
| Pearson’s
correlation | Kendall’s
correlation | Spearman’s
correlation |
|---|
| iNOS | 0.950b | 0.726b | 0.901b |
| COX-2 | 0.882b | 0.765b | 0.908b |
| Caspase-9 | 0.715b | 0.705a | 0.881b |
Discussion
Glaucoma is the second leading cause of blindness
worldwide (17). It is imperative
to explore the mechanisms of retinal damage in glaucoma. In the
present study, EVC was used for the construction of chronic ocular
hypertension rats. The mRNA and protein expression levels of
HIF-1α, iNOS, COX-2 and caspase-9 were investigated in chronic
ocular hypertension rats. Moreover, a correlation analysis of
HIF-1α with IOP, ERG-b, iNOS, COX-2 and caspase-9 was also
conducted.
First, an inducible rat model of elevated IOP was
developed using the EVC method. Following cauterization of two
episcleral veins in the rats, IOP increases of 50–80% were reported
in treated eyes, with a loss of 4% of the retinal ganglion cells
per week (18). Cauterization of
three episcleral veins produced an IOP increase of 120–150%, with a
loss of 5–6% of retinal ganglion cells per week (19). Two or three of the major episcleral
veins were coagulated in the experimental group of the present
study. The peak IOP (33.942±9.208, P<0.01) was obtained 7 days
after the EVC procedure, and was 2.66-fold higher than that at 0
days. This result was in accordance with previous studies (18,19).
The mRNA expression levels of HIF-1α, iNOS, COX-2
and caspase-9 in EVC-treated rats were increased significantly
compared with those in normal rats. The peak expression levels of
HIF-1α, iNOS, COX-2 and caspase-9 were obtained 7, 7, 7 and 14 days
after surgery. The correlation analysis indicated that HIF-1α had a
significant positive correlation with iNOS, COX-2 and caspase-9 at
both the mRNA and protein level. Moreover, HIF-1α also demonstrated
a significant positive correlation with IOP and ERG-b.
COX-2 is an inducible enzyme with a significant role
in inflammation. It is overexpressed in a variety of cancer types
and plays an essential role in the angiogenesis of gastric
carcinoma (20,21). A previous study indicated that
COX-2 is transcriptionally induced by hypoxia via HIF-1, and the
upregulation of COX-2 contributes to maintaining tumor survival and
potentially promoting angiogenesis in colorectal tumor cells
(22). Lukiw et al reported
coordinated activation of HIF-1 and COX-2 expression in retinal
cells by hypoxia (12). Caspase-9
is the apex caspase of the mitochondrial pathway of apoptosis,
which plays a critical role in apoptotic initiation and progression
(14). Hänninen et al
investigated retinal ganglion cell death and activation of
caspase-9 in rats with experimental glaucoma and supported the
activation of caspase-9, the intrinsic caspase cascade, in retinal
ganglion cell death in experimental glaucoma (15). The upregulated caspase-9 in
experimental glaucoma may result in retinal ganglion cell death and
aggravate the development of glaucoma. These studies further
support the results revealed in the present study. Thus, COX-2 and
caspase-9 overexpression may be regarded as a critical adaptive
response to hypoxia in glaucoma. Several studies have examined the
observation that HIF-1α immunoreactivity is enhanced in the
glaucomatous retina in a canine glaucoma model and in human
glaucoma (4,6,9,23).
Upon activation by hypoxia and/or receptor-mediated signals, the
HIF-1α expression level was increased and several HIF-1
transcriptional targets were upregulated in the glaucomatous retina
in the present study and our previous study (9). Moreover, significant positive
correlations with HIF-1α, iNOS, COX-2, caspase-9, IOP and ERG-b
were identified in the current study. It furthermore indicates the
significant role of HIF-1α in the development and progression of
glaucoma.
Compared with EVC-treated rats, EPO administration
weakened the mRNA and protein expression levels of HIF-1α, iNOS,
COX-2 and caspase-9. Glaucoma is characterized by the progressive
death of retinal ganglion cells and visual field loss. EPO has been
reported to provide neuroprotection in cultured adult rat retinal
ganglion cells, with anti-oxidative, anti-apoptotic and
neurotrophic effects observed in several signaling pathways
(24,25). The neuroprotection of EPO in
retinal ganglion cells may be a potential reason for the
downregulated expression levels of HIF-1α, iNOS, COX-2 and
caspase-9 in experimental glaucoma rats.
In conclusion, the mRNA and protein expression
levels of HIF-1α, iNOS, COX-2 and caspase-9 were investigated in
EVC-treated chronic ocular hypertension rats, and the correlation
of HIF-1α with IOP, ERG-b, iNOS, COX-2 and caspase-9 was also
studied. Upregulated levels of HIF-1α, iNOS, COX-2 and caspase-9
were observed in experimental glaucoma rats. HIF-1α demonstrated a
significant correlation with iNOS, COX-2, caspase-9, IOP and ERG-b.
HIF-1α, iNOS, COX-2 and caspase-9 overexpression may be regarded as
a critical adaptive response to hypoxia in glaucoma. EPO
administration weakened the mRNA and protein expression levels of
HIF-1α, iNOS, COX-2 and caspase-9. To conclude, the protective
effect of EPO on the retina of chronic ocular hypertension rats may
be mediated by the HIF-1 signaling pathway involving iNOS, COX-2
and caspase-9.
References
|
1
|
Vohra R, Tsai JC and Kolko M: The role of
inflammation in the pathogenesis of glaucoma. Surv Ophthalmol.
58:311–320. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Nickells RW: Ganglion cell death in
glaucoma: from mice to men. Vet Ophthalmol. 10(Suppl 1): 88–94.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Semenza GL: HIF-1 and mechanisms of
hypoxia sensing. Curr Opin Cell Biol. 13:167–171. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Tezel G and Wax MB: Hypoxia-inducible
factor 1 alpha in the glaucomatous retina and optic nerve head.
Arch Ophthalmol. 122:1348–1356. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Jiang B-H, Semenza GL, Bauer C and Marti
HH: Hypoxia-inducible factor 1 levels vary exponentially over a
physiologically relevant range of O2 tension. Am J Physiol.
271:C1172–C1180. 1996.PubMed/NCBI
|
|
6
|
Ergorul C, Ray A, Huang W, et al: Hypoxia
inducible factor-1α (HIF-1α) and some HIF-1 target genes are
elevated in experimental glaucoma. J Mol Neurosci. 42:183–191.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zhu Y, Zhang L and Gidday JM: Role of
hypoxia-inducible factor-1alpha in preconditioning-induced
protection of retinal ganglion cells in glaucoma. Mol Vis.
19:2360–2372. 2013.
|
|
8
|
Whitlock NA, Agarwal N, Ma J-X and Crosson
CE: Hsp27 upregulation by HIF-1 signaling offers protection against
retinal ischemia in rats. Invest Ophthalmol Vis Sci. 46:1092–1098.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Gui DM, Yang Y, Li X and Gao DW: Effect of
erythropoietin on the expression of HIF-1 and iNOS in retina in
chronic ocular hypertension rats. Int J Ophthalmol. 4:40–43.
2011.PubMed/NCBI
|
|
10
|
Fu QL, Wu W, Wang H, Li X, Lee VW and So
KF: Up-regulated endogenous erythropoietin/erythropoietin receptor
system and exogenous erythropoietin rescue retinal ganglion cells
after chronic ocular hypertension. Cell Mol Neurobiol. 28:317–329.
2008. View Article : Google Scholar
|
|
11
|
Yanni SE, McCollum GW and Penn JS: Genetic
deletion of COX-2 diminishes VEGF production in mouse retinal
Müller cells. Exp Eye Res. 91:34–41. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lukiw WJ, Ottlecz A, Lambrou G, et al:
Coordinate activation of HIF-1 and NF-κB DNA binding and COX-2 and
VEGF expression in retinal cells by hypoxia. Invest Ophthalmol Vis
Sci. 44:4163–4170. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Soengas MS, Alarcón RM, Yoshida H, Giaccia
H, Hakem R, Mak TW and Lowe SW: Apaf-1 and caspase-9 in
p53-dependent apoptosis and tumor inhibition. Science. 284:156–159.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Nishiyama J, Yi X, Venkatachalam M and
Dong Z: cDNA cloning and promoter analysis of rat caspase-9.
Biochem J. 360:49–56. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Hänninen VA, Pantcheva MB, Freeman EE,
Poulin NR and Grosskreutz CL: Activation of caspase 9 in a rat
model of experimental glaucoma. Curr Eye Res. 25:389–395. 2002.
View Article : Google Scholar
|
|
16
|
Goldblum D and Mittag T: Prospects for
relevant glaucoma models with retinal ganglion cell damage in the
rodent eye. Vision Res. 42:471–478. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Quigley HA and Broman AT: The number of
people with glaucoma worldwide in 2010 and 2020. Br J Ophthalmol.
90:262–267. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Laquis S, Chaudhary P and Sharma SC: The
patterns of retinal ganglion cell death in hypertensive eyes. Brain
Res. 784:100–104. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ahmed FA, Chaudhary P and Sharma SC:
Effects of increased intraocular pressure on rat retinal ganglion
cells. Int J Dev Neurosci. 19:209–218. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Huang SP, Wu MS, Shun CT, et al:
Cyclooxygenase-2 increases hypoxia-inducible factor-1 and vascular
endothelial growth factor to promote angiogenesis in gastric
carcinoma. J Biomed Sci. 12:229–241. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wang D and Dubois RN: The role of COX-2 in
intestinal inflammation and colorectal cancer. Oncogene.
29:781–788. 2010. View Article : Google Scholar
|
|
22
|
Kaidi A, Qualtrough D, Williams AC and
Paraskeva C: Direct transcriptional up-regulation of
cyclooxygenase-2 by hypoxia-inducible factor (HIF)-1 promotes
colorectal tumor cell survival and enhances HIF-1 transcriptional
activity during hypoxia. Cancer Res. 66:6683–6691. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Savagian CA, Dubielzig RR and Nork TM:
Comparison of the distribution of glial fibrillary acidic protein,
heat shock protein 60, and hypoxia-inducible factor-1α in retinas
from glaucomatous and normal canine eyes. Am J Vet Res. 69:265–272.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Chang ZY, Yeh MK, Chiang CH, Chen YH and
Lu DW: Erythropoietin protects adult retinal ganglion cells against
NMDA-, trophic factor withdrawal-, and TNF-α-induced damage. PLoS
One. 8:e552912013. View Article : Google Scholar
|
|
25
|
Zhong L, Bradley J, Schubert W, et al:
Erythropoietin promotes survival of retinal ganglion cells in
DBA/2J glaucoma mice. Invest Ophthalmol Vis Sci. 48:1212–1218.
2007. View Article : Google Scholar : PubMed/NCBI
|
















