Introduction
Ionizing radiation breaks DNA double strands and
results in the induction of various responses in cancer cells,
including altered susceptibility to immune cells. It achieves this
through the upregulation of immunomodulatory surface and secretory
molecules, such as the major histocompatibility complex (MHC),
co-stimulatory molecules and cytokines (1). It has previously been shown that
irradiation of malignant cells enhanced the natural killer (NK)
cell-mediated cytotoxicity in autologous models (2) and the susceptibility of cancer cells
to NK cells has been shown to increase following irradiation
(3). However, despite the
beneficial effects of radiotherapy in the treatment of cancer,
there are some adverse effects, including facilitating invasion and
metastasis (4,5). It has previously been observed that
matrix metalloproteinases (MMPs) are upregulated by irradiation and
their expression has been associated with poor prognosis of
patients with cancer (6–8). Furthermore, it has been shown that
several proteases, including MMPs and a disintegrin and
metalloproteinase domain-containing proteins (ADAMs), may increase
the soluble form of NK group 2 member D ligands (NKG2DLs) (9–13).
This may result in cancer cell evasion of NK cell-mediated immune
responses, through the reduction of surface NKG2DLs on cancer cells
and the downregulation of NKG2D receptors on NK cells (11). Therefore, it may be beneficial to
reduce the activity of MMPs and decrease the expression levels of
soluble NKG2DLs in cancer radiotherapy. In the present study, MMP
activity was inhibited using GM6001 or MMP inhibitor III (MMPi
III), and the alterations to the surface and soluble NKG2DLs
expression levels were investigated in lung cancer cells, following
irradiation.
Materials and methods
Cell lines and reagents
NCI-H23, human non-small cell lung cancer cells,
were obtained from the Korean Cell Line Bank (Seoul, South Korea).
The cells were maintained in RPMI-1640 media, supplemented with 10%
fetal bovine serum (FBS; Gibco Life Technologies, Carlsbad, CA,
USA), 2 mM L-glutamine (Life Technologies, Carlsbad, CA, USA), 100
mg/ml streptomycin (USB Corporation, Cleveland, OH, USA), and 100
U/ml penicillin (USB corporation). The NK-92 natural killer cell
line was obtained from the American Type Culture Collection
(Manassas, VA, USA) and maintained in α-Minimum Essential Modified
medium, supplemented with 12.5% (v/v) FBS, 12.5% (v/v) horse serum
(Life Technologies), 2 mM L-glutamine, 0.1 mM 2-mercaptoethanol
(Sigma-Aldrich, St. Louis, MO, USA), 200 U/ml recombinant human
interleukin-2 (Proleukin; Novartis Pharmaceuticals, Camberley, UK),
100 mg/ml streptomycin, and 100 U/ml penicillin. All of the cells
were cultured at 37°C, in a humidified atmosphere containing 5%
CO2.
Total RNA extraction and
semi-quantitative polymerase chain reaction (qPCR)
Total RNA extraction and qPCR were performed as
described by previous methods (14). Briefly, total RNA was extracted
from the cells using the RNeasy® Mini kit (Qiagen,
Hilden, Germany), according to the manufacturer’s instructions.
cDNA was synthesized from 1 μg extracted total RNA, using 100 pmol
random primers (Takara Bio Inc., Otsu, Japan) and 100 U M-MLV
reverse transcriptase (Promega Corporation, Madison, WI, USA). The
resulting cDNA was used in the PCR reaction, which was performed
using the QIAGEN Multiplex PCR kit (Qiagen). Numerous primer pairs
were used to investigate the mRNA expression levels of NKG2DLs: MHC
class-I polypeptide-related chain proteins (MIC)A and MICB, UL-16
binding proteins (ULBP)1-3; MMPS: MMP2, 9 and 14; and ADAMs: ADAM10
and 17 (Bioneer Corporation, Daejeon, Korea). β-actin and ribosomal
protein L19 were used as the loading control and degradation
marker, respectively. The PCR products were separated by
electrophoresis on an ethidium bromide-stained 2.0% agarose gel and
quantified using Quantity One Image Analysis Software (Bio-Rad
Laboratories, Hercules, CA, USA).
Flow cytometry
To determine the surface NKG2D ligands on cancer
cells, the cells were incubated with 10 mg/ml mouse anti-MICA,
anti-MICB and anti-ULBP1-3 (R&D Systems, Inc., Minneapolis, MN,
USA), which are NKG2DL-specific monoclonal antibodies (mAbs), or
the corresponding isotype controls for 10 min at room temperature.
Subsequently, the cells were incubated with a goat
anti-mouse-phycoerythrin conjugated antibody (BD Phamingen, San
Diego, CA, USA) for 30 min at 4°C. The analysis was performed using
a BDFACSCalliber™ (Becton Dickinson, Franklin Lakes, NJ, USA) using
CellQuest™ software (Becton Dickinson). The cell surface expression
was quantified as the value of the mean fluorescence intensities
obtained with the specific mAbs.
Irradiation and co-treatment with MMP
inhibitors
To irradiate the cancer cells, a ClinaciX Linear
Accelerator (Varian Medical Systems, Palo Alto, CA, USA) was used,
under the guidance of Dr. Jiho Nam (Pusan National University
Yangsan Hospital, South Korea). The 1 * 106 cells were seeded at
prior day to 100mm diameter culture dishes with 10 ml PRMI-1640
medium and irradiated at a rate of 8 Gy/min to 200 * 200 mm area,
under a 10 mm depth-coverage of RPMI-1640 medium. The irradiated
NCI-H23 cells were allowed to recover for 6 h after reduction of
the medium to 10 ml. Subsequently, MMPi III and GM6001 were treated
for 18 h. Following an incubation for 18 h, the cells and
supernatants were harvested for further experimentation.
NK cell-mediated cytotoxicity assay
NK cell-mediated cytotoxicity was determined using
flow cytometry. Briefly, the irradiated only or irradiation/MMP
inhibitor co-treated NCI-H23 cells were harvested. The cells were
stained with 50 mM carboxyfluorescein succinimidyl ester (CFSE) for
30 min at 37°C and washed three times with phosphate buffered
saline (Gibco Life Technologies, Carlsbad, CA, USA). NK-92 cells
and CFSE-stained NCI-H23 cells were co-cultured for 4 h. Propidium
iodide (PI) was added to the co-cultured samples for identification
of the dead cells. The dead cells were quantified using the
following formula: (CFSE + PI + cells/CFSE + cells × 100).
ELISA
NCI-H23 cells were treated with MMP inhibitors
following irradiation, and the media was harvested. The soluble
MICA in the media was evaluated using a Human ELISA kit (Abcam,
Cambridge, United Kingdom), according to the manufacturer’s
instructions. Briefly, standard MICA and 100 ml supernatants, which
were prepared according to the manufacturer’s instructions, were
added to anti-MICA coated microplates. Following a 24 h incubation
at 4°C, biotinylated-MICA detection antibody and horseradish
peroxidase-streptavidin solution were sequentially added. After the
addition of the substrate reagent, soluble MICA was detected using
microplate reader fluorometry at a wavelength of 450 nm
(MicroQuant; MTX Lab Systems, Inc., Vienna, VA, USA).
Statistical analyses
To evaluate the altered gene expression levels, the
mean fold of gene expression among the groups and the standard
error of the mean were calculated. For comparisons of the groups, a
paired Student’s t-test was performed. P<0.05 was considered to
indicate a statistically significant difference in all of the
experiments.
Results
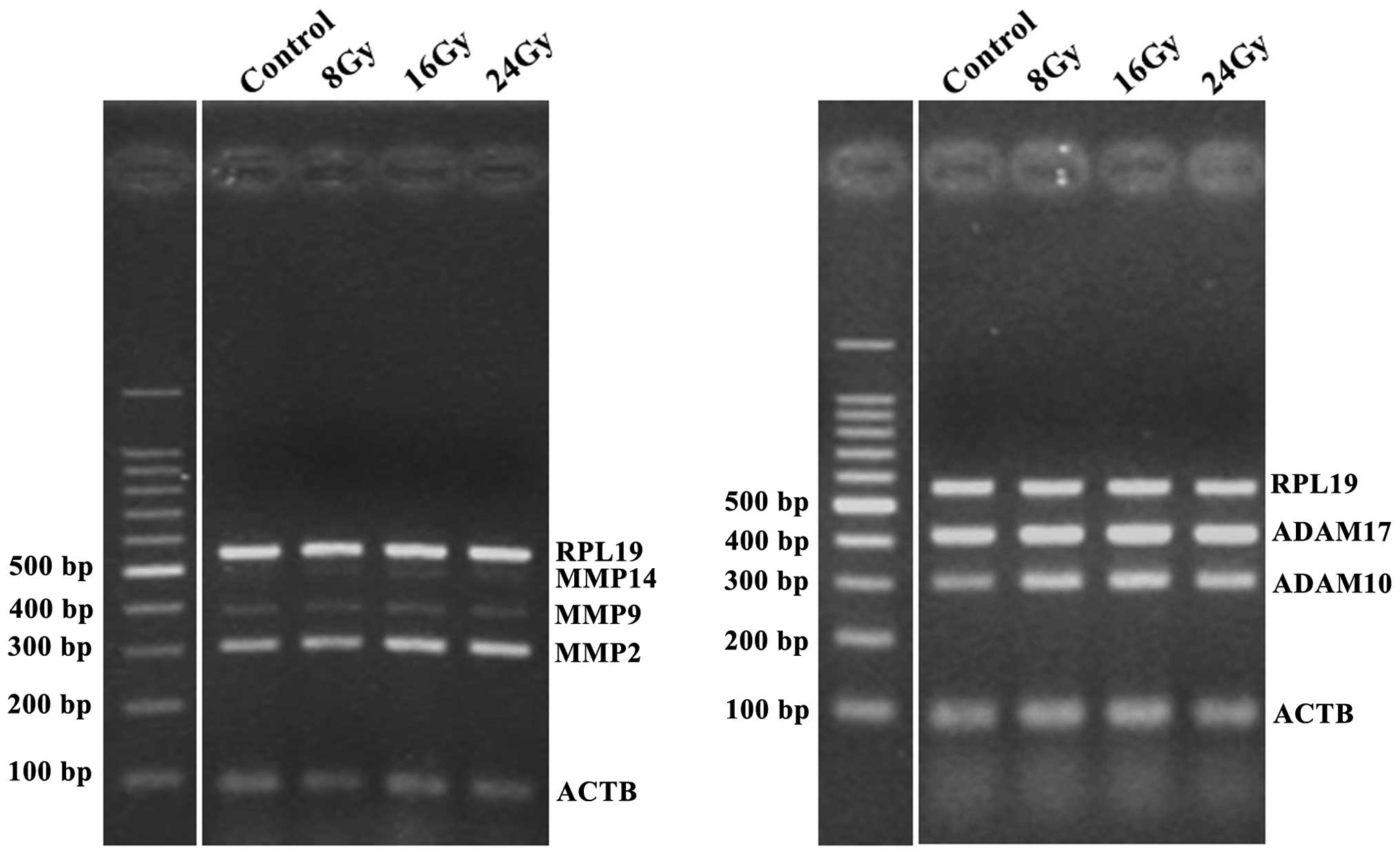
Ionizing radiation increases the
expression levels of MMP2 and ADAM 10, in a dose-dependent
manner
Previous reports have shown that three MMPs (MMP2, 9
and 14) and two ADAMs (ADAM10 and 17) are associated with the
shedding of MICA and MICB (9–13).
Therefore, in the present study, the alterations to the expression
levels of these proteases, following ionizing radiation, were
analyzed by qPCR. NCI-H23 cells were irradiated using a ClinaciX
Linear Accelerator (Varian Medical Systems, Inc.) at 8, 16 and 24
Gy doses. Following a 24 h incubation of the cells, the mRNA
expression levels of three MMPs (MMP2, 9 and 14) and two ADAMs
(ADAM10 and 17) were analyzed. MMP2 and ADAM10 were markedly
upregulated by ionizing radiation, in a dose-dependent manner
(Fig. 1). The expression levels of
MMP9 and MMP14 were low and the expression levels of ADAM17 were
high, and the alterations to these proteases, in response to
irradiation of the cells are not shown.
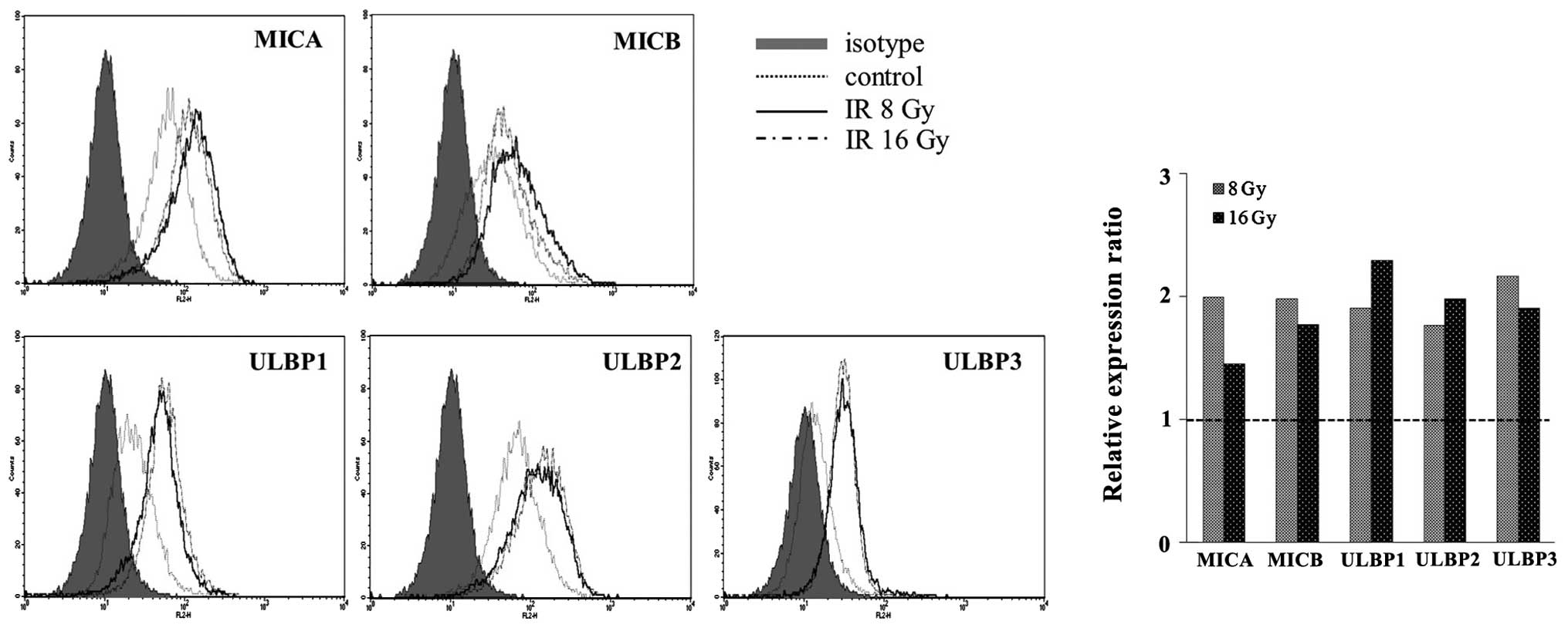
Surface NKG2DL levels are increased by
ionizing radiation, in a dose-independent manner
The surface expression of NKG2DLs in NCI-H23 cells
was analyzed using flow cytometric analysis. The surface expression
levels of NKG2DLs were markedly increased following 8 and 16 Gy
irradiation, in a dose-independent manner (Fig. 2). An irradiation dose of 16 Gy was
no more effective at inducing NKG2DLs surface expression, as
compared with a 8 Gy irradiation. These results suggest that
ionizing radiation may be a potent inducer of NKG2DLs and that high
dose irradiation may have adverse effects, especially in the
induction of MICA, MICB and ULBP3. Previous reports have suggested
that this upregulation is associated with the ATM-ATR pathway at
the translational level (15,16).
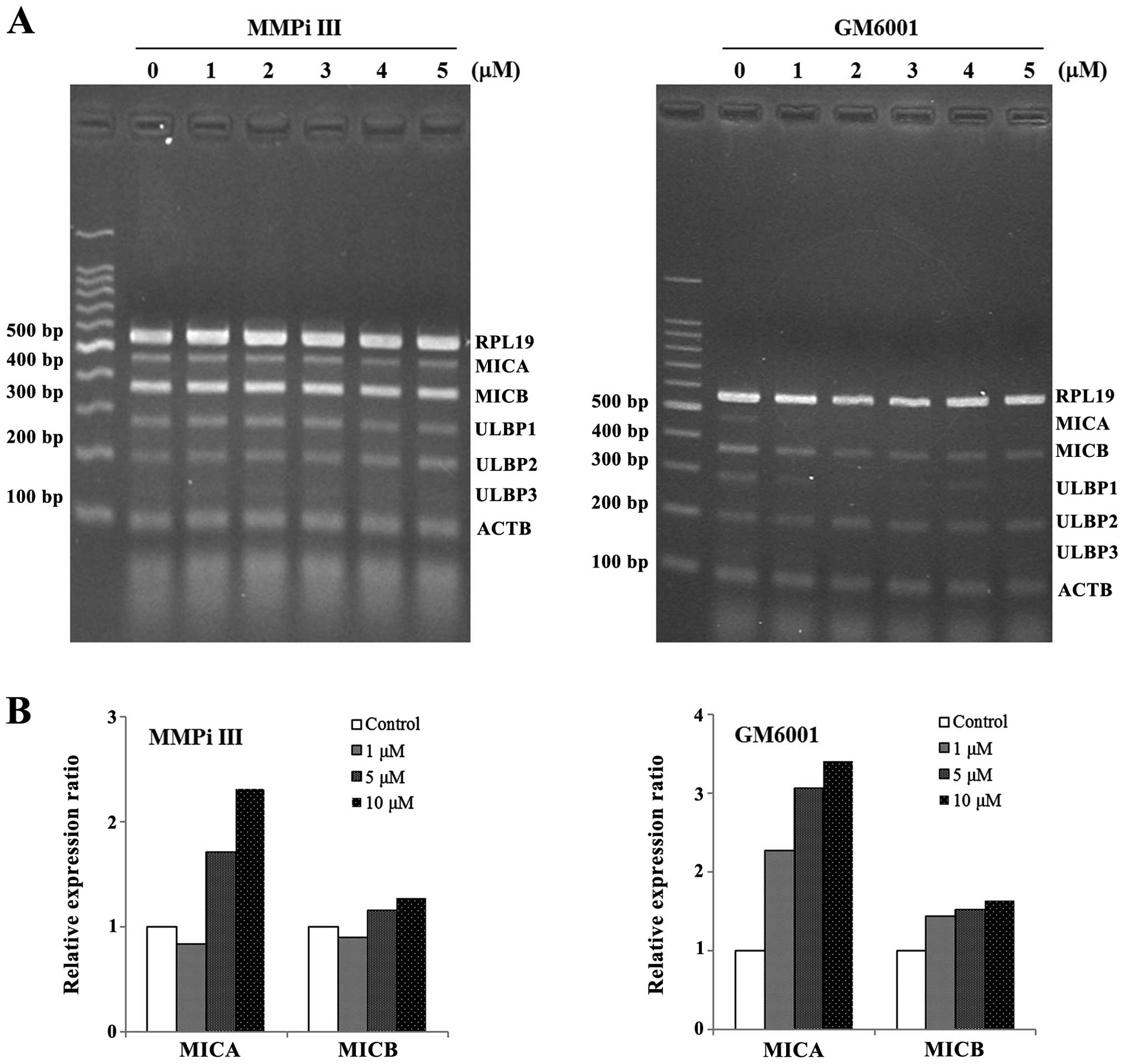
MMP inhibitors do not increase the mRNA
expression levels of NKG2DLs, but they do increase the levels of
surface NKG2DLs
To determine whether the inhibition of MMPs
modulated the expression of NKG2DLs, two MMP inhibitors, MMPi III
and GM6001, were used to treat the NCI-H23 cells. At the mRNA
level, the expression levels of NKG2DLs were not markedly
increased, in response to MMP inhibition (Fig. 3A). However, the surface protein
levels of MICA and MICB were increased in the cells treated with
MMP inhibitors. The surface MICA levels were markedly increased in
response to treatment with both of the MMP inhibitors, whereas the
surface MICB levels were only slightly increased (Fig. 3B).
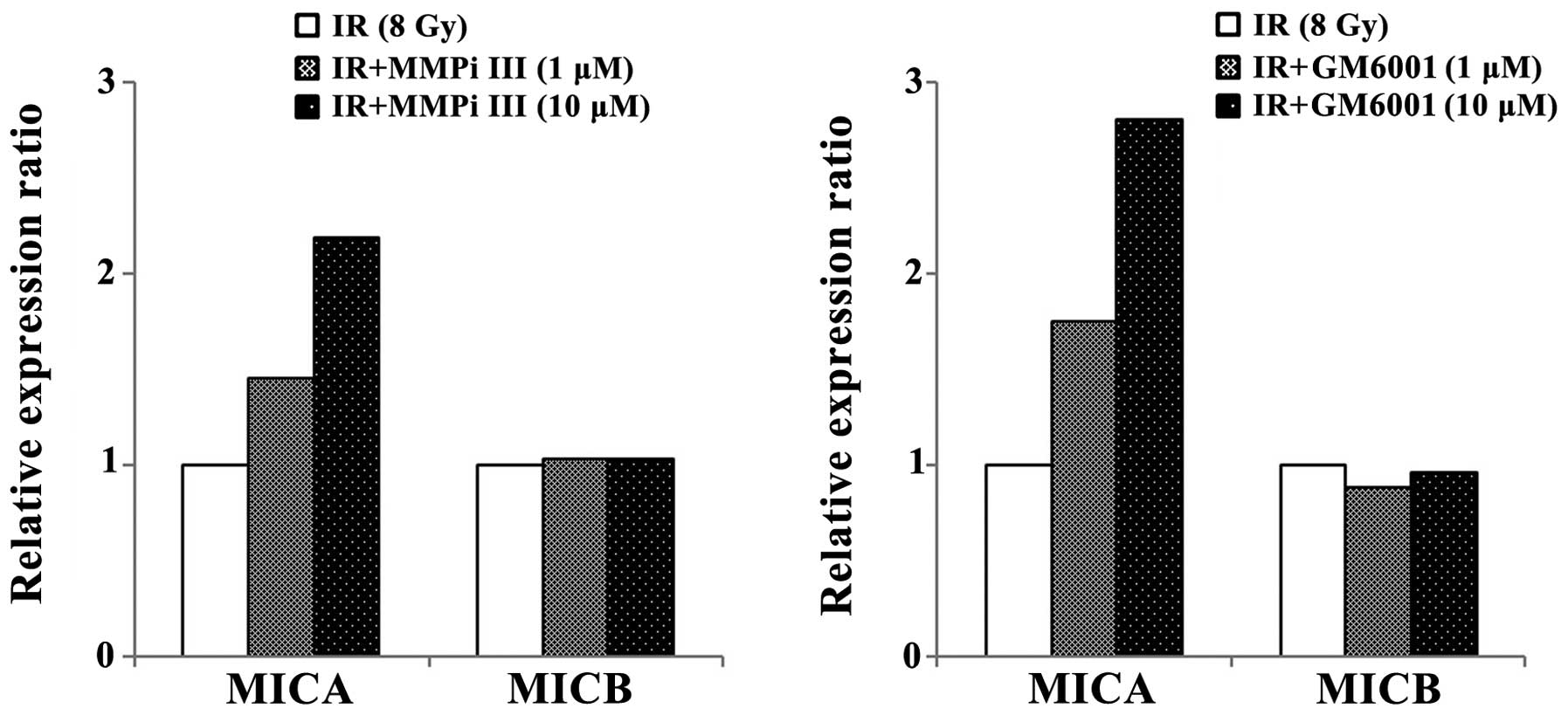
Combined treatment of ionizing radiation
and MMP inhibitors increases surface MICA levels
To determine whether the inhibition of MMPs, which
were induced by ionizing radiation, could increase surface MICA and
MICB levels, ionizing radiation and MMP inhibitors were combined
and used to treat NCI-H23 cells (Fig.
4). The inhibition of MMPs further enhanced the surface
expression levels of MICA, which were induced by ionizing
radiation. However, the relative surface expression levels of MICB
were not markedly altered by MMP inhibitors and irradiation
co-treatment, as compared with irradiation treatment only. From
these results, it was hypothesized that the preexisting MMPs may
contribute the shedding of MICB and radiation induced MMPs only
affected the shedding of MICA.
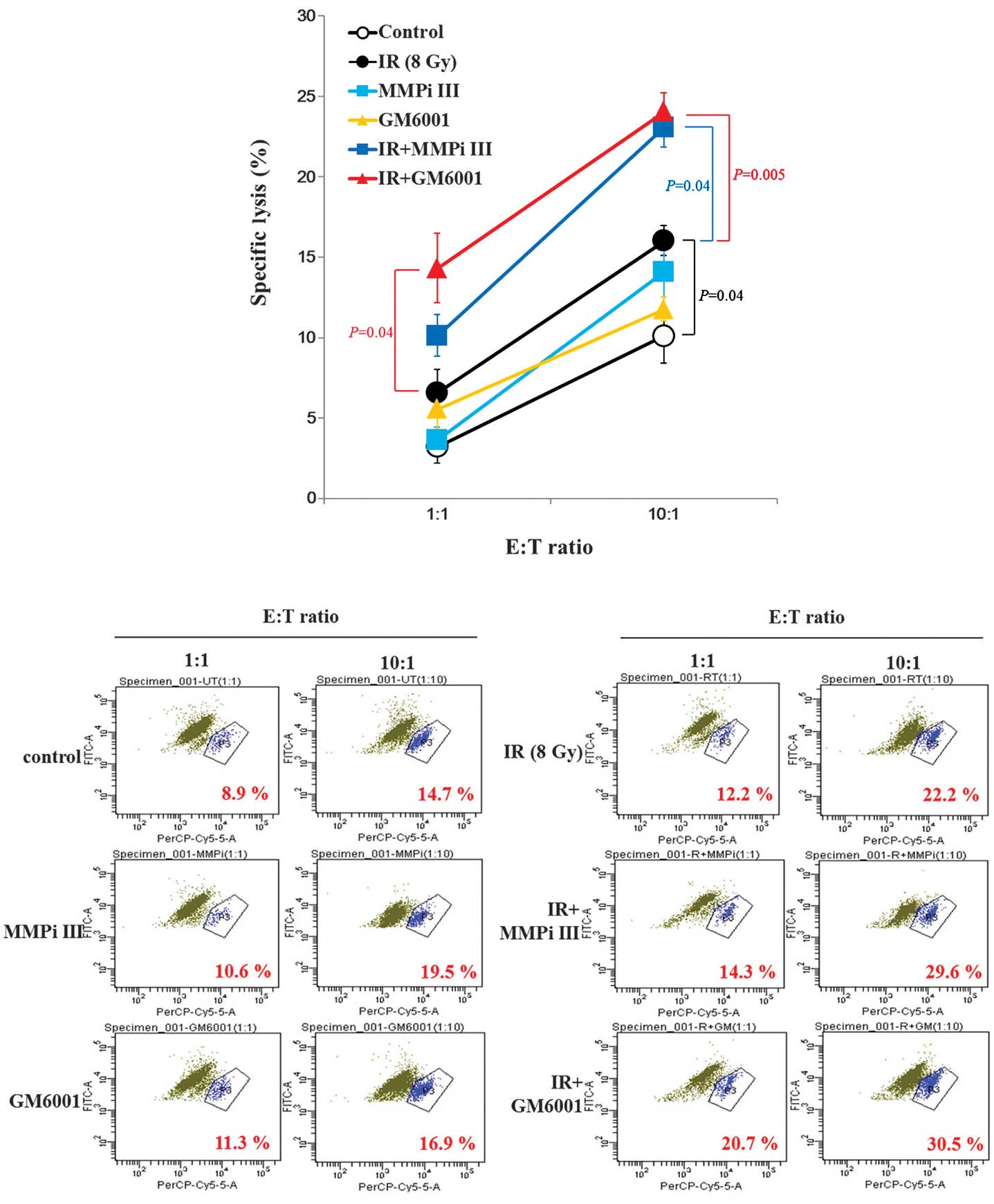
Susceptibility of NCI-H23 cells to NK
cells is synergistically increased by the combination of ionizing
radiation and MMP inhibitors
Consequently, it was determined whether the
co-treatment of MMP inhibitors and irradiation, could increase the
cytotoxic effects of NK cells on cancer cells. The MMP inhibitor
treatment alone did not alter the cytotoxic NK effects and ionizing
radiation treatment alone only minimally increased the cytolysis of
cancer cells. However, the combined treatment synergistically
increased the cytotoxicity of NK cells to NCI-H23 cells (Fig. 5). It remains unclear as to why the
induction of surface expression levels of MICA and MICB, through
the inhibition of MMPs, could not alter the cytotoxicity of NK
cells.
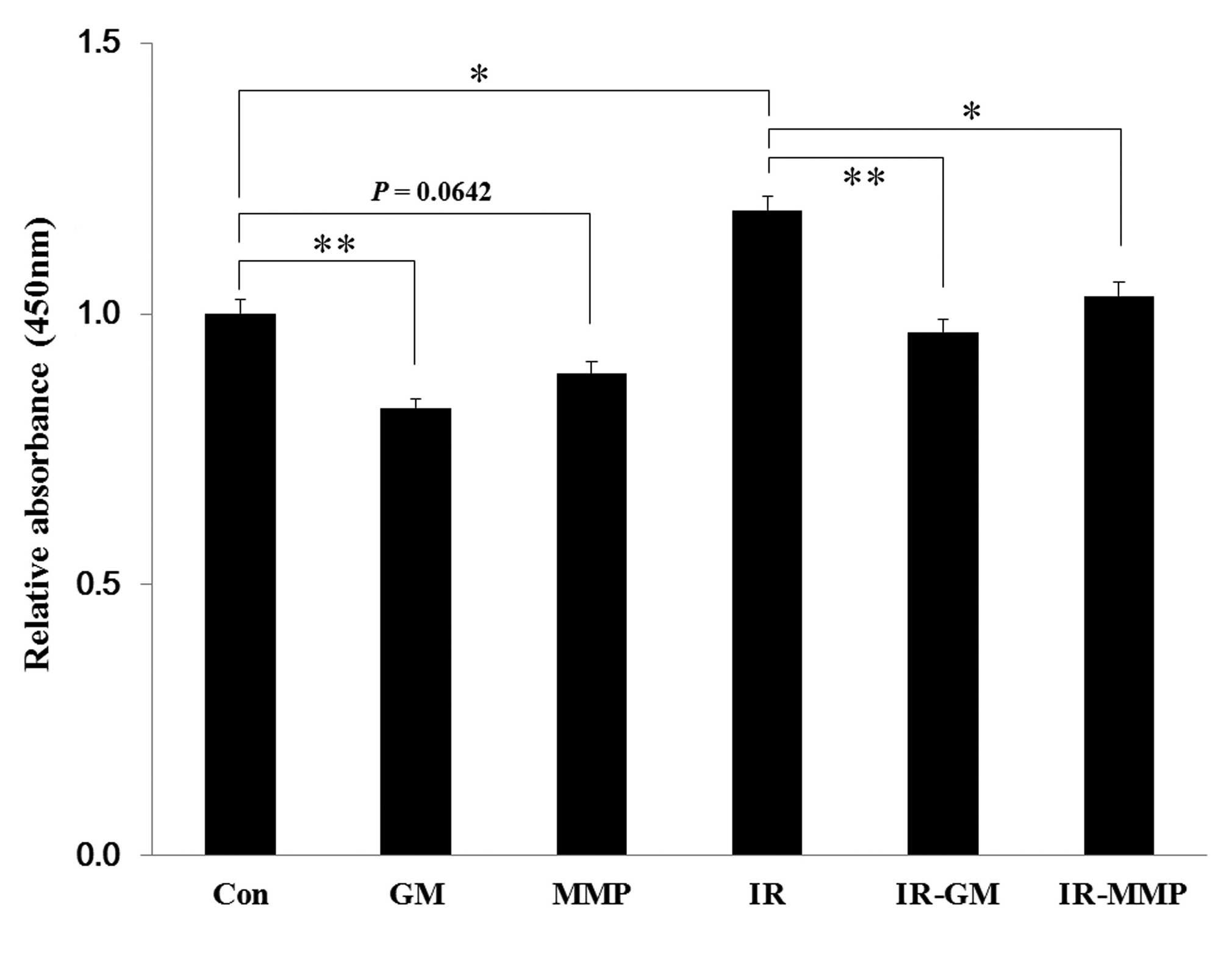
Soluble NKG2D levels are increased by
ionizing radiation, but blocked by MMP inhibitors
It was hypothesized that the increased levels of
surface MICA, by treatment with MMP inhibitors, was due to the
reduction in the shedding of soluble MICA. The levels of soluble
MICA in the supernatants, which were collected from irradiated or
MMP inhibitor-treated NCI-H23 cells, were quantified by ELISA.
Treatment with the MMP inhibitors decreased the levels of soluble
MICA in both the non-irradiated and irradiated samples, and
ionizing radiation treatment alone increased the soluble MICA
levels, as compared with the control (Fig. 6).
Discussion
NK cells exert cytotoxic effects on numerous cancer
cells; however, anti-cancer immunity is generally ineffective
against established cancer cells. Cytotoxicity of NK cells needs to
be potentiated (17) and is
controlled by the balance of signaling between stimulatory and
inhibitory receptors. Therefore, the induction of activating
ligands, such as NKG2DLs, is a potential approach to reactivate NK
cells and increase cytolysis of cancer cells (18). The precise mechanisms of NKG2DL
regulation however, remains unclear. It has previously been
observed that there are discordances between the mRNA and surface
protein expression levels of NKG2DLs, in response to numerous cell
stresses (3,19). Furthermore, the surface protein
levels of NKG2DLs were increased, without alteration of mRNA
expression, by ionizing radiation in A549 lung cancer cells
(20). These results suggest that
the expression levels of NKG2DLs are strictly regulated at the
transcriptional level, as well as the post-transcriptional level.
Prior to the present experiments, ionizing radiation was identified
as being capable of markedly increasing the surface NKG2DLs levels,
this may be through the ATM-ATR pathway at the post-transcriptional
level, despite minimal alterations to the mRNA expression levels,
in radioresistant cells (20).
However, despite the observed significant induction of NKG2DLs, the
enhancement of the susceptibility of the cancer cells to NK cells
was minimal (3). This may be due
to the increased expression levels of soluble NKG2DLs, MHC
molecules and heat shock proteins (HSPs). The downregulation of MHC
molecules and HSPs in previous studies, through the use of histone
deacetylase inhibitors and heat shock factor inhibitors,
respectively, have been shown to further enhance NK cell-mediated
anticancer immunity (20,21). Soluble NKG2DLs are shed through the
proteolysis of extramembrane domains in membrane bound-proteins by
numerous proteases including MMPs and ADAMs, or directly secreted
by exosomes (22). The secreted
and soluble NKG2DLs bind to NKG2D receptors on NKG2D+ immune cells,
including NK cells, and downregulate the surface expression levels
of NKG2D receptors (23).
Eventually, this results in the lack of response of the immune
cells to NKG2DLs+ cancer cells. The reduction of soluble NKG2DLs
through the inhibition of MMPs may be an effective strategy to
minimize the adverse effects of radiotherapy.
The present study aimed to determine whether
ionizing radiation, a potent NKG2DL-inducer, may effectively
enhance the susceptibility of cancer cells to NK cells, with the
assistance of MMP inhibitors, through the reduction of soluble
NKG2DLs. Although the mRNA expression levels of NKG2DLs were not
increased by treatment with two MMP inhibitors, and MMP2 was
upregulated by irradiation in NCI-H23 cells, the surface NKG2DLs
levels synergistically increased, in response to the co-treatment
of ionizing radiation and MMP inhibitors. Simultaneously, the
concentration of soluble NKG2DLs, sMICA and sMICB, were increased
by irradiation but reduced in response to MMP inhibition.
Therefore, the combined effects of treatment of cancer cells with
ionizing radiation and MMP inhibitors, may increase the
cytotoxicity of NK cells.
Ionizing radiation efficiently induced the surface
expression levels of NKG2DLs; however, it also increased the
soluble expression levels of NKG2DLs and suppressed NK activity.
Furthermore, MMPs have a role in cancer invasion and metastasis,
and are associated with a poor prognosis in patients with cancer.
Therefore, when radiotherapy is used in the treatment of patients
with cancer, it may be beneficial to also block the activity of
MMPs. The present study showed that MMP inhibition may further
enhance the efficacy of NK cell-based anticancer immunotherapy,
when combined with radiotherapy.
Acknowledgements
The present study was supported by a grant from the
Pusan National University (2011–2012). Support was also provided by
the National R&D Program, through the Dongnam Institute of
Radiological & Medical Sciences, funded by the Ministry of
Science, ICT and Future Planning (no. 50590-2014). Support was also
provided by the National Research and Development Program for
Cancer Control, Ministry for Health and Welfar, Republic of Korea
(no. 0920050).
References
|
1
|
Friedman EJ: Immune modulation by ionizing
radiation and its implications for cancer immunotherapy. Curr Pharm
Des. 8:1765–1780. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Nakagawa K, Yoshida F, Omori N, Tsunoda T
and Nose T: The effect of radiation therapy combined with natural
killer cells against spontaneous murine fibrosarcoma. Biotherapy.
2:69–75. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kim JY, Son YO, Park SW, et al: Increase
of NKG2D ligands and sensitivity to NK cell-mediated cytotoxicity
of tumor cells by heat shock and ionizing radiation. Exp Mol Med.
38:474–484. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
von Essen CF: Radiation enhancement of
metastasis: a review. Clin Exp Metastasis. 9:77–104. 1991.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wang CJ, Chen HC, Huang EY and Lee SP:
Elective neck irradiation for nasopharyngeal carcinoma. Chang Gung
Med J. 23:387–395. 2000.PubMed/NCBI
|
|
6
|
Davidson B, Goldberg I, Kopolovic J, et
al: MMP-2 and TIMP-2 expression correlates with poor prognosis in
cervical carcinoma - a clinicopathologic study using
immunohistochemistry and mRNA in situ hybridization. Gynecol Oncol.
73:372–382. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ekinci T, Ozbay PO, Yiğit S, Yavuzcan A,
Uysal S and Soylu F: The correlation between immunohistochemical
expression of MMP-2 and the prognosis of epithelial ovarian cancer.
Ginekol Pol. 85:121–130. 2014.PubMed/NCBI
|
|
8
|
Liu WW, Zeng ZY, Wu QL, Hou JH and Chen
YY: Overexpression of MMP-2 in laryngeal squamous cell carcinoma: a
potential indicator for poor prognosis. Otolaryngol Head Neck Surg.
132:395–400. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Boutet P, Agüera-González S, Atkinson S,
et al: Cutting edge: the metalloproteinase
ADAM17/TNF-alpha-converting enzyme regulates proteolytic shedding
of the MHC class I-related chain B protein. J Immunol. 182:49–53.
2009. View Article : Google Scholar
|
|
10
|
Kohga K, Takehara T, Tatsumi T, et al:
Anticancer chemotherapy inhibits MHC class I-related chain a
ectodomain shedding by downregulating ADAM10 expression in
hepatocellular carcinoma. Cancer Res. 69:8050–8057. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lee BK, Kim MJ, Jang HS, et al: A high
concentration of MMP-2/gelatinase A and MMP-9/gelatinase B reduce
NK cell-mediated cytotoxicity against an oral squamous cell
carcinoma cell line. In Vivo. 22:593–597. 2008.PubMed/NCBI
|
|
12
|
Liu G, Atteridge CL, Wang X, Lundgren AD
and Wu JD: The membrane type matrix metalloproteinase MMP14
mediates constitutive shedding of MHC class I chain-related
molecule A independent of A disintegrin and metalloproteinases. J
Immunol. 184:3346–3350. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Sun D, Wang X, Zhang H, Deng L and Zhang
Y: MMP9 mediates MICA shedding in human osteosarcomas. Cell Biol
Int. 35:569–574. 2011. View Article : Google Scholar
|
|
14
|
Park SW, Bae JH, Kim SD, et al: Comparison
of level of NKG2D ligands between normal and tumor tissue using
multiplex RT-PCR. Cancer Invest. 25:299–307. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Eissmann P, Evans JH, Mehrabi M, Rose EL,
Nedvetzki S and Davis DM: Multiple mechanisms downstream of TLR-4
stimulation allow expression of NKG2D ligands to facilitate
macrophage/NK cell crosstalk. J Immunol. 184:6901–6909. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Gasser S, Orsulic S, Brown EJ and Raulet
DH: The DNA damage pathway regulates innate immune system ligands
of the NKG2D receptor. Nature. 436:1186–1190. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lanier LL: NK cell recognition. Annu Rev
Immunol. 23:225–274. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ljunggren HG and Malmberg KJ: Prospects
for the use of NK cells in immunotherapy of human cancer. Nat Rev
Immunol. 7:329–339. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
19
|
Bae JH, Kim JY, Kim MJ, et al: Quercetin
enhances susceptibility to NK cell-mediated lysis of tumor cells
through induction of NKG2D ligands and suppression of HSP70. J
Immunother. 33:391–401. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Son CH, Keum JH, Yang K, et al:
Synergistic enhancement of NK cell-mediated cytotoxicity by
combination of histone deacetylase inhibitor and ionizing
radiation. Radiat Oncol. 9:492014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kim JY, Bae JH, Lee SH, et al: Induction
of NKG2D ligands and subsequent enhancement of NK cell-mediated
lysis of cancer cells by arsenic trioxide. J Immunother.
31:475–486. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Chitadze G, Bhat J, Lettau M, Janssen O
and Kabelitz D: Generation of soluble NKG2D ligands: proteolytic
cleavage, exosome secretion and functional implications. Scand J
Immunol. 78:120–129. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Groh V, Wu J, Yee C and Spies T:
Tumour-derived soluble MIC ligands impair expression of NKG2D and
T-cell activation. Nature. 419:734–738. 2002. View Article : Google Scholar : PubMed/NCBI
|