Introduction
Neural stem cells (NSCs) are a subtype of stem cells
in the nervous system that are able to self-renew and generate
neurons and glia (1). Although the
location of NSCs in the adult brain and the brain regions to which
their progeny migrate in order to differentiate remain to be fully
elucidated, it is hypothesized that the majority of NSCs are
located in the subventricular zone of the forebrain and the
subgranular layer of the hippocampal dentate gyrus in the adult
mammalian brain (2). Under certain
pathophysiological conditions, NSCs are activated to generate
progenitor cells, which then migrate to the subventricular zone
where they differentiate and replace degenerate neural cells
(1,2). Although the therapeutic application
of NSCs is limited due to the relatively low regenerative capacity
of the adult central nervous system and the difficulties associated
with isolating patient NSCs, NSC-based approaches have been
considered for the potential treatment of neurodegenerative
disorders, ischemic stroke and cerebral traumatic injury.
Therefore, identifying efficient endogenous or exogenous factors,
which enhance the survival and growth of NSCs may be important for
NSC-based therapeutic approaches.
Previous studies have demonstrated that adipose
tissue is not only a depot of lipid, but is also an active
endocrine organ, producing biologically active substances termed
‘adipokines’, including leptin, adiponectin, resistin and
angiotensin (3–7). Omentin is a novel adipokine, which is
expressed in and released from omental adipose tissue (8,9).
Using a large-scale in situ hybridization screening method,
the omentin gene has been cloned in small intestinal paneth cells
in mice (10). Notably, this gene
was found to regulate insulin-stimulated glucose uptake in human
adipocytes (8–9). Omentin can be detected in human blood
with a physiological concentration ranging between 300 and 600
ng/ml (11,12). In patients with obesity (12) or obesity-linked disorders,
including type 2 diabetes (13),
endothelial dysfunction (14),
carotid atherosclerosis (15) and
coronary artery disease (16), the
blood omentin levels are decreased. However, few studies have
investigated the role of omentin in the nervous system. Brunetti
et al demonstrated that injection of omentin into the
arcuate nucleus of the hypothalamus did not modify hypothalamic
feeding behavior-associated peptide gene expression (17). In the present study, the effect of
omentin on NSCs was investigated.
Materials and methods
Animals
Pregnant C57BL/6J mice were purchased from Vital
River Laboratories Animals Technology Co., Ltd. (Beijing, China).
All experiments were performed according to the guidelines of the
Experimental Animal Care Committee of Jilin University (Changchun,
China).
Reagents
Recombinant human omentin-1 and LY294002 were
purchased from Enzo Life Sciences, Inc. (Farmingdale, NY, USA).
Mouse monoclonal antibody against nestin, rabbit monoclonal
antibody against vimentin and rabbit polyclonal antibody against
SIRT1 were purchased from Abcam (Cambridge, UK). Mouse monoclonal
antibody against phospho-AktThr308, rabbit polyclonal
antibody against total-Akt (t-Akt), rabbit monoclonal antibody
against phospho-AS160Ser318 and rabbit polyclonal
antibody total-AS160 (t-AS160) were purchased from Cell Signaling
Technology, Inc. (Beverly, MA, USA). Mouse monoclonal antibody
against tubulin was obtained from Sigma-Aldrich (St. Louis, MO,
USA). Tumor necrosis factor (TNF)-α, brain-derived neurotrophic
factor (BDNF) and glial cell line-derived neurotrophic factor
(GDNF) were purchased from PeproTech, Inc. (Rocky Hill, NJ, USA).
The Cell Counting kit (CCK)-8 viability assay was obtained from
Dojindo Molecular Technologies, Inc. (Kumamoto, Japan). DAPI was
purchased from Invitrogen Life Technologies (Carlsbad, CA, USA).
Enhanced chemiluminescence for western blot analysis was purchased
from Pierce Biotechnology, Inc. (Rockford, IL, USA).
NSC isolation and culture
NSC isolation and culture was performed, as
described previously (18).
Briefly, the cortex was dissected and triturated until a single
cell suspension was obtained. The cells were then grown in
non-adherent conditions in NeuroCult® NSC Basal Medium
(mouse; Stem Cell Technologies, Vancouver, Canada) with 20 ng/ml
fibroblast growth factor 2 and 20 ng/ml epidermal growth factor
(PeproTech, Inc.) for 7 days to enable the formation of
neurospheres. Neurospheres were passaged every 5 days. For
subcloning, the neurospheres were collected and dissociated using
papain for 20 min at 37°C by gentle agitation. The cells were
replated at the same cell density (5×105/l) for each
condition. At the time points indicated in the Figures, the number
and size of the neurospheres and the total number of cells were
analyzed.
Immunochemistry
Immunochemistry was performed, as described
previously (19). Briefly, the
neurospheres were seeded onto poly-l-lysine-coated coverslips
(Sigma-Aldrich) for 5 days and then fixed using 4% paraformaldehyde
(Sigma-Aldrich) for 30 min at room temperature prior to
immunostaining (20). The cells
were treated with 0.1% Triton X-100 (Sigma-Aldrich) at room
temperature for 20 min, followed by incubation with an antibody
against nestin or vimentin at 37°C for 2 h and incubation with
Alexa Fluor 488 and Alexa Fluor 555-conjugated secondary antibodies
at room temperature for 1 h (21).
The cell nuclei were counterstained using DAPI and visualized under
a fluorescent microscope (Olympus IX71; Olympus, Tokyo, Japan).
Assessment of NSC survival
The survival of NSCs was evaluated using a
non-radioactive CCK-8 assay, as described previously (22,23).
The neurospheres were treated with omentin (100 ng/ml or 1 μg/ml),
TNF-α (100 ng/ml) or LY294002 (10 μM) (24) for the indicated time periods. The
medium was then discarded and the cells were incubated with 10 μl
CCK-8 solution for 1 h at 37°C. The optical density at 450 nm was
analyzed using a microplate reader (Infinite 200; Tecan, Männedorf,
Switzerland). The experiments were performed in duplicate.
Western blotting
Western blotting was performed, as described
previously (25,26). Briefly, the cells were lysed using
lysis buffer (50 mM Tris; pH 7.4, 150 mM sodium chloride, 1%
Nonidet P-40, 1 mM EDTA, 1 mM sodium orthovanate, 1 mM sodium
fluoride, 1 mg/ml leupeptin, 1 mg/ml aprotinin, 1 mg/ml pepstatin A
and 1 mM phenylmethanesulfonyl fluoride (Sigma-Aldrich) (27). The cell lysates were centrifuged at
10,000 × g for 10 min and the supernatants were collected. The
samples were then boiled for 10 min. The protein concentration was
determined using a bicinchoninic acid assay (28). Equal quantities of the proteins
were separated using 10% SDS-PAGE and transferred onto
nitrocellulose membranes using standard procedures (29). The membranes were incubated
overnight at 4°C with antibodies against p-Akt (1:200), t-Akt
(1:500), SIRT1 (1:250) or tubulin (1:1,000). Following incubation
with the corresponding secondary antibodies (1:5,000), the
membranes were washed three times and the bands were detected using
the enhanced chemiluminescence kit (30).
Statistical analysis
All statistical analyses were performed using the
GraphPad Prism 5 software program (GraphPad Software, Inc., La
Jolla, CA, USA). Data are expressed as the mean ± standard error of
the mean and were compared using analysis of variance with Tukey’s
correction test. P<0.05 was considered to indicate a
statistically significant difference.
Results
Omentin promotes NSC growth in a
dose-dependent manner
In the present study, the neurospheres were
initially stained using two well-accepted NSC markers, nestin and
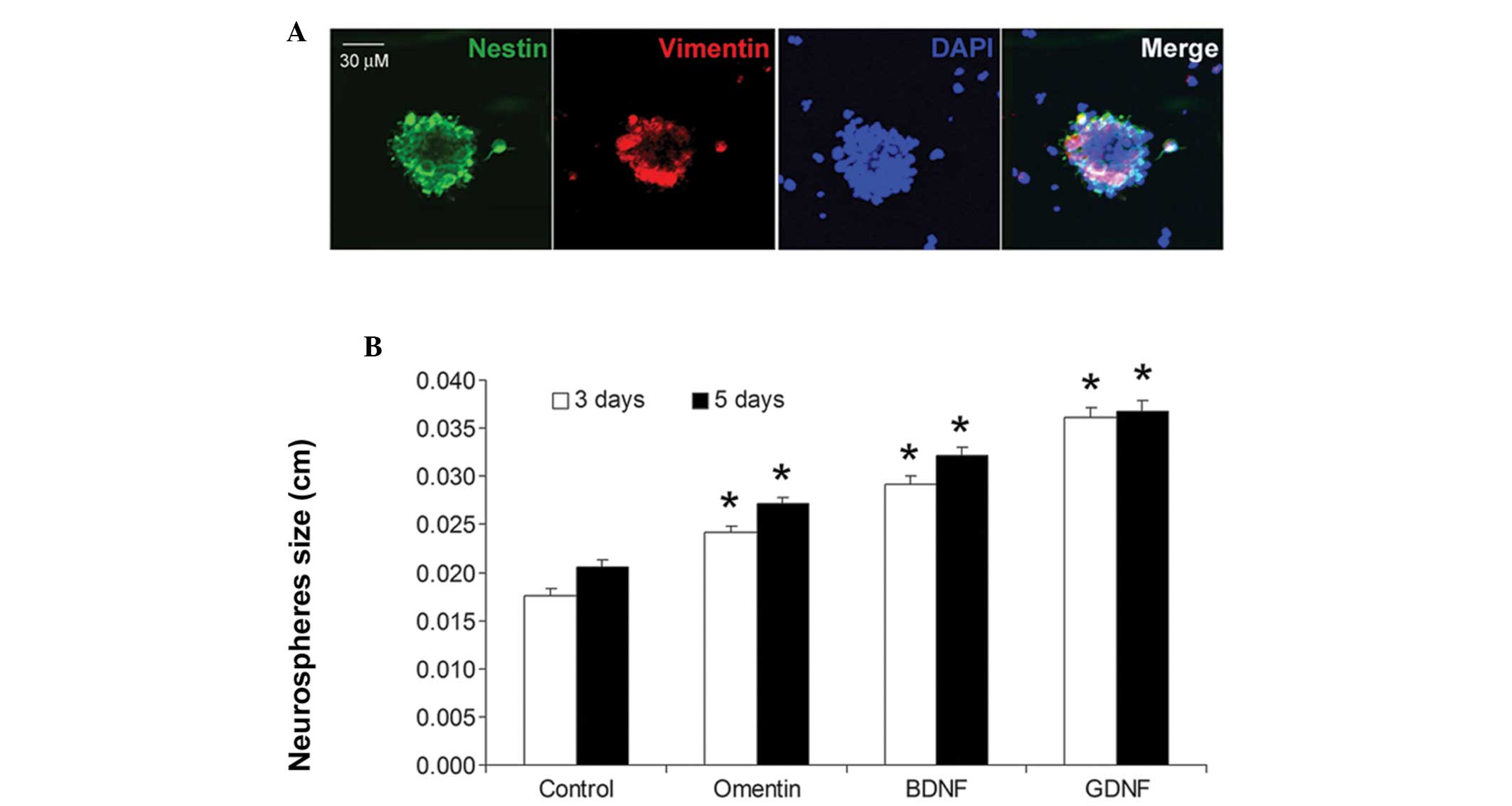
vimentin. As shown in Fig. 1A, the
neurospheres were positive for nestin and vimentin staining,
matching the NSC phenotype. Notably, treatment with omentin (100
ng/ml) for 3 and 5 days significantly increased the size of the NSC
neurospheres (P<0.01; Fig. 1B).
The proliferative effects on the NSCs were also compared between
omentin and two other neurotrophic factors, BDNF and GDNF. The
proliferative effect of omentin on the NSCs was weaker compared
with those of BDNF and GDNF (Fig.
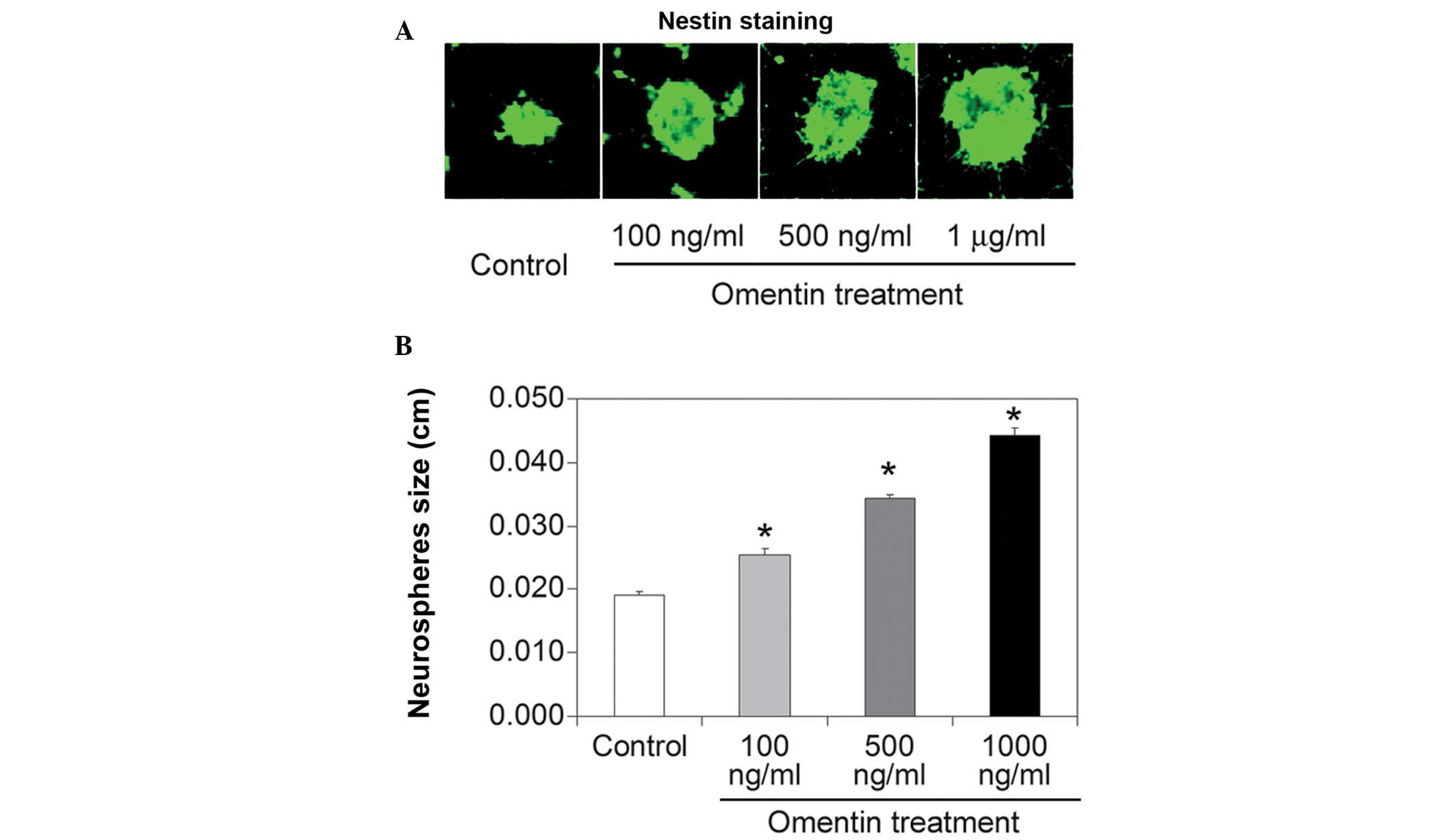
1B). The effect of different concentrations of omentin (100 and
500 ng/ml and 1 μg/ml) on neurosphere size was also investigated.
All three concentrations of omentin increased neurosphere size
(Fig. 2A and B), however, the
proliferative effect of omentin was most significant at the highest
concentration (1 μg/ml). These results indicated that omentin
promoted NSC growth in a dose-dependent manner.
Omentin increases NSC viability in normal
conditions and conditions of stress
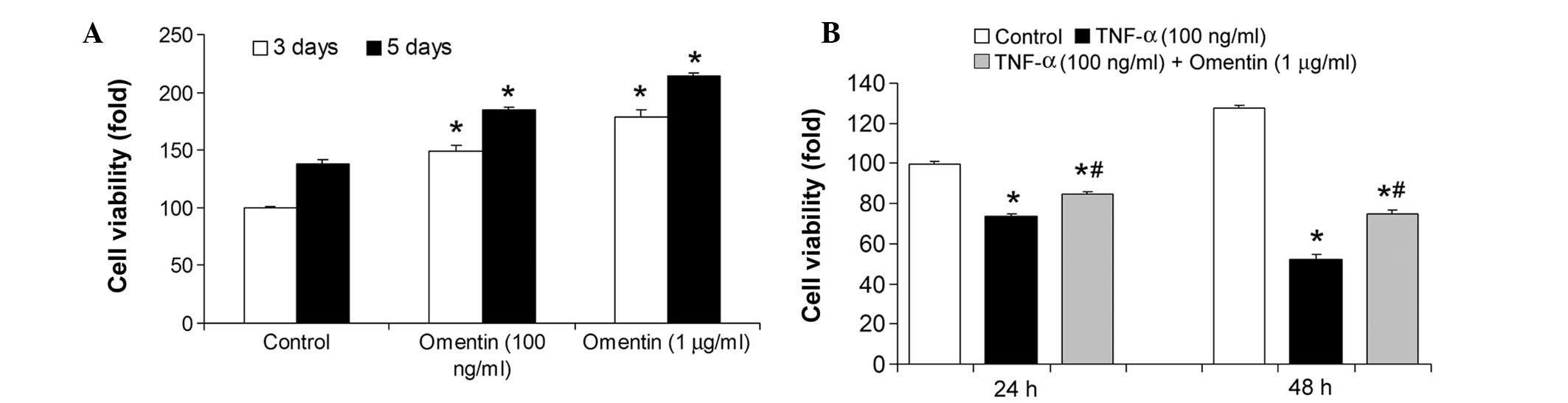
The present study then investigated the effect of
omentin on NSC viability. Two concentrations of omentin (100 ng/ml
and 1 μg/ml) were supplemented for 3 and 5 days. The viability of
the NSCs was significantly increased (P<0.01) at these
concentrations of omentin (Fig.
3A). The ability of omentin to protect NSCs under conditions of
stress was also investigated. TNF-α, a pro-inflammatory cytokine,
caused a marked decrease in cell viability, which was partly
inhibited by omentin (Fig. 3B).
These results indicate that omentin increased NSC viability in
normal conditions and conditions of stress.
Omentin activates Akt signaling in
NSCs
Since Akt is a serine/threonine protein kinase,
which is an important regulator of cell survival and proliferation
(31), the present study
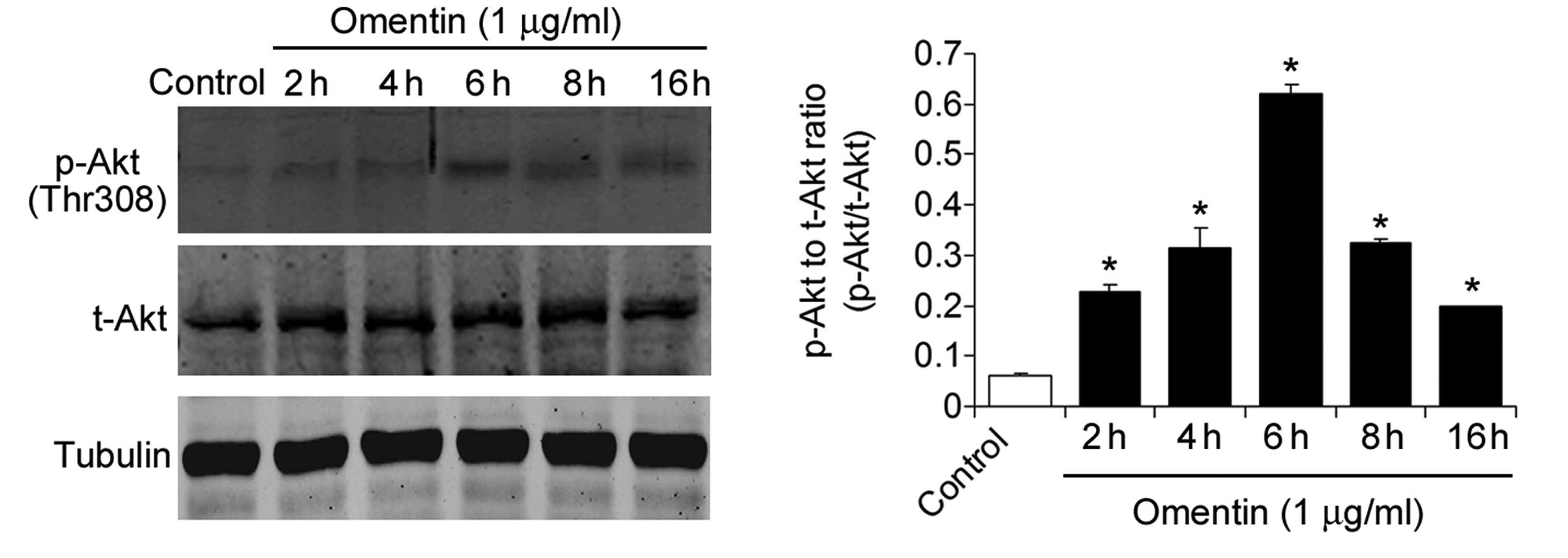
investigated the effect of omentin on Akt signaling. The NSCs were
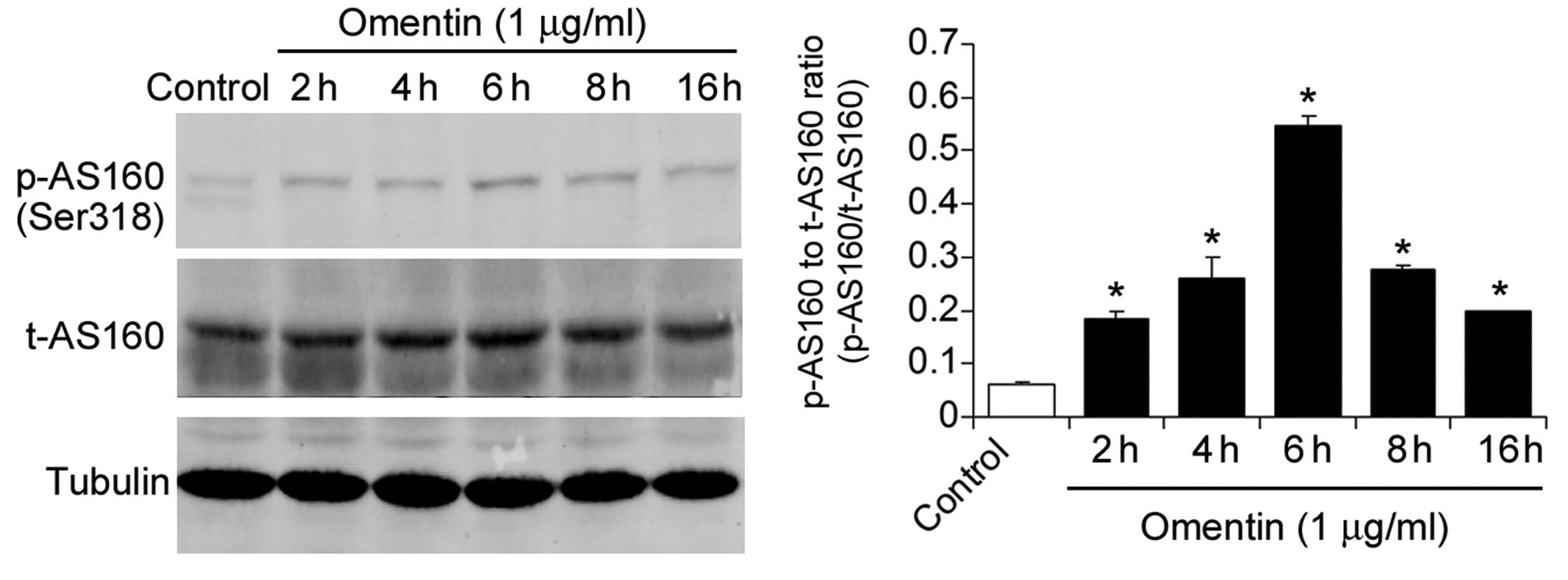
treated with omentin (1 μg/ml) for 2, 4, 6, 8 and 16 h. Omentin
treatment significantly increased the level of
phospho-AktThr308, peaking at 6 h (Fig. 4). Furthermore, the phosphorylation
of AS160, the substrate of phosphorylated Akt was measured.
Treatment with omentin also significantly increased the level of
phospho-AS160Ser318, peaking at 6 h (Fig. 5). These results suggested that
omentin activated Akt signaling in the NSCs.
Activation of Akt signaling by omentin is
required for its pro-survival effect on NSCs
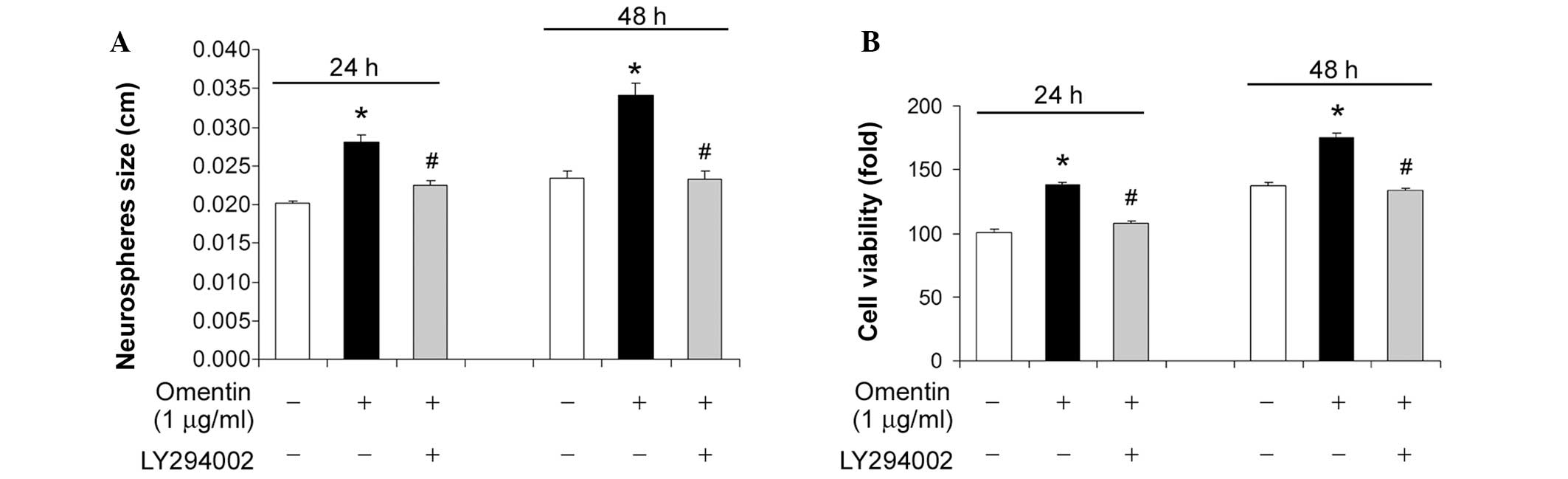
To evaluate the importance of Akt signaling by
omentin, LY294002, a specific inhibitor of Akt signaling, was used.
Inhibition of Akt signaling by treatment with LY294002 (10 μM for
24 and 48 h) markedly suppressed the increase in neurosphere size
promoted by omentin (Fig. 6A).
Similarly, LY294002 eradicated the promoting effect of omentin on
NSC viability (Fig. 6B). These
results indicated that the activation of Akt signaling by omentin
was required for its pro-survival effect on NSCs.
Discussion
The present study demonstrated that omentin promoted
the growth of NSCs and protected them against inflammatory
factor-induced damage. Additional investigation revealed that
omentin markedly increased Akt phosphorylation (Thr308) and AS160
phosphorylation (Ser318) in NSCs. Treatment with the PI3K/Akt
inhibitor, LY294002, in NSCs eliminated the promoting effect of
omentin.
To the best of our knowledge, the present study is
the first to provide evidence that omentin promotes NSC growth. As
an adipokine released by adipose tissue, omentin has been observed
to exhibit proliferating and anti-proliferative effects in certain
types of tissues/cells (9).
Omentin is present in the fetus and neonate, the concentration of
which is higher than that in maternal serum (32), suggesting it may be crucial to
enhance a growth-promoting effect. Xie et al demonstrated
that omentin (25–200 ng/ml) stimulates proliferation and inhibits
differentiation in primary mouse osteoblasts (33). In addition, omentin was
demonstrated to inhibit matrix mineralization in osteoblasts
(33) and 25–200 ng/ml omentin has
been found to promote the proliferation of human osteoblasts
through the PI3K/Akt signaling pathway (34). By contrast, Zhang and Zhou provided
evidence that omentin upregulates p53 to induce apoptosis in human
hepatocellular carcinoma cells (35). In the present study, the effects of
three different concentrations of omentin (100 and 500 ng/ml and 1
μg/ml) in NSCs were assessed. All three concentrations of omentin
had a significant promoting effect on the growth of the NSCs in
vitro. Of note, these concentrations were similar to the
physiological concentration (300–600 ng/ml) (11,12).
Therefore, the neurotrophic effect of omentin under physiological
conditions may be important in the development and maintenance of
NSCs. In addition, these results provide new information regarding
the potential inherent connection between adipose and brain
tissues.
The present study also identified that the
activation of Akt signaling by omentin underlies the molecular
mechanism of the promoting effect of omentin on NSCs. Previously,
it has been found that omentin is able to trigger intracellular
signaling pathways, among which the Akt signaling pathway has been
extensively investigated (34,36).
In human adipocytes, omentin increases Akt phosphorylation in the
absence and presence of insulin (9). Omentin inhibits the osteoblastic
differentiation of calcifying vascular smooth muscle cells and
attenuates the arterial calcification and loss of bone observed in
osteoprotegerin-deficient mice through the PI3K/Akt pathway
(33,36). Additionally, omentin stimulates
endothelial cell function and ischemia-induced revascularization
through its ability to stimulate the Akt-endothelial nitric oxide
synthase (eNOS) signaling pathway (37). A previous study reported that
omentin did not induce Akt phosphorylation in endothelial cells
(38). The present study observed
significant increases in the expression of Akt and its downstream
factor AS160 in NSCs. Inhibition of PI3K/Akt signaling by LY294002
eliminated the promoting effect of omentin on NSCs, suggesting that
the activation of Akt signaling is essential for its promoting
effect on NSCs. In addition, these results support the hypothesis
that Akt mediates the pro-survival effect of omentin in NSCs.
Previous studies have demonstrated that omentin activates other
signaling pathways, including the extracellular signal-regulated
kinase (39), AMP-activated
protein kinase (40), c-Jun
N-terminal kinase (40) and p38
(41) signaling pathways. These
findings indicate that omentin may have multiple receptors to
induce diverse intracellular signaling cascades, for which further
investigation is warranted.
In conclusion, the present study demonstrated for
the first time, to the best of our knowledge, that omentin promotes
the growth and survival of NSCs in vitro through activation
of the Akt signaling pathway. These results may improve current
understanding on the role of omentin in the nervous system.
Acknowledgements
This study was supported by the National Natural
Science Foundation of China (grant no. 81272999).
References
|
1
|
Temple S: The development of neural stem
cells. Nature. 414:112–117. 2001. View
Article : Google Scholar : PubMed/NCBI
|
|
2
|
Doetsch F, Caillé I, Lim DA,
García-Verdugo JM and Alvarez-Buylla A: Subventricular zone
astrocytes are neural stem cells in the adult mammalian brain.
Cell. 97:703–716. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Maury E and Brichard SM: Adipokine
dysregulation, adipose tissue inflammation and metabolic syndrome.
Mol Cell Endocrinol. 314:1–16. 2010. View Article : Google Scholar
|
|
4
|
Guerre-Millo M: Adipose tissue and
adipokines: for better or worse. Diabetes Metab. 30:13–19. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Markofski MM, Carrillo AE, Timmerman KL,
et al: Exercise training modifies ghrelin and adiponectin
concentrations and is related to inflammation in older adults. J
Gerontol A Biol Sci Med Sci. 69:675–681. 2014. View Article : Google Scholar
|
|
6
|
Brown-Borg HM and Bartke A: GH and IGF1:
roles in energy metabolism of long-living GH mutant mice. J
Gerontol A Biol Sci Med Sci. 67:652–660. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Hozawa A, Sugawara Y, Tomata Y, et al:
Relationship between serum adiponectin levels and disability-free
survival among community-dwelling elderly individuals: The
Tsurugaya Project. J Gerontol A Biol Sci Med Sci. 67:530–536. 2012.
View Article : Google Scholar
|
|
8
|
Schaffler A, Neumeier M, Herfarth H, Furst
A, Scholmerich J and Buchler C: Genomic structure of human omentin,
a new adipocytokine expressed in omental adipose tissue. Biochim
Biophys Acta. 1732:96–102. 2005. View Article : Google Scholar
|
|
9
|
Yang RZ, Lee MJ, Hu H, et al:
Identification of omentin as a novel depot-specific adipokine in
human adipose tissue: possible role in modulating insulin action.
Am J Physiol Endocrinol Metab. 290:E1253–E1261. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Komiya T, Tanigawa Y and Hirohashi S:
Cloning of the novel gene intelectin, which is expressed in
intestinal paneth cells in mice. Biochem Biophys Res Commun.
251:759–762. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
de Souza Batista CM, Yang RZ, Lee MJ, et
al: Omentin plasma levels and gene expression are decreased in
obesity. Diabetes. 56:1655–1661. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Shibata R, Takahashi R, Kataoka Y, et al:
Association of a fat-derived plasma protein omentin with carotid
artery intima-media thickness in apparently healthy men. Hypertens
Res. 34:1309–1312. 2012. View Article : Google Scholar
|
|
13
|
Pan HY, Guo L and Li Q: Changes of serum
omentin-1 levels in normal subjects and in patients with impaired
glucose regulation and with newly diagnosed and untreated type 2
diabetes. Diabetes Res Clin Pract. 88:29–33. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Moreno-Navarrete JM, Ortega F, Castro A,
Sabater M, Ricart W and Fernandez-Real JM: Circulating omentin as a
novel biomarker of endothelial dysfunction. Obesity (Silver
Spring). 19:1552–1559. 2011. View Article : Google Scholar
|
|
15
|
Liu R, Wang X and Bu P: Omentin-1 is
associated with carotid atherosclerosis in patients with metabolic
syndrome. Diabetes Res Clin Pract. 93:21–25. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Shang FJ, Wang JP, Liu XT, et al: Serum
omentin-1 levels are inversely associated with the presence and
severity of coronary artery disease in patients with metabolic
syndrome. Biomarkers. 16:657–662. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Brunetti L, Di Nisio C, Recinella L, et
al: Effects of vaspin, chemerin and omentin-1 on feeding behavior
and hypothalamic peptide gene expression in the rat. Peptides.
32:1866–1871. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Agostini M, Tucci P, Chen H, et al: p73
regulates maintenance of neural stem cell. Biochem Biophys Res
Commun. 403:13–17. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Guo R, Hu N, Kandadi MR and Ren J:
Facilitated ethanol metabolism promotes cardiomyocyte contractile
dysfunction through autophagy in murine hearts. Autophagy.
8:593–608. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Lev N, Barhum Y, Pilosof NS, et al: DJ-1
protects against dopamine toxicity: implications for Parkinson’s
disease and aging. J Gerontol A Biol Sci Med Sci. 68:215–225. 2013.
View Article : Google Scholar
|
|
21
|
Kumar V, Atherton PJ, Selby A, et al:
Muscle protein synthetic responses to exercise: effects of age,
volume, and intensity. J Gerontol A Biol Sci Med Sci. 67:1170–1177.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wang P, Xu TY, Guan YF, Su DF, Fan GR and
Miao CY: Perivascular adipose tissue-derived visfatin is a vascular
smooth muscle cell growth factor: role of nicotinamide
mononucleotide. Cardiovasc Res. 81:370–380. 2009. View Article : Google Scholar
|
|
23
|
Wang P, Guan YF, Du H, Zhai QW, Su DF and
Miao CY: Induction of autophagy contributes to the neuroprotection
of nicotinamide phosphoribosyltransferase in cerebral ischemia.
Autophagy. 8:77–87. 2012. View Article : Google Scholar
|
|
24
|
Tucsek Z, Gautam T, Sonntag WE, et al:
Aging exacerbates microvascular endothelial damage induced by
circulating factors present in the serum of septic patients. J
Gerontol A Biol Sci Med Sci. 68:652–660. 2013. View Article : Google Scholar :
|
|
25
|
Wang P, Zhang RY, Song J, et al: Loss of
AMP-activated protein kinase-alpha2 impairs the insulin-sensitizing
effect of calorie restriction in skeletal muscle. Diabetes.
61:1051–1061. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Wang P, Xu TY, Guan YF, et al:
Nicotinamide phosphoribosyltransferase protects against ischemic
stroke through SIRT1-dependent adenosine monophosphate-activated
kinase pathway. Ann Neurol. 69:360–374. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Oh JM, Choi EK, Carp RI and Kim YS:
Oxidative stress impairs autophagic flux in prion protein-deficient
hippocampal cells. Autophagy. 8:1448–1461. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Conte TC, Silva LH, Silva MT, et al: The
beta2-adrenoceptor agonist formoterol improves structural and
functional regenerative capacity of skeletal muscles from aged rat
at the early stages of postinjury. J Gerontol A Biol Sci Med Sci.
67:443–455. 2012. View Article : Google Scholar
|
|
29
|
Zhang T, Li Y, Park KA, et al:
Cucurbitacin induces autophagy through mitochondrial ROS production
which counteracts to limit caspase-dependent apoptosis. Autophagy.
8:559–576. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Song YM, Song SO, Jung YK, et al: Dimethyl
sulfoxide reduces hepatocellular lipid accumulation through
autophagy induction. Autophagy. 8:1085–1097. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Song G, Ouyang G and Bao S: The activation
of Akt/PKB signaling pathway and cell survival. J Cell Mol Med.
9:59–71. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Briana DD, Boutsikou M, Baka S, et al:
Omentin-1 and vaspin are present in the fetus and neonate, and
perinatal concentrations are similar in normal and
growth-restricted pregnancies. Metabolism. 60:486–490. 2011.
View Article : Google Scholar
|
|
33
|
Xie H, Xie PL, Wu XP, et al: Omentin-1
attenuates arterial calcification and bone loss in
osteoprotegerin-deficient mice by inhibition of RANKL expression.
Cardiovasc Res. 92:296–306. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Wu SS, Liang QH, Liu Y, Cui RR, Yuan LQ
and Liao EY: Omentin-1 stimulates human osteoblast proliferation
through PI3K/Akt signal pathway. Int J Endocrinol. 2013:3689702013.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Zhang YY and Zhou LM: Omentin-1, a new
adipokine, promotes apoptosis through regulating Sirt1-dependent
p53 deacetylation in hepatocellular carcinoma cells. Eur J
Pharmacol. 698:137–144. 2013. View Article : Google Scholar
|
|
36
|
Duan XY, Xie PL, Ma YL and Tang SY:
Omentin inhibits osteoblastic differentiation of calcifying
vascular smooth muscle cells through the PI3K/Akt pathway. Amino
Acids. 41:1223–1231. 2011. View Article : Google Scholar
|
|
37
|
Maruyama S, Shibata R, Kikuchi R, et al:
Fat-derived factor omentin stimulates endothelial cell function and
ischemia-induced revascularization via endothelial nitric oxide
synthase-dependent mechanism. J Biol Chem. 287:408–417. 2012.
View Article : Google Scholar :
|
|
38
|
Yamawaki H, Tsubaki N, Mukohda M, Okada M
and Hara Y: Omentin, a novel adipokine, induces vasodilation in rat
isolated blood vessels. Biochem Biophys Res Commun. 393:668–672.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Zhong X, Li X, Liu F, Tan H and Shang D:
Omentin inhibits TNF-alpha-induced expression of adhesion molecules
in endothelial cells via ERK/NF-kappaB pathway. Biochem Biophys Res
Commun. 425:401–406. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Yamawaki H, Kuramoto J, Kameshima S, Usui
T, Okada M and Hara Y: Omentin, a novel adipocytokine inhibits
TNF-induced vascular inflammation in human endothelial cells.
Biochem Biophys Res Commun. 408:339–343. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Kazama K, Usui T, Okada M, Hara Y and
Yamawaki H: Omentin plays an anti-inflammatory role through
inhibition of TNF-alpha-induced superoxide production in vascular
smooth muscle cells. Eur J Pharmacol. 686:116–123. 2012. View Article : Google Scholar : PubMed/NCBI
|