Introduction
Malignant glioma is the most common type of primary
brain tumor in adults and is associated with a disproportionately
high morbidity and mortality (1).
Despite advances in understanding the molecular pathogenesis of
malignant glioma, the prognosis and therapy of this tumor type
remains poor. A subpopulation of glioma cells, termed
glioma-initiating cells (GICs), which are brain tumor-initiating
cells or glioma stem cells, has been previously identified in
glioma (2–4). These cells have the ability to
undergo self-renewal and initiate tumorigenesis. They are
considered to be responsible for the initiation, propagation and
recurrence of tumors. In addition, it appears that tumor stem-like
cells may be resistant to numerous conventional cancer therapies,
which may explain the limitations of traditional agents in curing
human malignancy (4,5). Therefore, therapeutic strategies
aimed at targeting these cells may be more effective in providing
durable responses.
Salinomycin is a polyether antibiotic isolated from
Streptomyces albus, which acts as an ionophore with a high
affinity for potassium (6,7). In addition to its well-established
antimicrobial activities, it has previously been demonstrated to
act as a specific inhibitor of breast cancer stem cells (8). Although the mechanism of action of
salinomycin remains to be elucidated, it has been reported that
salinomycin may serve as a permeability-glycoprotein inhibitor,
thus impairing the viability of cancer stem cells (9). By contrast, salinomycin has also been
demonstrated to induce apoptosis in cancer cells and overcome
apoptotic resistance in human breast cancer cells (10). Nevertheless, the activity of
salinomycin in growth suppression and tumorsphere formation in
glioma, particularly GICs, remains to be elucidated.
In the present study, the in vitro and in
vivo effects of salinomycin on GL261 glioma cells were
investigated. The present study may provide valuable insights into
understanding the pathogenesis of GICs and may offer a novel
therapeutic approach for the treatment of human malignant
glioma.
Materials and methods
Reagents
Dulbecco’s modified Eagle’s medium (DMEM)/F12
culture medium was purchased from Life Technologies (Gaithersburg,
MD, USA). Fetal bovine serum (FBS) and B27 supplement were
purchased from Gibco-BRL (Grand Island, NY, USA). Basic fibroblast
growth factor (bFGF) and epidermal growth factor (EGF) were
obtained from PeproTech (Rocky Hill, NJ, USA). Normal goat serum
was provided by Wuhan Boshide Biotechnology Co., Ltd. (Wuhan,
China). Poly-L-lysine, 4′,6-diamidino-2-phenylindole (DAPI),
fluorescein isothiocyanate (FITC)-conjugated goat anti-rabbit
antibody and Cy3-conjugated goat anti-rabbit antibody were provided
by Sigma (St. Louis, MO, USA). Rabbit anti-mouse CD133 monoclonal
antibody and glial fibrillary acidic protein (GFAP) polyclonal
antibody were purchased from Abcam (Cambridge, MA, USA) and
Zhongshan Jinqiao Biotechnology, Ltd., (Beijing, China),
respectively. Anti-caspase-3 antibody (rabbit polyclonal against
mouse, rat or human) and anti-β-actin antibody were obtained from
Abcam Inc. (Cambridge, MA, USA). Cell Counting kit-8 (CCK-8) was
purchased from Dojindo Laboratories (Tokyo, Japan).
Cell culture
GL261 cells were obtained from the American Type
Culture Collection (ATCC, Manassas, VA, USA) and were cultured in
DMEM/F12 culture medium containing 10% FBS. Cells were maintained
at 37°C in a humidified environment containing 5% CO2.
The medium was changed every 4 days. To induce the formation of
neurospheres (NS), FBS was gradually withdrawn from culture medium
in a gradient reduction pattern (10, 5, 2 and 0%). Cells were then
maintained in serum free DMEM/F12 medium supplemented with 20 ng/ml
bFGF, 20 ng/ml EGF, B27 (1X), 2 mM L-glutamine and 4 U/l insulin.
The floating cells formed NS-like clones and secondary spheres
derived from single cells of these clones were used to induce cell
differentiation. To induce differentiation, the GL261-NS cells were
seeded onto coverslips and cultured in DMEM/F12 culture medium
containing 10% FBS. These cells were defined as GL261 adherent
cells (AC).
Immunocytochemistry analysis
The spheres were placed onto coverslips precoated
with poly-L-lysine (Sigma) and then fixed with 4% paraformaldehyde
for 20 min at room temperature. Cell samples were blocked with
normal goat serum and then incubated with the following respective
primary antibodies: Rabbit anti-mouse CD133/1 monoclonal antibody
(1:50 dilution) and GFAP polyclonal antibody (1:100 dilution)
overnight. The cells were then washed three times in
phosphate-buffered saline (PBS) and then stained with
Cy3-conjugated goat anti-rabbit (1:100 dilution) or FITC-conjugated
goat anti-rabbit secondary antibody (1:50 dilution) for 30 min.
Cell samples were then counterstained with 100 mg/ml DAPI for 10
min to visualize nuclei and then analyzed with a confocal laser
scanning microscope (Leica, Mannheim, Germany).
Determination of cell viability
The effect of drug treatment on cell viability was
determined using the CCK-8 kit. Briefly, cells were seeded onto a
96-well-plate and then treated 24 h after seeding with different
concentrations (0.01, 0.03, 0.1, 0.3, 1, 3, 10, 30 μM) of drug.
Dimethyl sulfoxide (DMSO) was used as a negative control. Four days
after drug incubation, 10 μl of thawed CCK-8 solution was added to
each well. Plates were incubated for 4 h at 37°C and the absorbance
was read at 450 nm with a reference wavelength of 600 nm using the
Thermo Scientific™ Varioskan™ Flash Multimode Reader (Thermo Fisher
Scientific, Waltham, MA, USA).
Colony formation assay
In order to evaluate the effects of agents on the
colony formation of GL261-NS, cells were seeded onto a 96-well
plate at a density of 1×103 cells/ml. The cells were
then incubated with 0.1 μM salinomycin, 30 μM
1-(4-amino-2-methyl-5-pyrimidyl)-methyl-3-(2-chloro
ethyl)-3-nitrosourea hydrochloride (ACNU) or 10 μM vincristine
(VCR) dissolved in DMSO. DMSO was used as a negative control. A
total of 12 wells were assessed for each treatment group. Six days
after incubation, the colony forming ability of cells were examined
and the number of colonies was counted under an Olympus CX22
microscope (Olympus Corp., Inc., Tokyo, Japan).
Flow cytometric analysis
Apoptotic cell death was measured using the Annexin
V-FITC/propidium iodide Apoptosis Detection kit (Dojindo Molecular
Technologies, Inc., Kunamoto, Japan) according to the
manufacturer’s instructions and assessed on a fluorescence
activated cell sorting Calibur instrument (Beckman Coulter Inc.,
Miami, FL, USA). Data were analyzed using CellQuest software
(Becton-Dickinson, San Jose, CA, USA).
Western blot analysis
Cells were washed with ice-cold PBS and then lysed
in ice-cold lysis buffer (50 mM Tris, pH 7.4, 150 mM NaCl, 1%
Triton X-100, 1 mM EDTA, 1 mM ethylene glycol tetraacetic acid, 1
mM phenylmethylsulfonyl fluoride, 10 μg/ml aprotinin, 10 μg/ml
leupeptin, 1 mM sodium orthovandate and 1 mM NaF) for 30 min.
Following this, samples were centrifuged at 16,000 × g at 4°C for
30 min. The supernatant was collected and the protein concentration
was determined using the Bradford protein assay. Total cell protein
was separated by 12% SDS-PAGE and transferred onto a polyvinylidene
difluoride membrane (Millipore, Billerica, MA, USA). Membranes were
blocked with 5% non-fat milk then incubated with anti-caspase-3
primary antibody at 4°C overnight. On the following day, the
membrane was washed with 0.1% Tween 20 in PBS and probed with
horseradish peroxidase-conjugated secondary antibody for 1 h at
room temperature. The bound antibody complexes were detected using
an electrochemiluminesence reagent (GE Healthcare, Amersham, UK).
β-actin was used as an internal control. Band images were analyzed
with a gel imaging analysis system (Kodak ID, Kodak, Rochester, NY,
USA). The intensities of the immunoreactive bands were quantified
by densitometric analysis.
Establishment of a tumor-bearing animal
model
A total of 40 six-week-old specific pathogen free
level C57BL/6 male mice weighing 22±2 g were obtained from the
Laboratory Animal Center, Third Military Medical University
(Chongqing, China). Animals were randomly divided into the
following four groups each including 10 animals: Sham-surgery,
sham-surgery plus salinomycin, tumor-bearing plus placebo and
tumor-bearing plus salinomycin. In tumor-bearing groups, animals
received orthotopic transplantation of GL261-NS cells. Briefly,
mice were anesthetized with 400 mg/kg chloral hydrate via
intraperitoneal injection. Animal experiment procedures were
approved by the Ethics Committee of Southwest Hospital, Third
Military Medical University (Chongqing, China). The cell density
was adjusted to 2×106 cells/ml in serum free DMEM/F12
culture medium and then 5 μl of cell suspension was injected into
the right caudate nucleus. Mice were placed on a stereotaxic
instrument. Following sterilization and skin incision, a hole with
a diameter of 3 mm was made with a micro-electrical drill (RWD Life
Science, Inc., Shenzhen, China) on the skull at the position of 1.4
mm anterior to the anterior fontanel and 2.0 mm lateral to the
sagittal suture. Stereotaxic coordinates were obtained from the
bregma and the dura mater according to a mouse brain atlas
(11). In the sham surgery group,
mice received 1 ml of normal saline by intraperitoneal injection 24
h after cell injection and for salinomycin treatment, mice were
subjected to intraperitoneal administration of salinomycin. The two
groups of animals received daily injections for 10 days. The animal
survival time was assessed for up to 60 days post-surgery. Animal
experiments were conducted according to the guidelines of the
Institutional Committee for Laboratory Animal Usage. The study was
approved by the Laboratory Animal Welfare and Ethics Committee of
the Third Military Medical University (Chongqing, China).
Statistical analysis
Statistical analyses were performed using the SPSS
13.0 statistical software package (SPSS, Inc., Chicago, IL, USA).
Data are presented as the mean ± standard error of the mean. The
results were analyzed using a one-way analysis of variance with
Fisher’s Least Significant Difference test. LogRank (Mantel-Cox)
analysis was used to compare the significance of median survival
time of animals. P<0.05 was considered to indicate a
statistically significant difference.
Results
Identification and characterization of
cultured GL261-NS and GL261-AC cells
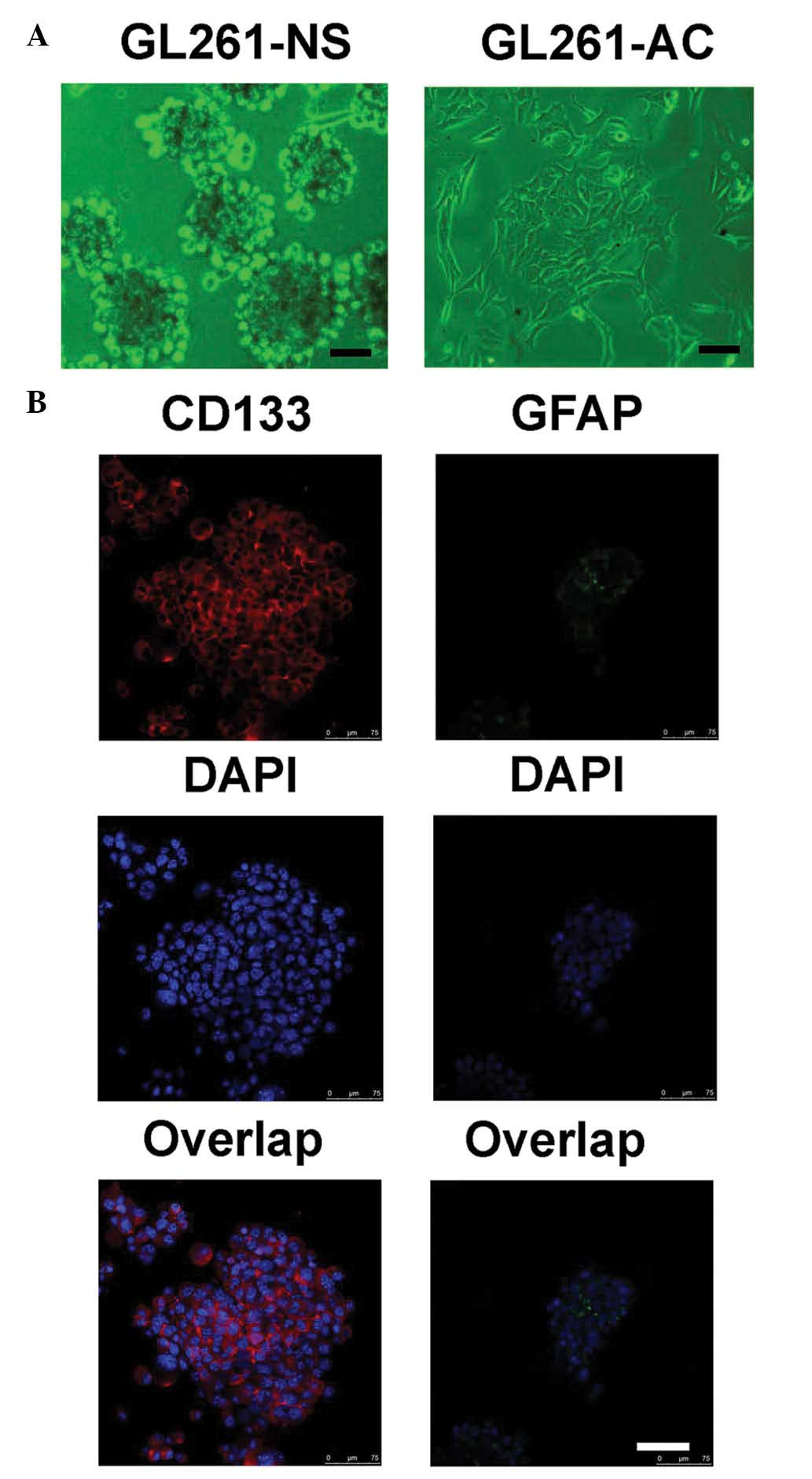
Neurosphere-like clones formed when the cells were
cultured in serum-free culture medium supplemented with bFGF, EGF,
L-glutamine and insulin (Fig. 1A).
In addition, abundant expression of the stem cell marker, CD133,
was observed in these neurosphere-like GL261-NS cells (Fig. 1B). In addition, the level of GFAP
expression, which is a biomarker for differentiated glial cells,
was relatively low. In order to induce cell differentiation, the
culture medium was changed and cells were maintained in DMEM/F12
culture medium containing 10% FBS. The majority of the cells became
adherent and began to differentiate, which were defined as GL261-AC
cells.
Salinomycin reduces the cell viability of
GL261-NS and GL261-AC cells
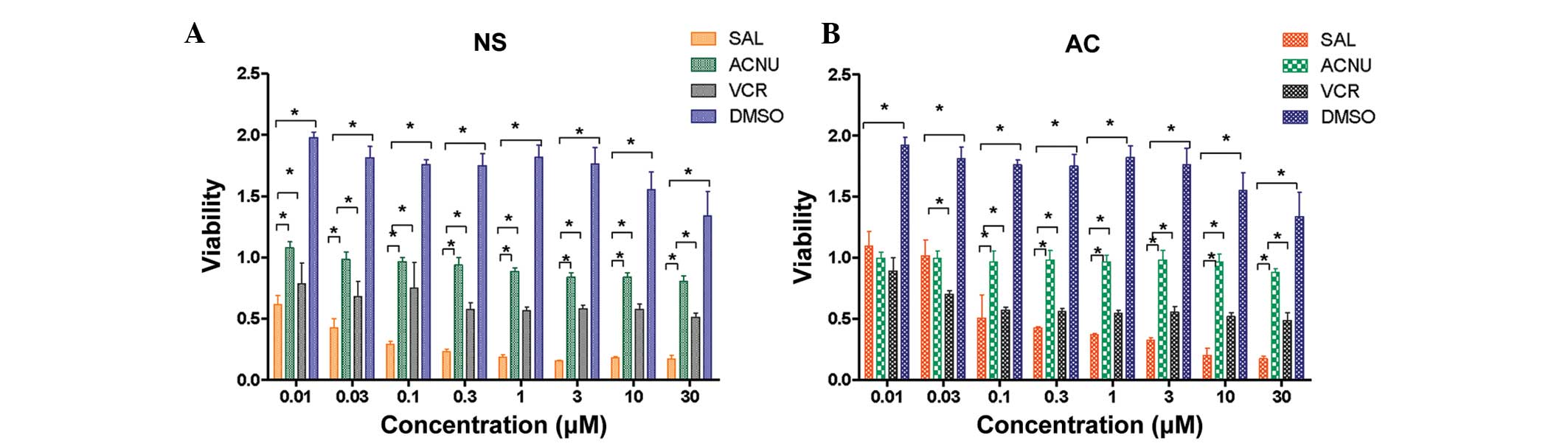
The effects of salinomycin on the cell viability of
GL261-NS and GL261-AC cells were then determined. Two commonly used
anti-cancer agents, ACNU and VCR, were also used as controls. As
shown in Fig. 2, salinomycin (0.01
μM-30 mM) significantly decreased the cell viability of GL261-NS
and GL261-AC cells in a dose-dependent manner (P<0.05, compared
with the DMSO control). Salinomycin appeared to preferentially
inhibit the cell growth and viability of GL261-NS cells compared
with GL261-AC cells with IC50 values of 0.06 and 0.58 μM,
respectively. In addition, a salinomycin concentration of 0.1 μM or
higher was more effective in decreasing cell viability than ACNU or
VCR (P<0.05).
Salinomycin suppresses GL261-NS colony
formation
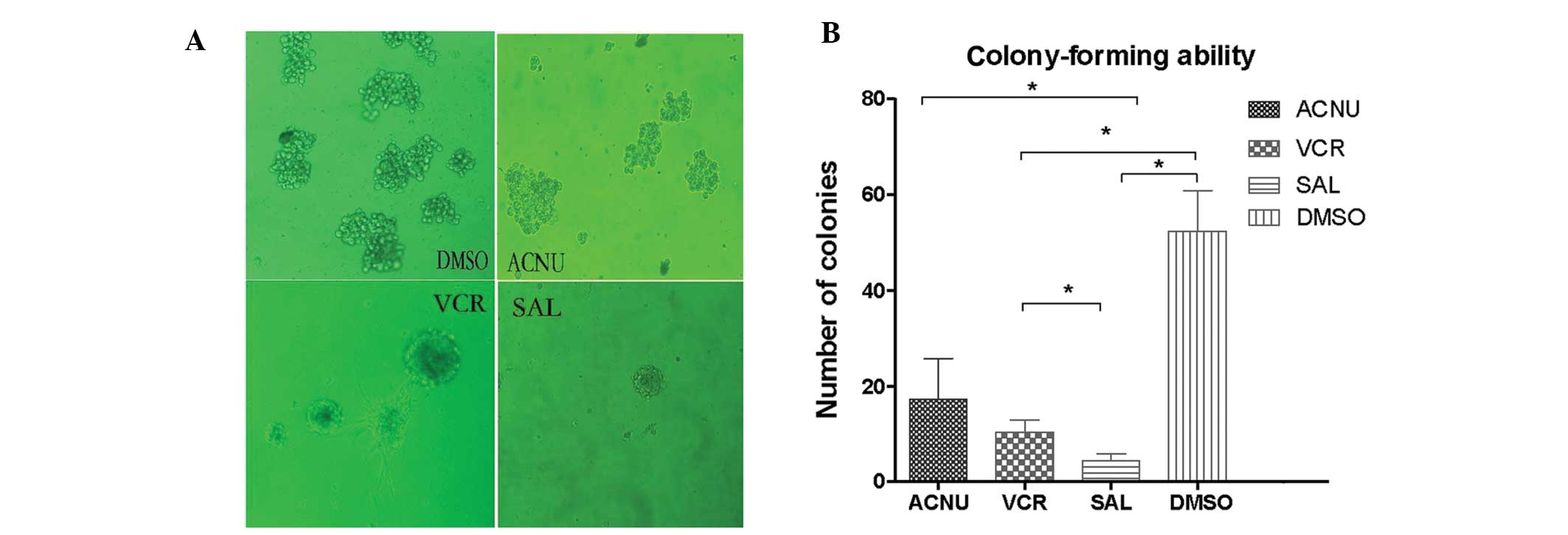
The effect of drug treatment on GL261-NS colony
formation was then assessed. Although 30 μM ACNU or 10 μM VCR
significantly decreased the sphere-forming ability of GL261-NS
cells after 6 days of treatment, the administration of only 0.1 μM
salinomycin markedly inhibited GL261-NS colony formation (SAL, 4.31
± 1.53%; ACNU, 17.31 ± 8.53%; VCR, 10.41 ± 2.53%; DMSO, 52.31 ±
8.53%; P<0.05, SAL compared with the other three groups;
Fig. 3). These results indicate
that salinomycin is significantly more effective at reducing colony
formation than standard chemotherapy.
Salinomycin promotes GL261-NS
apoptosis
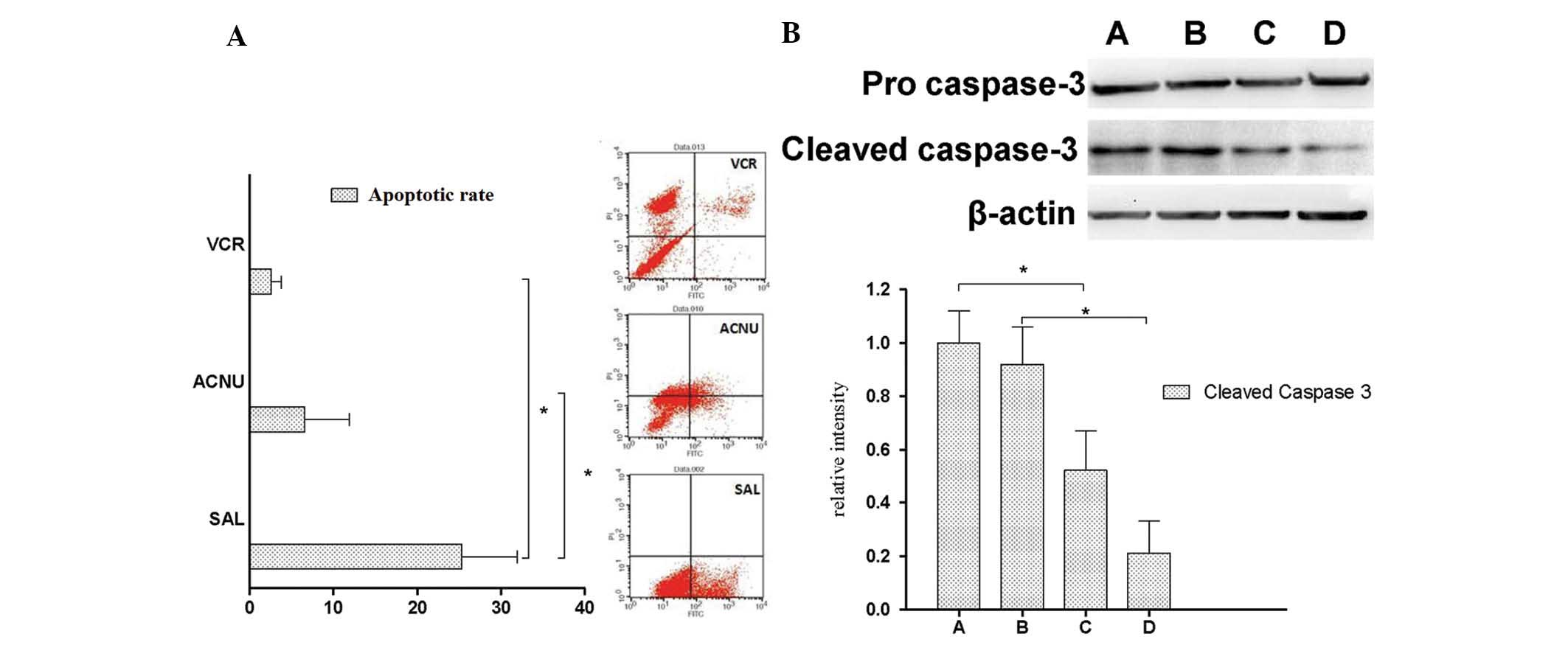
Following this, the effect of salinomycin on
GL261-NS apoptosis was assessed. The administration of SAL markedly
increased apoptotic cell death (P<0.05; Fig. 4A). The expression of the apoptotic
cascade protein caspase-3 was further examined in GL261-NS cells
following 12 or 24 h of salinomycin treatment. Significantly
elevated expression of activated caspase-3 was observed in cells
treated with 10 μM compared with 0.1 μM salinomycin (P<0.05;
Fig. 4B). The level of activated
caspase-3 was also significantly reduced after 24 h of treatment
compared with 12 h (P<0.05). Collectively, these data indicate
that salinomycin promotes apoptosis of GL261-NS cells.
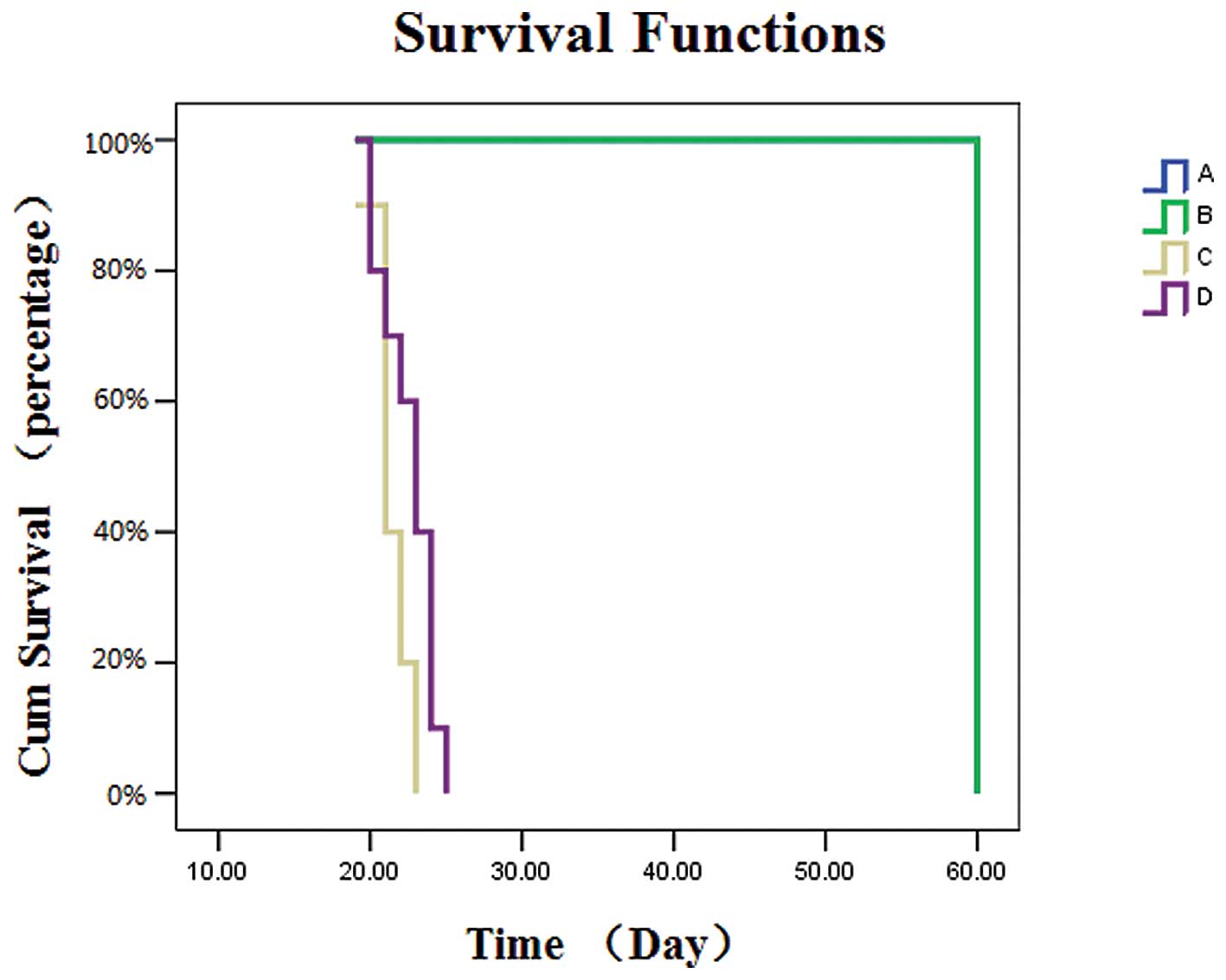
Salinomycin prolongs the median survival
time of tumor-bearing mice
In order to determine the in vivo effects of
salinomycin on the survival time of tumor-bearing mice, xenografts
were treated with salinomycin and the overall survival rate was
monitored. Treatment with salinomycin significantly prolonged the
median overall survival of mice injected with GL261-NS cells
compared with untreated controls (23 vs. 21 days,
respectively; P<0.05; Fig. 5).
All animals in the sham-surgery and sham-surgery plus salinomycin
groups survived for the entire study period (60 days). These
findings suggest that salinomycin may be an effective therapy for
targeting GICs in glioma, reducing tumor burden and extending
survival.
Discussion
GICs are a small population of cells that have the
ability to undergo self-renewal and recapitulate the original tumor
in vivo. Accumulating evidence indicates that GICs express
the cell surface protein CD133 (prominin-1) (12–16).
However, certain CD133-negative cells also possess tumor-initiating
potential (17,18), indicating the complexity in the
identification of cancer cells and underscoring the requirement for
more refined biomarkers. In the present study, morphological
identification accompanied with biomarker examination was applied
to identify GICs from the GL261 cell line. The results demonstrated
that in stem-cell culture medium, GL261 glioma cells formed NS-like
clones and expressed a high level of CD133. However, these cells
could be induced to differentiate in culture medium supplemented
with FBS and the majority of the differentiated GL261-AC cells
became adherent and expressed the glial cell marker GFAP. These
data confirmed the characteristics of the cultured GL261-NS cells
(GICs) and GL261-AC cells (differentiated cells) and our ability to
induce these effects in vitro. In addition, GL261-NS was
significantly more aggressive compared with GL261-AC in vivo
and this effect was enhanced further in syngeneic mice compared
with immunodeficient mice as previously reported (19).
Alkylating agents, including temozolomide and
nitrosourea derivatives, for example ACNU, remain the most commonly
used chemotherapy agents for the treatment of patients with
malignant glioma. However, intrinsic or acquired chemoresistance to
alkylating agents is recognized as the major cause of treatment
failure (20). In addition,
plant-derived anticancer compounds, including vincristine and
vinblastine, are antimitotic drugs that are also commonly used in
anti-cancer therapy. Nevertheless, these agents have limited
utility due to the development of drug resistance. Tumor stem cells
have been reported to be intrinsically resistant to conventional
chemotherapies. These cells can regenerate from the original tumor
eradicated by such treatments, ultimately leading to tumor
recurrence (21). Therefore, the
development of novel treatment approaches targeting this population
of cells appears to be critical for more effective
chemotherapies.
Salinomycin has been verified to be a specific
inhibitor of breast cancer stem cells (8), however, its anti-cancer activity
against malignant glioma and GICs remains to be elucidated. The
current results demonstrated that salinomycin significantly reduced
the cell viability of GL261-NS and GL261-AC in a dose-dependent
manner, with a more substantial inhibition of GL261-NS
proliferation, indicating that salinomycin may efficiently target
GICs in vitro. In addition, salinomycin eventually induced
cell death by promoting apoptosis of GL261-NS cells. Fuchs et
al previously reported that salinomycin induced apoptosis and
overcame apoptotic resistance in human cancer cells (10). Notably, salinomycin activated a
distinct apoptotic pathway not associated with cell cycle arrest,
the tumor suppressor protein p53 or caspase activation, which may
contribute to the apoptotic resistance observed in cancer cells
(10). By contrast, Kim et
al identified that salinomycin induced apoptosis of human
prostate cancer cells by elevating the intracellular reactive
oxygen species (ROS) level, decreasing mitochondrial membrane
potential, triggering cytochrome c release to the cytoplasm and
activating caspase-3 (22).
Consistent with this study, it was identified that salinomycin
induced cell apoptosis of GL261-NS cells by inducing caspase-3
activity. Future studies are to continue to examine the exact
mechanism involved in salinomycin-mediated GICs apoptosis.
In conclusion, the present study demonstrated that
salinomycin significantly reduces cell viability of GL261-NS and
GL261-AC cells, with a more substantial inhibition of GL261-NS
proliferation. It depletes GL261-NS from tumorspheres and induces
apoptotic cell death. Additionally, salinomycin prolongs the median
survival time of glioma-bearing mice compared with control animals,
suggesting that the drug may represent a valuable cancer
therapeutic option for the treatment of malignant glioma.
Acknowledgements
This study was supported by the National Basic
Research Program of China (973 Program, grant no. 2010CB529403),
Joint Fund of the National Natural Science Foundation of China and
the China Academy of Engineering Physics (grant no. U1230128),
National Natural Science Foundation of China (grant nos. 30901538
and 81402067) and Natural Science Foundation Project of CQ
Chongqing Science & Technology Commission (grant no.
cstc2012jjB0081).
References
|
1
|
Wen PY and Kesari S: Malignant gliomas in
adults. N Engl J Med. 359:492–507. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Stiles CD and Rowitch DH: Glioma stem
cells: a midterm exam. Neuron. 58:832–846. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Vescovi AL, Galli R and Reynolds BA: Brain
tumour stem cells. Nat Rev Cancer. 6:425–436. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Natsume A, Kinjo S, Yuki K, et al:
Glioma-initiating cells and molecular pathology: implications for
therapy. Brain Tumor Pathol. 28:1–12. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Eramo A, Ricci-Vitiani L, Zeuner A, et al:
Chemotherapy resistance of glioblastoma stem cells. Cell Death
Differ. 13:1238–1241. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Miyazaki Y, Shibuya M, Sugawara H,
Kawaguchi O and Hirsoe C: Salinomycin, a new polyether antibiotic.
J Antibiot (Tokyo). 27:814–821. 1974. View Article : Google Scholar
|
|
7
|
Mitani M, Yamanishi T, Miyazaki Y and
Ōtake N: Salinomycin effects on mitochondrial ion translocation and
respiration. Antimicrob Agents Chemother. 9:655–660. 1976.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Gupta PB, Onder TT, Jiang G, et al:
Identification of selective inhibitors of cancer stem cells by
high-throughput screening. Cell. 138:645–659. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Riccioni R, Dupuis Ml, Bernabei M, et al:
The cancer stem cell selective inhibitor salinomycin is a
p-glycoprotein inhibitor. Blood Cells Mol Dis. 45:86–92. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Fuchs D, Heinold A, Opelz G, Daniel V and
Naujokat C: Salinomycin induces apoptosis and overcomes apoptosis
resistance in human cancer cells. Biochem Biophys Res Commun.
390:743–749. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Franklin KBJ and Paxinos G: The Mouse
Brain in Stereotaxic Coordinates. 2nd edition. Academic Press; San
Diego: pp. 49–95. 1997
|
|
12
|
Bao S, Wu Q, McLendon RE, et al: Glioma
stem cells promote radioresistance by preferential activation of
the DNA damage response. Nature. 444:756–760. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Piccirillo SG, Reynolds BA, Zanetti N, et
al: Bone morphogenetic proteins inhibit the tumorigenic potential
of human brain tumour-initiating cells. Nature. 444:761–765. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Galli R, Binda E, Orfanelli U, et al:
Isolation and characterization of tumorigenic, stem-like neural
precursors from human glioblastoma. Cancer Res. 64:7011–7021. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Singh SK, Hawkins C, Clarke ID, et al:
Identification of human brain tumour initiating cells. Nature.
432:396–401. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Singh SK, Clarke ID, Terasaki M, et al:
Identification of a cancer stem cell in human brain tumors. Cancer
Res. 63:5821–5828. 2003.PubMed/NCBI
|
|
17
|
Beier D, Hau P, Proescholdt M, et al:
CD133(+) and CD133(−) glioblastoma-derived cancer stem cells show
differential growth characteristics and molecular profiles. Cancer
Res. 67:4010–4015. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Joo KM, Kim SY, Jin X, et al: Clinical and
biological implications of CD133-positive and CD133-negative cells
in glioblastomas. Lab Invest. 88:808–815. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Yi L, Zhou C, Wang B, et al: Implantation
of GL261 neurospheres into C57/BL6 mice: A more reliable syngeneic
graft model for research on glioma-initiating cells. Int J Oncol.
43:477–484. 2013.PubMed/NCBI
|
|
20
|
Sarkaria JN, Kitange GJ, James CD, et al:
Mechanisms of chemoresistance to alkylating agents in malignant
glioma. Clin Cancer Res. 14:2900–2908. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Singh A and Settleman J: EMT, cancer stem
cells and drug resistance: an emerging axis of evil in the war on
cancer. Oncogene. 29:4741–4751. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kim KY, Yu SN, Lee SY, et al:
Salinomycin-induced apoptosis of human prostate cancer cells due to
accumulated reactive oxygen species and mitochondrial membrane
depolarization. Biochem Biophys Res Commun. 413:80–86. 2011.
View Article : Google Scholar : PubMed/NCBI
|