Introduction
Glioma is the most prevalent type of brain tumor,
accounting for >50% of all brain tumors (1), with one of the highest mortality
rates of all cancers. Gliomas have a highly vascularized phenotype
and at present, the primary treatment method is surgical therapy;
however, complete surgical resection is difficult due to the
infiltrative growth and invasiveness of gliomas. Radiotherapy has
been used in combination with surgical therapy approaches; however,
despite the successful development of novel radiotherapeutic
strategies for the treatment of glioma, radioresistance remains a
dominant and unresolved problem.
A previous study revealed that cofilin-1 (CFL1) was
significantly upregulated in radioresistant astrocytomas (2), which indicated that CFL1 may be
involved in the radioresistant phenotype and therefore may be a
target for increasing radiosensitivity.
Cofilin genes have two subtypes which encode
different proteins in mammals. CFL1, a member of the
actin-depolymerizing factor family, is a small (19 kDa), ubiquitous
cytoskeletal protein which is expressed in non-muscular cells,
including nerve and liver cells (3). CFL1 is essential for the promotion of
actin depolymerization/polymerization and the rapid turnover of
actin filaments (4).
Reorganization of the actin cytoskeleton is essential for tumor
development as well as cell motility, adhesion, invasion and
angiogenesis. A previous study demonstrated that CFL1 inhibition in
carcinoma cells decreased cell motility (5); in addition, downregulation of cofilin
reduced assembly and stability of the invadopodia, therefore
indicating its critical role in cell invasion (6). Angiogenesis was found to be dependent
on the CFL1-induced regulation of actin cytoskeletal dynamics;
furthermore, CFL1 was reported to be the target of several
angiogenesis inhibitors (7).
Apart from surgical resection, radiotherapy is the
most effective method of glioma treatment; however, the major
obstacle for effective radiotherapy is radioresistance. Previous
studies have identified numerous factors which have been reported
to influence the effectiveness of radiotherapy (8–13);
however, to the best of our knowledge, there are no studies that
have investigated an association between CFL1 and radiotherapy. The
aim of the present study was to examine the potential association
between CFL1 and radioresistance in human glioma cells.
Materials and methods
Cell culture
Human U251 cells, purchased from Nanjing KeyGEN
Biotech Co., Ltd (Nanjing, China), were cultured in Dulbecco’s
modified Eagle’s Medium (DMEM; Gibco-BRL, Carlsbad, CA, USA)
supplemented with 10% fetal calf serum (Gibco-BRL). Cells were
incubated at 37°C in 5% CO2 and routinely subcultured
every day unless otherwise stated.
Establishment of radioresistant U251
cells (RR-U251)
U251 cells were seeded at a density of
1×105 in a T25 flask (Corning Inc., Corning, NY, USA) in
complete medium. When cells reached 50% confluence they were
treated with 5 Gy of radiation using a 60Co source
(RuiDi Biotechnology, Nanjing, China) at 0.5 Gy/min. When cells
reached 80% confluence, they were trypsinized (Trypsin;
Sigma-Aldrich Shanghai Trading Co., Ltd, Shanghai, China) and
subcultured into new flasks. When cells reached 50% confluence, the
cells were serially irradiated with 5 Gy until 60 Gy of irradiation
was reached, as previously described (14).
Transfection
The sequences of CFL1-small interfering (si)RNA
duplexes and the high expression plasmid pcDNA3.1-CFL1 were
synthesized by GenePharma Co. Ltd (Shanghai, China). siRNA1
(5′-AGCGCAAGAAGGCGGUGCUTT-3′), siRNA2 (5′-GAGGAUCUGGUGUUUAUCUTT-3′)
and siRNA3 (5′-GGUGUCAUCAAGGUGUUCATT-3′) were designed to target
different coding regions of the human CFL1 messenger (m)RNA
sequence (Gene ID, 1072).
U251 cells were seeded onto six-well plates in DMEM
containing 10% fetal calf serum without penicillin or streptomycin
and then incubated overnight. Cells were then transfected with
CFL1-siRNAs or pcDNA3.1-CFL1 using Lipofectamin™ 2000 (Invitrogen
Life Technologies, Carlsbad, CA, USA) according to the
manufacturer’s instructions. Following 6 h of transfection, the
medium was replaced with complete medium and the transfection
efficiency was evaluated using a fluorescence microscope (Axiovert
40 CFL; Carl Zeiss, Oberkochen, Germany). CFL1 expression was
analyzed at 24, 48 and 72 h following transfection. Cells
transfected with pcDNA3.1-CFL1 were selected for stable clones
using DMEM containing 400 μg/ml G418 (Sigma-Aldrich).
Cell viability assay
Cell viability was determined using an MTT assay
(Sigma-Aldrich, St Louis, MO, USA). Following radiotherapy, normal
U251 cells, RR-U251 cells and the treated cells were seeded onto
96-well plates (5.0×103 cells/well; n=6 for each
condition). After 48 h, 20 μl MTT was added and incubated for 4 h
prior to the addition of 150 μl DMSO. Then the optical density (OD)
in each individual well was recorded at 570 nm using a microplate
reader (Multiskan Ascent, model no. 354; Thermo Fisher Scientific,
Shanghai, China). Cell viability was calculated as follows: Cell
viability (100%) =
(ODtreatment/ODcontrol)x100%.
Cell migration assays
Wound healing assays were used to evaluate cell
migration ability. Normal U251 and RR-U251 cells treated with
CFL1-siRNA and pcDNA3.1-CFL1 were seeded onto six-well plates.
Following radiotherapy, monolayers were disrupted to generate a
linear wound using a 10-μl pipette tip. The six-well plates were
washed twice with PBS and incubated with fresh medium. Images were
captured at 0 and 24 h at identical sites using a fluorescence
microscope, and the migration distance was measured. The migration
ratio was calculated using the following formula: Migration ratio =
[(Width0 h-Width24 h)/Width0 h] ×
100%.
Cell invasion assay
A cell invasion assay was performed using 24-well
Transwell chambers (Corning, Inc.) and the inserts were coated with
50 μl Matrigel® (Dilution, 1:8 with DMEM; BD
Biosciences, Franklin Lakes, NJ, USA). Normal U251 and RR-U251
cells treated with CFL1-siRNA and pcDNA3.1-CFL1 were cultured in
six-well plates. Following radiotherapy, the monolayer cells were
trypsinized and transferred to the upper Matrigel chamber in 100 μl
serum-free DMEM at a density of 1×105/ml. DMEM
supplemented with 15% fetal bovine serum (Gibco, Invitrogen Life
Technologies) was added to the lower chamber as the
chemoattractant. Following incubation for 24 h, cells remaining in
the upper chamber were removed using cotton swabs, while invaded
cells were fixed using dehydrated alcohol (Sigma-Aldrich), stained
with crystal violet (Sigma-Aldrich) and then counted under a
microscope (Axiovert 40 CFL). Images were captured in five randomly
selected fields for each well (magnification, ×100). Three separate
experiments were performed.
Western blot analysis
Total protein was extracted from an equal number of
cells in each group using radioimmunoprecipitation assay lysis
buffer (Thermo Scientific, Waltham, MA, USA). Total protein (20 μg)
was separated using 10% SDS-PAGE (Sunshine Biotechnology, Nanjing,
China). The fractionated proteins were electro-transferred to a
polyvinylidene fluoride membrane (Sunshine Biotechnology). The
membrane was blocked in 5% skimmed milk (GuangMing, Nanjing, China)
and probed with rabbit anti-CFL1 polyclonal primary antibodies
(Abcam, Cambridge, MA, USA) diluted in Tris-buffered saline with
Tween20 (1:500; Sunshine Biotechnology) overnight at 4°C. The
membrane was then incubated with the appropriate horseradish
peroxidase-conjugated polyclonal goat anti-rabbit secondary
antibodies (1:10,000; Sunshine Biotechnology) for 2–3 h at room
temperature. Immunoreactive bands were detected using a Supersignal
west Pico Trial enhanced chemiluminescence kit (Thermo Fisher
Scientific) and visualized using a Gel Image Analysis system
(3400Mini; CLINX Science Instruments Co., Ltd, Shanghai,
China).
Reverse transcription quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was extracted from an equal number of
cells in each group using the SV Total RNA Isolation System
(Promega, Madison, WI, USA) according to the manufacturer’s
instructions. Total RNA was then reverse transcribed to
complementary DNA with the Reverse Transcription System (Promega).
mRNA expression was determined by qPCR using GoTaq® qPCR
Master Mix (Promega) under standard thermocycler conditions (AG
22331; Eppendorf, Hamburg, Germany).
The primers used were as follows: CFL1 forward,
5′-TGTGGCTGTCTCTGATGGAG-3′ and reverse, 5′-TTGTCTGGCAGCATCTTGAC-3′;
GAPDH forward, 5′-GTTCCAGTATGACTCTACCC-3′ and reverse,
5′-AGTCTTCTGAGGCAGTGATG-3′.
The following experimental run protocol was used:
Denaturation program, 95°C for 1 min; and an amplification and
quantification program, 45 cycles of 95°C for 45 sec, 58°C for 45
sec, 72°C for 45 sec with final fluorescence measurement.
Inhibition was evaluated by quadruplication assay.
The inhibitory effect was measured using the
following formula: Relative gene expression value =
2-ΔΔCt; ΔCt = CtCFL1 - CtGAPDH;
ΔΔCt = ΔCtexperimental group - ΔCtcontrol
group.
Statistical analysis
Statistical analysis was performed using SPSS 13.0
software (SPSS, Inc., Chicago, IL, USA). Differences were analyzed
using Student’s t-test and the Mann-Whitney U test. Values are
presented as the mean ± standard deviation. P<0.05 was
considered to indicate a statistically significant difference
between values.
Results
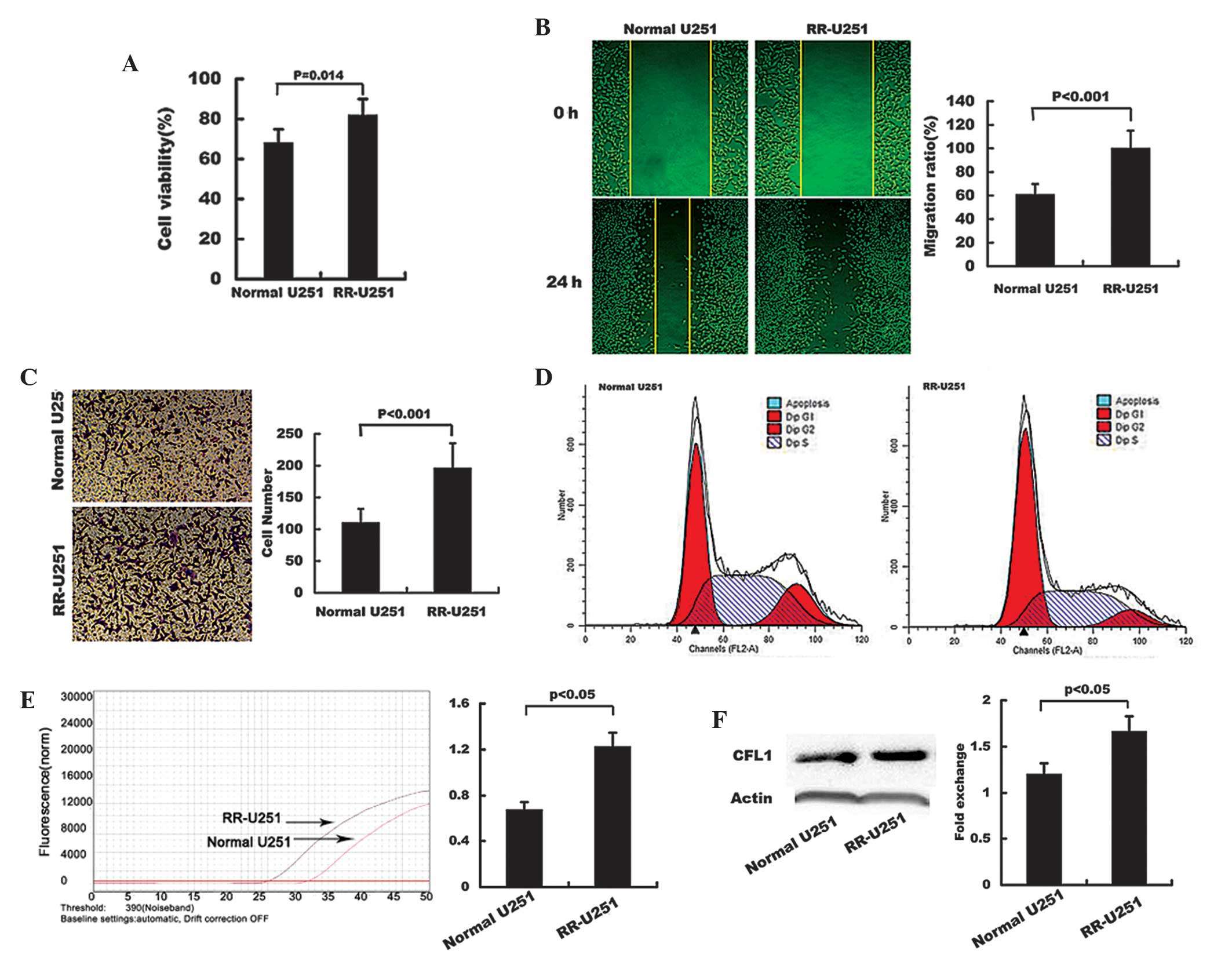
Establishment of RR-U251 cells
RR-U251 cells were established from normal U251
cells irradiated using a 60Co source at 0.5 Gy/min for
10 min per exposure until the accumulated exposure was 60 Gy.
Radiosensitivity was characterized by measuring cell viability,
cell cycle distribution as well as migration and invasion abilities
following radiotherapy. The results showed that the cell viability,
migration and invasion were significantly increased in RR-U251
cells compared with those of the normal U251 cells (Fig. 1A–C, respectively). Following
radiotherapy, the percentage of cells arrested in G2 phase was
16.20% in U251 cells, compared with 8.44% in RR-U251 cells
(Fig. 1D); this therefore
suggested that radiosensitivity was decreased in RR-U251 cells.
Elevated mRNA and protein expression levels of CFL1 were observed
in RR-U251 cells compared with those of normal U251 cells (Fig. 1E and F, respectively).
Establishment of CFL1-silenced U251 cells
and CFL1-overexpressing U251 cells
Transfection of siRNA-CFL1 duplexes led to stable
exogenous gene expression in U251 cells, with ~85–90% efficiency as
indicated by the green fluorescent protein reporter (Fig. 2AI, II). Compared with those of the
control group, all three duplexes significantly inhibited CFL1 mRNA
and protein expression (Fig. 2B and
D, respectively). Of note, siRNA2 had a more potent silencing
effect compared with that of siRNA1 and siRNA3. As shown in
Fig. 2E, CFL1 protein expression
was significantly silenced at 24 h following transfection compared
with that of the 0, 48 and 72 h groups; therefore, CFL1-siRNA2
transfected for 24 h was used for all subsequent experiments.
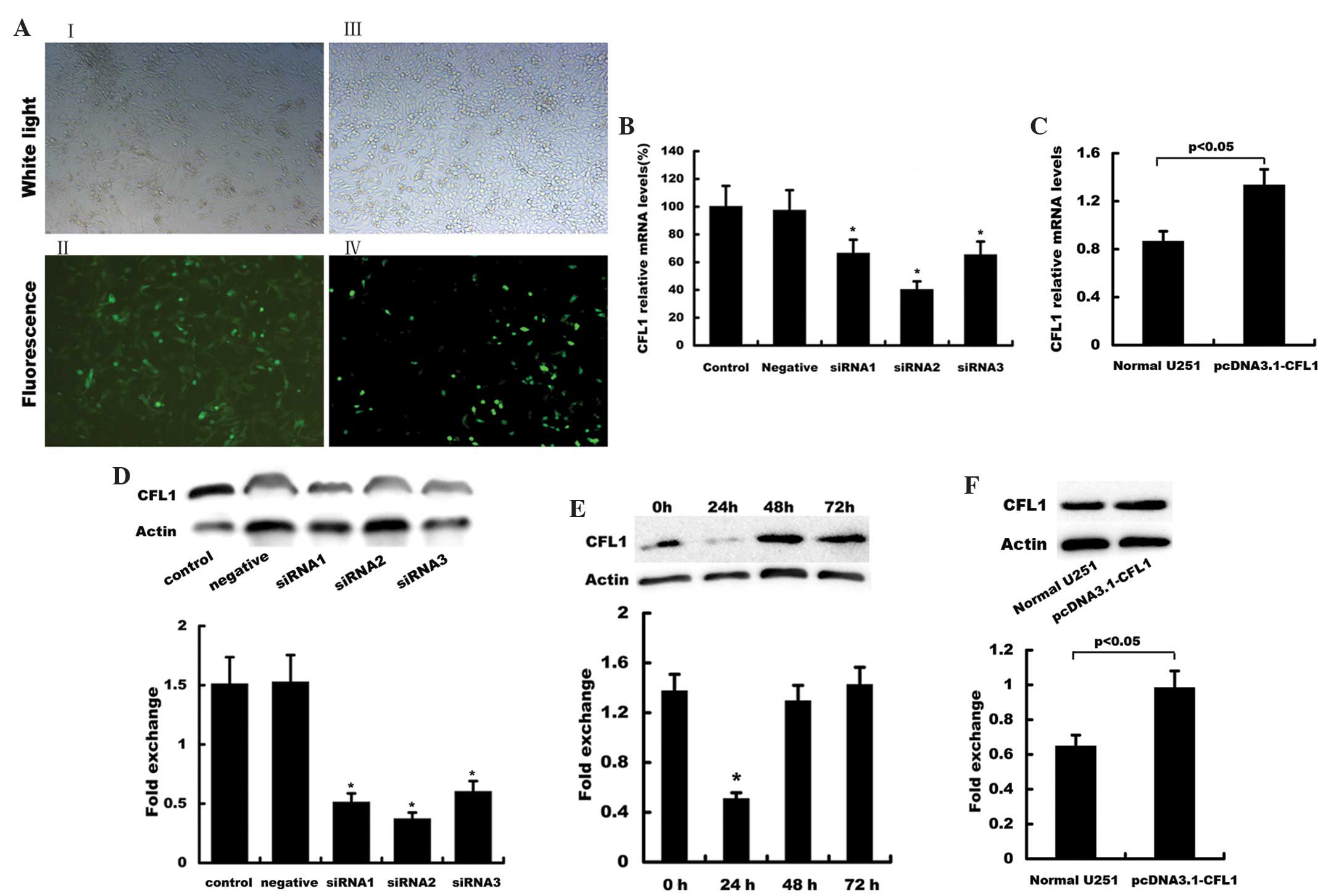 | Figure 2Establishment of CFL1-silenced and
CFL1-overexpressing U251 cells. (A) Cells were transfected with (I,
II) green fluorescent protein-siRNA or (III, IV) pcDNA3.1-CFL1
using Lipofectamine™ 2000. Following 24 h of transfection, plates
were observed under bright field and fluorescence microscope
systems. Reverse transcription quantitative polymerase chain
reaction was used to evaluate the mRNA expression of CFL1 in U251
cells following transfection with (B) siRNA1, siRNA2 and siRNA3 as
well as (C) pcDNA3.1-CFL1. Results are presented as the fold
increase relative to the expression of human GAPDH, determined
using densitometric analysis (n=3). Western blot analysis was used
to detect protein expression of CFL1 in U251 cells following
transfection with (D) siRNA1, siRNA2 and siRNA3, (E) siRNA2 for 24,
48 and 72 h, as well as (F) pcDNA3.1-CFL1. β-actin served as the
loading control. CFL1, cofilin-1; siRNA; small interfering RNA;
mRNA, messengerRNA. |
Transfection with pcDNA3.1-CFL1 led to a stable
exogenous gene expression in U251 cells with ~30–40% efficiency
(Fig. 2AIII, IV). Three weeks
following G418 selection for stable clones, mRNA and protein
expression levels of CFL1 were found to be significantly
upregulated in U251 cells treated with pcDNA3.1-CFL1, as confirmed
by RT-qPCR and western blot analysis (Fig. 2C and F, respectively).
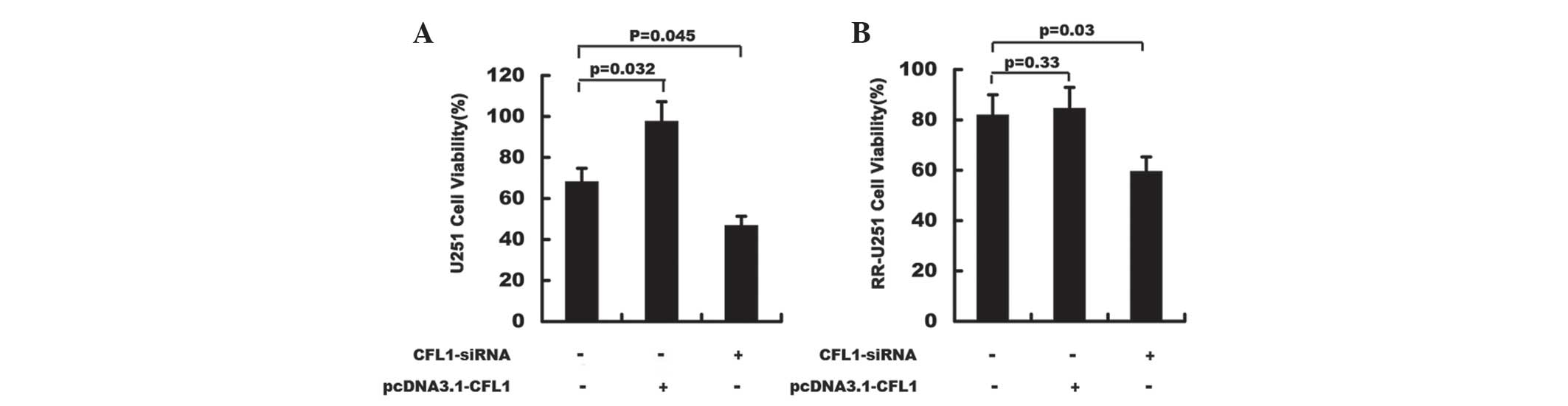
Radiotherapy and cell viability
Following radiotherapy, the cell viability of U251
cells was assessed using the MTT method. As shown in Fig. 3, cell viability was significantly
decreased in CFL1-silenced U251 and CFL1-silenced RR-U251 cells
compared to that of the control groups; in addition, overexpression
of CFL1 through transfection of pcDNA3.1-CFL1 resulted in
significantly enhanced proliferation in normal U251 cells (Fig. 3). These results indicated that
downregulation of CFL1 may significantly elevate the
radiosensitivity of U251 and RR-U251 cells.
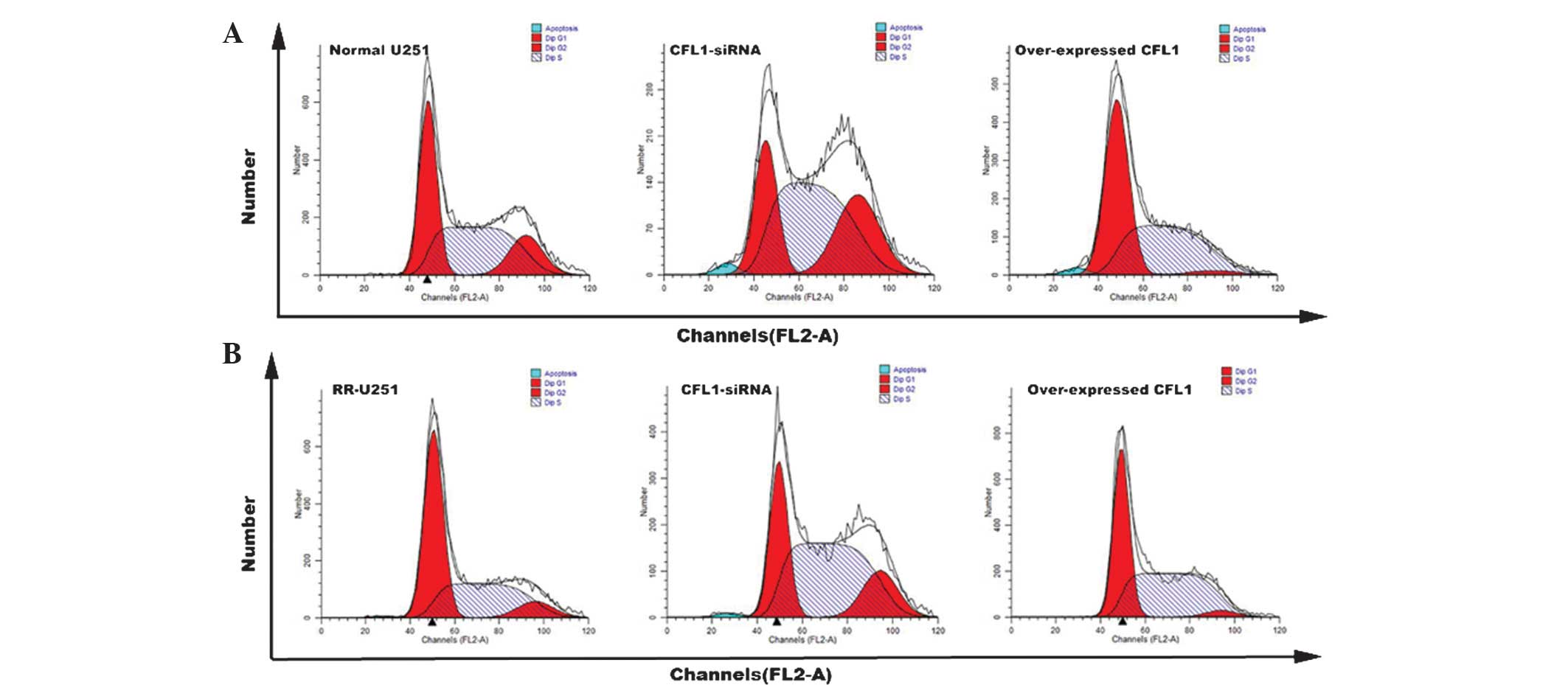
Radiotherapy and cell cycle
distribution
Flow cytometry was used to analyze cell cycle
distribution following radiotherapy. Compared with that of the
control groups, the number of cells arrested in G2 phase was
significantly increased in normal U251 and RR-U251 cells
transfected with CFL1-siRNA (Fig.
4); in addition, the number of cells arrested in G2 phase was
significantly decreased in normal U251 and RR-U251 cells
transfected with pcDNA3.1-CFL1. These results demonstrated that
CFL1 expression affected the cell cycle in human U251 cells
following radiotherapy. Cells arrested in G2 phase may be prone to
apoptosis; therefore, the reduction of CFL1 may increase the number
of apoptotic cells as well as increase radiosensitivity.
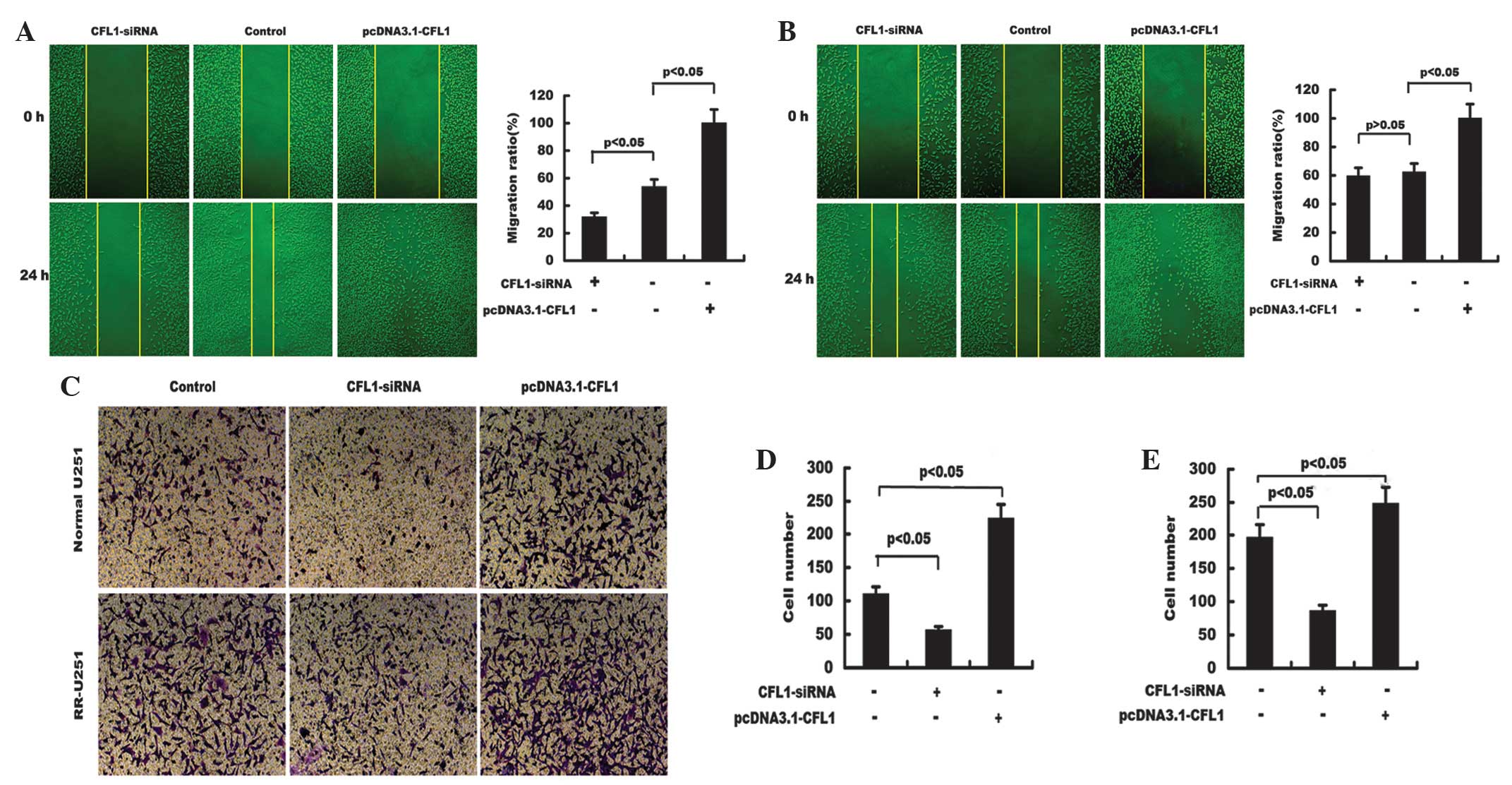
Radiotherapy and cell migration
ability
The migration ability of untreated cells and cells
treated with CFL1-siRNA or pcDNA3.1-CFL1 were examined using a
wound healing assay. As shown in Fig.
5A and B, following radiotherapy, CFL1-silenced U251 cells
showed significantly decreased migration ability compared with that
of the control cells. By contrast, CFL1-overexpressing cells showed
significantly enhanced migration abilities in normal U251 and
RR-U251 cells. These results indicated that downregulation of CFL1
significantly reduced the migration ability of U251 cells and
elevated the radiosensitivity of normal U251 and RR-U251 cells.
Radiotherapy and cell invasion
ability
Cell invasion ability was determined using a
Transwell chamber system. Compared with that of the control, the
invasion potential of U251 cells transfected with CFL1-siRNA was
significantly decreased in normal U251 and RR-U251 cells, whereas
cells transfected with pcDNA3.1-CFL1 demonstrated markedly
increased invasive abilities (Fig.
5C–E). These results indicated that downregulation of CFL1
significantly reduced the invasion ability of U251 cells and
elevated the radiosensitivity of normal U251 and RR-U251 cells.
Discussion
Human intracranial glioma is the most common type of
primary malignant tumor; it is highly invasive and has unclear
boundaries with surrounding tissues (15). Six months following surgery,
infiltrative tumor cells may invade other issues, rapidly resulting
in glioma recurrence (16).
Surgery is the preferred treatment for glioma, and is combined with
chemotherapy or radiotherapy in order to eradicate the tumor
metastatic small lesions. Compared with chemotherapy, radiotherapy
is a more effective treatment for conformal therapy to the target
irregular sections without the limits of the blood-brain barrier
(17); therefore, it has become
the most important treatment for malignant glioma following
surgery.
However, numerous factors have been shown to
restrict the effects of radiotherapy. Previous studies have
suggested that radioresistance may be caused by interactions
between tumors and their microenvironment through angiogenesis
(18), hypoxia (19) and immunosuppressive processes
(20). Conversely, other studies
have shown that radiotherapy may induce cell cycle arrest, DNA
repair and apoptosis (21),
therefore indicating that these factors critical for
radiosensitivity were the result of interactions between
intracellular proteins or genes.
A previous study reported that CFL1 was
significantly upregulated in radioresistant astrocytomas (2); these findings suggested that CFL1 may
be correlated with radiosensitivity in glioma. CFL1, an
actin-binding protein, has a critical role in the cell cytoskeleton
maintaining cellular homeostasis and participates in numerous
physiological activities (22).
Studies have shown that cofilin was a critical factor for tumor
metastasis and drug resistance to chemotherapy (23–25).
Cofilin acts as an important regulatory factor in tumor cell
invasion and metastasis via the formation of lamellipodia, which
therefore promote cell migration (26). Castro et al (27) identified CFL1 as a potential
biomarker for the prognosis of non-small cell lung cancer, where it
was found to be associated with resistance to alkylating drugs. In
addition, CFL1 was reported to be highly expressed in highly
invasive cells (28–31), including breast cancer, colon
cancer and malignant glioma cells. These previous studies therefore
indicated that cofilin was essential for tumor progression, cell
motility, cell adhesion, cell invasion and angiogenesis.
Studies have shown that the extent of malignancy and
recurrence of gliomas was associated with cell motility. CFL1, a
key protein in cell movement, may promote the formation of
filopodia and enhanced cell motility (32). CFL1 was reported to be
overexpressed in cells with high metastatic and invasion abilities,
including hepatoma carcinoma, breast cancer and colon carcinoma
cells. The results of the present study showed that CFL1 was
overexpressed in RR-U251 cells, and that the migration and invasion
abilities of these cells were significantly enhanced. Furthermore,
these results indicated that CFL1 overexpression decreased
radiosensitivity via increasing the metastasis and invasiveness of
U251 cells.
Cell cycle arrest, DNA repair and apoptosis induced
by radiotherapy are the key factors which contribute to
radiosensitivity (33). The
results of the present study demonstrated that the number of cells
arrested in G2 phase was significantly reduced in RR-U251 and
CFL1-overexpressing U251 cells. This therefore indicated that the
number of apoptotic cells declined and the radiosensitivity of U251
cells with high CFL1 expression decreased. In addition,
CFL1-silencing in U251 cells resulted in increased
radiosensitivity. These results suggested that the regulation of
CFL1 in tumor cells may occur due to the priming of cell
transformation, reinforcement of cell mobility in cell metastasis
and the division of tumor cells. Cofilin and Lim kinase, its
regulatory protein, have been shown to have critical roles in cell
motility (34). The results of the
present study indicated that downregulation of CFL1 may increase
the radiosensitivity of U251 cells through reducing cellular
migration and invasion abilities.
In conclusion, the results of the present study
demonstrated that downregulation of CFL1 may increase
radiosensitivity in U251 cells in vitro; however, further
studies are required in order to elucidate the exact molecular
mechanism of this. In addition, further studies are required in
order to determine the role of CFL1 in vivo.
Acknowledgements
The present study was supported by grants from the
National Natural Science Foundation of China (no. NSFC81172390) and
the Health Bureau of Nanjing (no. ZKX10021).
References
|
1
|
Louis DN, Ohgaki H, Wiestler OD, et al:
The 2007 WHO classification of tumours of the central nervous
system. Acta Neuropathol. 114:97–109. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Yan H, Yang K, Xiao H, Zou YJ, Zhang WB
and Liu HY: Over-expression of cofilin-1 and phosphoglycerate
kinase 1 in astrocytomas involved in pathogenesis of
radioresistance. CNS Neurosci Ther. 18:729–736. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Bagheri-Yarmand R, Mazumdar A, Sahin AA
and Kumar R: LIM kinase 1 increases tumor metastasis of human
breast cancer cells via regulation of the urokinase-type
plasminogen activator system. Int J Cancer. 118:2703–2710. 2006.
View Article : Google Scholar
|
|
4
|
Bernstein BW and Bamburg JR: ADF/cofilin:
a functional node in cell biology. Trends Cell Biol. 20:187–195.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hotulainen P and Hoogenraad CC: Actin in
dendritic spines: connecting dynamics to function. J Cell Biol.
189:619–629. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Yamaguchi H, Pixley F and Condeelis J:
Invadopodia and podosomes in tumor invasion. Eur J Cell Biol.
85:213–218. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Keezer SM, Ivie SE, Krutzsch HC, Tandle A,
Libutti SK and Roberts DD: Angiogenesis inhibitors target the
endothelial cell cytoskeleton through altered regulation of heat
shock protein 27 and cofilin. Cancer Res. 63:6405–6412.
2003.PubMed/NCBI
|
|
8
|
Francescone RA, Scully S, Faibish M, et
al: Role of YKL-40 in the angiogenesis, radioresistance, and
progression of glioblastoma. J Biol Chem. 286:15332–15343. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wang J, Wakeman TP, Lathia JD, et al:
Notch promotes radioresistance of glioma stem cells. Stem cells.
28:17–28. 2010.
|
|
10
|
Chautard E, Loubeau G, Tchirkov A,
Chassagne J, Vermot-Desroches C, Morel L and Verrelle P: Akt
signaling pathway: a target for radiosensitizing human malignant
glioma. Neuro Oncol. 12:434–443. 2010.PubMed/NCBI
|
|
11
|
Fedrigo CA, Grivicich I, Schunemann DP, et
al: Radioresistance of human glioma spheroids and expression of
HSP70, p53 and EGFr. Radiat Oncol. 6:1562011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kessler J, Hahnel A, Wichmann H, Rot S,
Kappler M, Bache M and Vordermark D: HIF-1α inhibition by siRNA or
chetomin in human malignant glioma cells: effects on hypoxic
radioresistance and monitoring via CA9 expression. BMC Cancer.
10:6052010. View Article : Google Scholar
|
|
13
|
Naidu MD, Mason JM, Pica RV, Fung H and
Pena LA: Radiation resistance in glioma cells determined by DNA
damage repair activity of Ape1/Ref-1. J Radiat Res. 51:393–404.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lin TY, Chang JT, Wang HM, et al:
Proteomics of the radioresistant phenotype in head-and-neck cancer:
Gp96 as a novel prediction marker and sensitizing target for
radiotherapy. Int J Radiation Oncology Biol Phys. 78:246–256. 2010.
View Article : Google Scholar
|
|
15
|
Clarke RH, Moosa S, Anzivino M, Wang Y,
Floyd DH, Purow BW and Lee KS: Sustained radiosensitization of
hypoxic glioma cells after oxygen pretreatment in an animal model
of glioblastoma and in vitro models of tumor hypoxia. PLoS One.
9:e1111992014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Fiorentini G, Giovanis P, Rossi S, Dentico
P, Paola R, Turrisi G and Bernardeschi P: A phase II clinical study
on relapsed malignant gliomas treated with electro-hyperthermia. In
Vivo. 20:721–724. 2006.
|
|
17
|
Dashti SR, Spalding A, Kadner RI, Yao T,
Kumar A, Sun Da and LaRocca R: Targeted intraarterial anti-VEGF
therapy for medically refractory radiation necrosis in the brain. J
Neurosurg Pediatr. Oct 31–2014.(Epub ahead of print). PubMed/NCBI
|
|
18
|
Folkes LK and O’Neill P: Modification of
DNA damage mechanisms by nitric oxide during ionizing radiation.
Free Radic Biol Med. 58:14–25. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Manda K, Glasow A, Paape D and Hildebrandt
G: Effects of ionizing radiation on the immune system with special
emphasis on the interaction of dendritic and T cells. Front Oncol.
2:1022012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Park E, Ahn G, Yun JS, et al: Dieckol
rescues mice from lethal irradiation by accelerating hemopoiesis
and curtailing immunosuppression. Int J Radiat Biol. 86:848–859.
2010.PubMed/NCBI
|
|
21
|
Chang HY, Shih MH, Huang HC, Tsai SR, Juan
HF and Lee SC: Middle infrared radiation induces G2/M cell cycle
arrest in A549 lung cancer cells. PLoS One. 8:e541172013.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Homma K, Niino Y, Hotta K and Oka K:
Ca(2+) influx through P2X receptors induces actin cytoskeleton
reorganization by the formation of cofilin rods in neurites. Mol
Cell Neurosci. 37:261–270. 2008. View Article : Google Scholar
|
|
23
|
Ashworth S, Teng B, Kaufeld J, et al:
Cofilin-1 inactivation leads to proteinuria-studies in zebrafish,
mice and humans. PLoS One. 5:el26262010. View Article : Google Scholar
|
|
24
|
Xu YL and Wang DB: Relationship between
cofilin-1 expression and implantation capacity in eutopic
endometrium of patient with endometriosis. Zhonghua Fu Chan Ke Za
Zhi. 45:252–255. 2010.PubMed/NCBI
|
|
25
|
Sidani M, Wessels D, Mouneimne G, et al:
Cofilin determines the migration behavior and turning frequency of
metastatic cancer cells. J Cell Bio. 179:777–791. 2007. View Article : Google Scholar
|
|
26
|
Lin CW, Yen ST, Chang HT, et al: Loss of
cofilin 1 disturbs actin dynamics, adhesion between enveloping and
deep cell layers and cell movements during gastrulation in
zebrafish. PLoS One. 5:e153312010. View Article : Google Scholar
|
|
27
|
Castro MA, Dal-Pizzol F, Zdanov S, et al:
CFL1 expression levels as a prognastic and drug resistanse marker
in nonsmall cell lung. Cancer. 116:3645–3655. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Spratley SJ, Bastea LI, Döppler H, Mizuno
K and Storz P: Protein kinase D regulates cofilin activity through
p21-activated kinase 4. J Biol Chem. 286:34254–34261. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Elliott CM, Stinner B and Venkataraman C:
Modelling cell motility and chemotaxis with evolving surface finite
elements. J R Soc Interface. 9:3027–3044. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Estornes Y, Gay F, Gevrey JC, et al:
Differential involvement of destrin and cofilin-1 in the control of
invasive properties of Isreco1 human colon cancer cells. Int J
Cancer. 121:2162–2171. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Ma Y, Wang B, Li W, et al: Intersectin1-s
is involved in migration and invasion of human glioma cells. J
Neurosci Res. 89:1079–1090. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Solbach TF, Konig J, Fromm MF and Zolk O:
ATP-binding cassette transporters in the heart. Trends Cardiovasc
Med. 16:7–15. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Zhang L, Luo J, Wan P, Wu J, Laski F and
Chen J: Regulation of cofilin phosphorylation and asymmetry in
collective cell migration during morphogenesis. Development.
138:455–464. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Vigil D, Kim TY, Plachco A, et al: ROCK1
and ROCK2 are required for non-small cell lung cancer
anchorage-independent growth and invasion. Cancer Res.
72:5338–5347. 2012. View Article : Google Scholar : PubMed/NCBI
|