Introduction
Angiogenesis is a complex process involving
angiogenic factor secretion, proteolytic enzyme secretion and
activation, extracellular matrix degradation, endothelial cell
activation, migration, proliferation, growth, sprouting and lumen
formation, new vessel differentiation and maturation, and
vasoganglion remodeling (1).
Choroidal neovascularization (CNV) is one of the most important
intraocular neovascular manifestations and is correlated with
numerous ocular diseases, including age-related macular
degeneration (AMD), which is the primary cause of vision loss among
people >60 years of age in developed countries (2,3), as
well as idiopathic chorioretinitis, ocular histoplasmosis, high
myopia macular degeneration, ophthalmic tumors and ocular
injury.
CNV is caused by fibrous vascular tissue formed by
choroidal neovascular buds passing through the Bruch’s membrane and
proliferating in the subretinal space (4). The mechanisms underlying CNV are
diverse and complex, involving numerous cellular factors and signal
transduction pathways that regulate the incidence and development
of CNV. Hypoxia-inducible factor (HIF), angiopoietin (Ang) and
numerous cytokines, including vascular endothelial growth factor
(VEGF), have been found to have an important role in CNV (2,5).
Several recent studies found that hypoxia/ischemia has an important
role in CNV, and HIF-1α and VEGF are key regulators of CNV under
hypoxic conditions (6,7).
Recently, an angiogenesis inhibitor has been
developed as a novel strategy for the treatment of CNV (8). Endostatin, a 20 kD potential
angiogenesis inhibitor, has been found to exert powerful effects
preventing endothelial vascular formation and tumor development
in vitro and in vivo, mainly in advanced solid tumors
and human umbilical vein endothelial cells (9,10).
Furthermore, Mori et al (11) compared the similarities between
tumor angiogenesis and CNV, and found that endostatins inhibit
occular neovascularization, and that the occurrence and development
of CNV were negatively correlated with the serum endostatin level.
In addition, they also confirmed that systemic application of
endostatin inhibited intraocular neovascularization. Tatar et
al (12) found that endostatin
was expressed in 92% of CNV samples obtained from patients with AMD
using immunohistochemical analysis. Our previous study reported
that Endostar was able to inhibit cell proliferation and migration
in normal RF/6A choroid-retinal endothelial cells through
regulating the expression of growth factors and inflammatory
factors in a dose- and time-dependent manner (13). Although Endostar may effectively
inhibit angiogenesis and tumor growth, its specific role and
mechanism in regulating CNV inhibition have not been well defined.
In the present study, RF/6A cells were cultured under hypoxic
conditions to simulate vascular endothelial cell growth,
proliferation and migration in vitro, and were used to
examine the possible mechanisms underlying the inhibitory effects
of Endostar on cell proliferation and migration.
Materials and methods
Cell viability assay
RF/6A rhesus choroid retinal endothelial cells
obtained from the Institute of Cell Biology, Chinese Academy of
Sciences (Shanghai, China) were cultured in Eagle’s minimum
essential medium (EMEM; Gibco-BRL, Grand Island, NY, USA)
supplemented with 10% fetal bovine serum (FBS; Hangzhou Sijiqing
Bioengineering Material Co., Ltd., Hangzhou, China). Hypoxia was
induced by exposing the cells to CoCl2 (Sigma-Aldrich,
St. Louis, MO, USA) at various concentrations (100–800 μM) in EMEM
medium with 0.5% FBS for 24 h, and the cell viability was
determined by a methylthiazol tetrazolium (MTT) assay
(Sigma-Aldrich). The absorbance at 570 nm was measured with a
Benchmark microplate reader (Bio-Rad, Hercules, CA, USA). The data
were reported as percentages of the absorbance in the control
cells.
Wound-healing assay
When the cells had been cultured to a monolayer, the
cells were wounded with 200 μl plastic pipette tips and incubated
in EMEM containing 0.5% FBS and 200 μM CoCl2, in the
absence or presence of Endostar (0.5, 1 or 10 μg/ml; Simcere
Pharmaceutical Group, Nanjing, China). Images were captured at 0,
24, 48 and 96 h using an Olympus IX-81 inverted microscope (Olympus
Corp., Tokyo, Japan). Migration was quantified by counting the
number of cells that had advanced into the cell-free space from the
initial wound border at 0 h.
Cell cycle analysis
Following treatment, the cells were washed with
ice-cold phopshate-buffered saline (PBS), trypsinized, resuspended
in PBS supplemented with 0.2% Triton X-100 and 1 mg/ml RNase A
(Sigma-Aldrich), and incubated with propidium iodide (PI;
Sigma-Aldrich) for 30 min at room temperature in the dark for DNA
staining. The cells were then analyzed using a flow cytometer
(FACSCalibur; Becton Dickinson, San Jose, CA, USA) with cell quest
software (Becton Dickinson).
Determination of the levels of secreted
VEGF using ELISA
Following overnight serum starvation, the cells in
the 24-well culture plates were pretreated with 0, 0.5, 1 or 10
μg/ml Endostar, for 1 h and then treated with 200 μM
CoCl2 for 24 h. The culture medium (200 μl) was
collected by centrifugation at 100 × g for 10 min and the VEGF
content was detected using a commercial human VEGF ELISA kit
(R&D Systems, Minneapolis, MN, USA) according to the
manufacturer’s instructions and calculated as pg/ml protein.
Reverse transcription-polymerase chain
reaction (RT-PCR)
To identify the mRNA expression levels of HIF-1α and
VEGF, the total RNA was extracted using TRIzol reagent (Invitrogen
Life Technologies, Carlsbad, CA, USA) according to the
manufacturer’s instructions and reverse-transcribed into cDNA in a
20 μl reaction, which was incubated at 42°C for 60 min, and then
heated to 72°C for 10 min to inactivate the reverse transcriptase.
cDNA was then used as a template in a 20-μl PCR system under the
following conditions: Denaturation at 94°C for 2 min followed by 35
cycles of denaturation at 94°C for 10 sec, annealing at 55°C for 10
sec and elongation at 72°C for 10 sec, and a final incubation at
72°C for 10 min. The amplified products were examined on 2% agarose
gels and densitometrically analyzed with a UVP gel analysis system
(Bio-Rad). The primer sequences were as follows: Forward: 5′-CAT
TAG AAA GCA GTT CCG CAA GC-3′ and reverse: 5′-CAG TGG TAG TGG TGG
CAT TAG C-3′ for human HIF-1α; forward: 5′-GAG CCT TGC CTT GCT GCT
CTA C-3′ and reverse: 5′-CAC CAG GGT CTC GAT TGG ATG-3′ for human
VEGF; and forward: 5′-TCA ACG GAT TTG GTC GTA TT-3′ and reverse:
5′-CTG TGG TCA TGA GTC CTT CC-3′ for human
glyceraldehyde-3-phosphate dehydrogenase (GAPDH). The primers were
synthesized by Sangon Biotech (Shanghai, China).
Statistical analysis
The data are expressed as the mean ± standard
deviation and were statistically analyzed by one-way analysis of
variance using Prism 4 software (GraphPad software Inc., San Diego,
CA, USA). P<0.05 was considered to indicate a statistically
significant difference.
Results
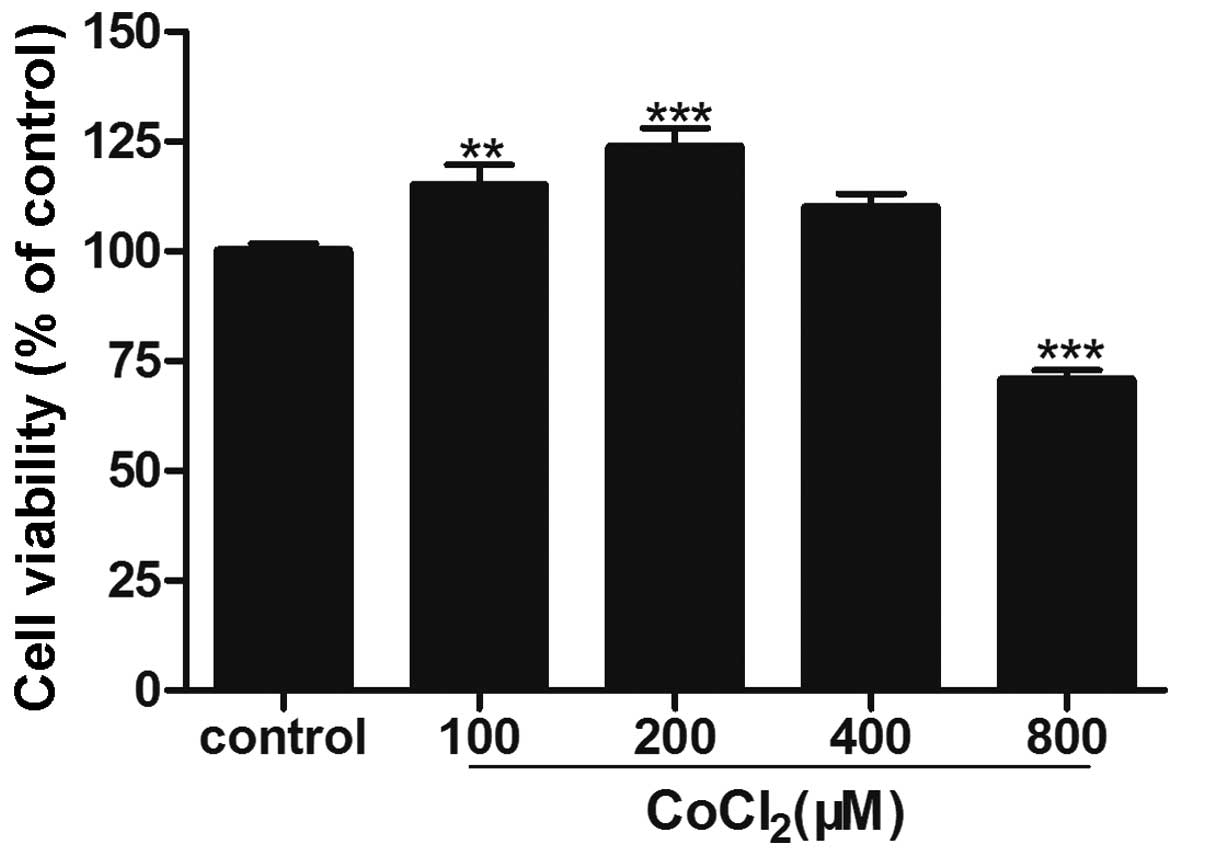
Induction of hypoxia with
CoCl2
Treatment with CoCl2 at doses <400 μM
for 24 h was able to induce cell proliferation, as measured by an
MTT assay. The cell viability was 114.9±10.1 and 123.6±9.6% in
cells treated with 100 and 200 μM CoCl2, respectively
(P<0.01). However, at a concentration >400 μM,
CoCl2 significantly inhibited cell proliferation
(Fig. 1).
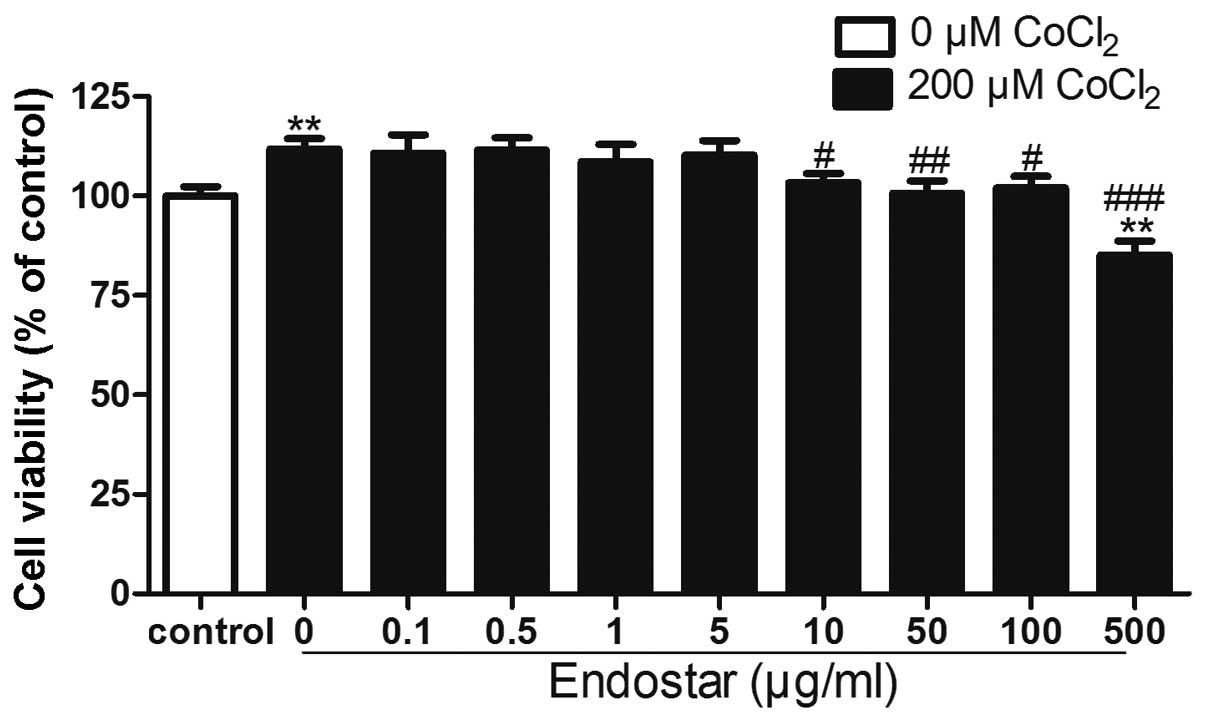
Effect of Endostar on the viability of
CoCl2-treated RF/6A cells
The viability of RF/6A cells treated with 200 μM
CoCl2 for 24 h was significantly increased compared with
the untreated control cells. Pre-treatment with Endostar at
concentrations of 1–500 μg/ml significantly attenuated
CoCl2-induced increase in cell viability (P<0.05);
however, treatment with 500 μg/ml Endostar resulted in a decline in
cell viability of RF/6A cells compared with that of the untreated
cells (Fig. 2). This therefore
suggested that concentrations of Endostar >100 μg/ml may have
toxic effects on these cells.
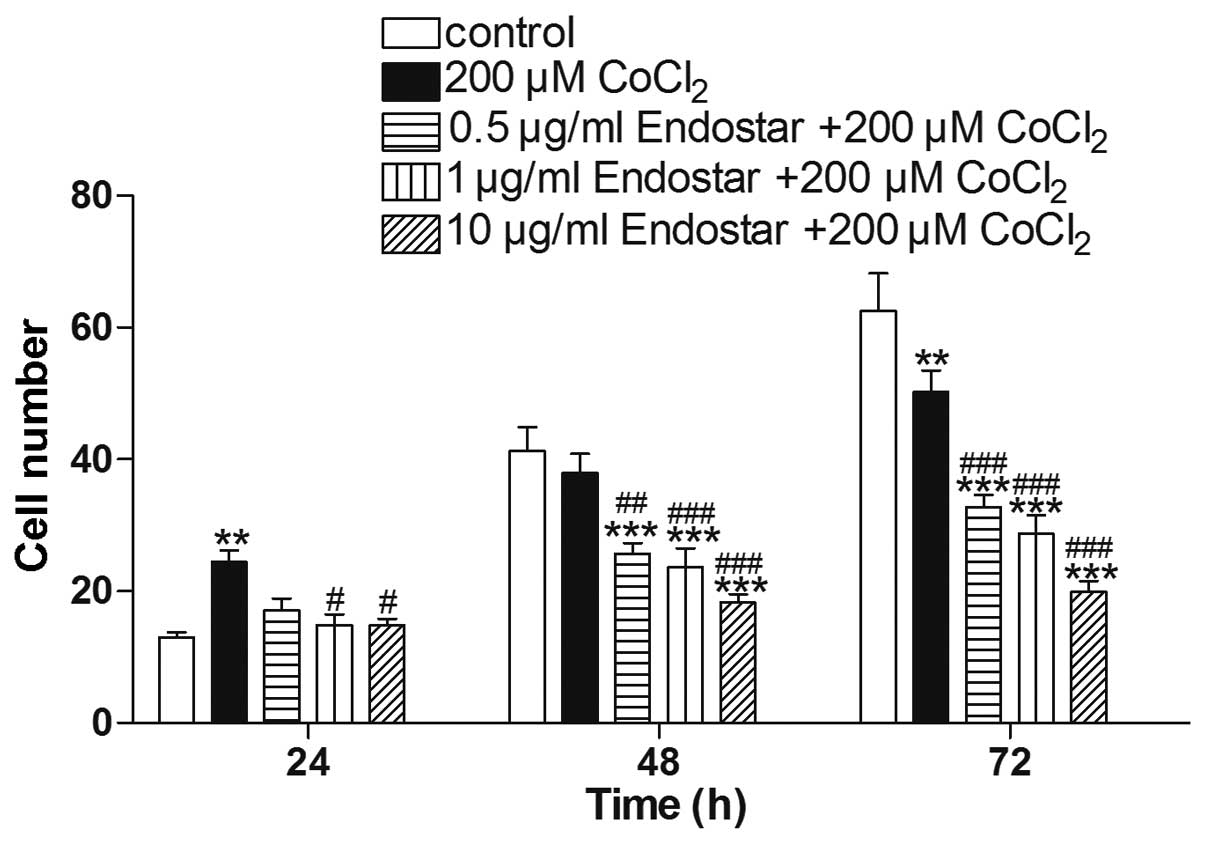
Effect of Endostar on the migration of
CoCl2-treated RF/6A cells
The effect of Endostar on the migration of
CoCl2-treated RF/6A cells was examined using a
wound-healing assay in vitro. It was identified that
CoCl2 treatment for 24 h significantly promoted cell
migration, which was inhibited by Endostar pretreatment at
different time points (Fig. 3). As
shown in Fig. 4, cell migration
was inhibited by Endostar treatment in a time and dose-dependent
manner. Under hypoxic conditions, the cell migration was increased
nearly 2-fold compared with the level in the control group after 24
h, by 92.0% compared with the control group after 48 h, and by
80.4% after 72 h. Compared with the group treated with
CoCl2 alone, cell migration was decreased to 70.0, 60.9
and 60.5% following 24 h, 67.8, 62.9 and 48.3% after 48 h, and
65.3, 57.1 and 39.6% after 72 h, following treatment with 0.5, 1
and 10 μg/ml Endostar, respectively.
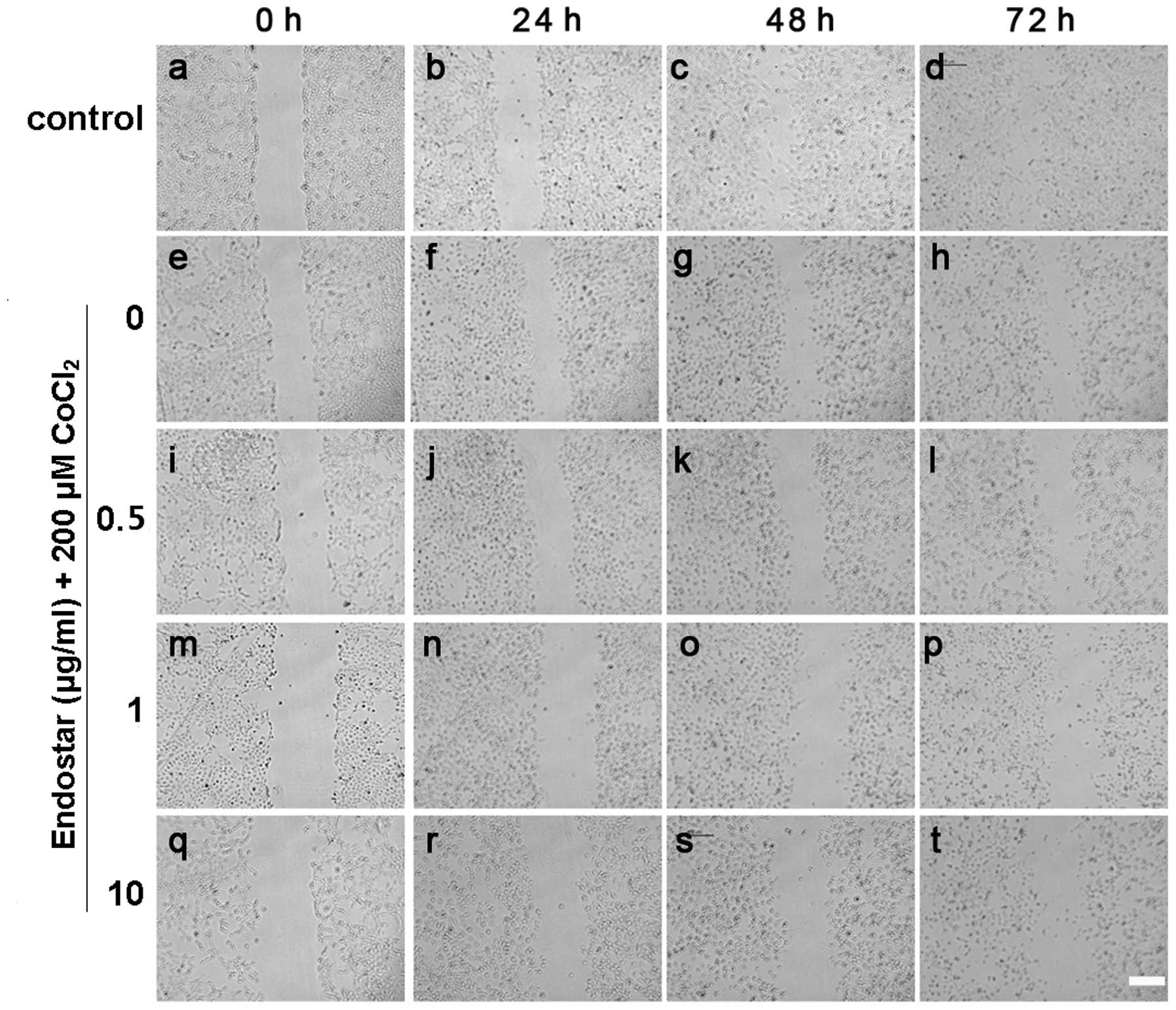 | Figure 3Inhibitory effect of Endostar on the
migration of hypoxic choroid-retinal endothelial cells (RF/6A).
(a-t) The cells as measured by a wound healing assay in
vitro. The migration of cells without any treatment at (a) 0,
(b) 24, (b) 48 and (d) 72 h; treated with CoCl2 at (e)
0, (f) 24, (g) 48 and (h) 72 h; pretreated with 0.5 μg/ml Endostar
and treated with CoCl2 at (i) 0, (j) 24, (k) 48 and (l)
72 h; pretreated with 1 μg/ml Endostar and treated with
CoCl2 at (m) 0, (n) 24, (o) 48 and (p) 72 h; pretreated
with 10 μg/ml Endostar and treated with CoCl2 at (q) 0,
(r) 24, (s) 48 and (t) 72 h. Scale bar, 100 μm. |
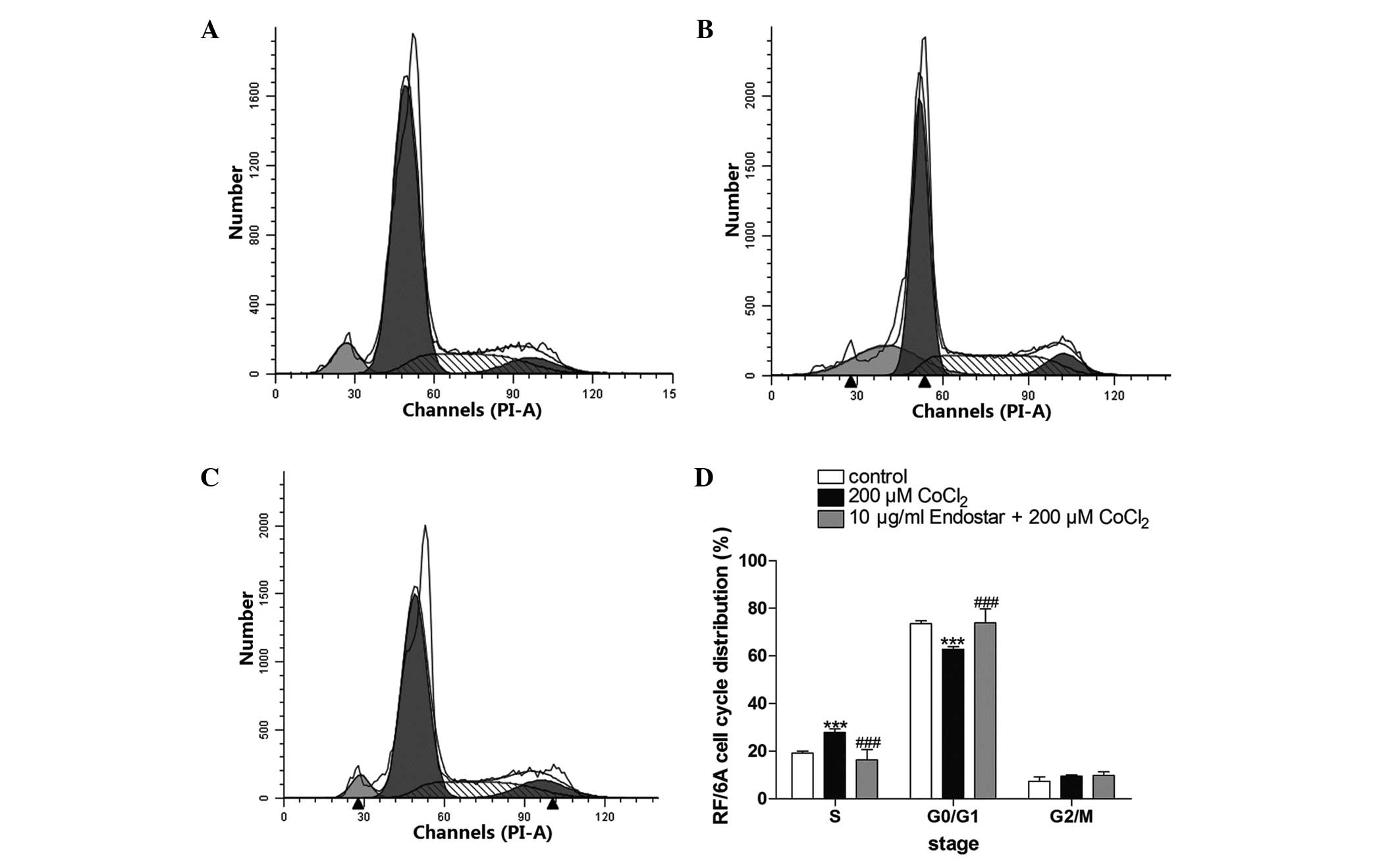
Effect of pretreatment with Endostar on
the cell cycle of CoCl2-treated RF/6A cells
The DNA content of CoCl2-treated RF/6A
cells was used for cell cycle analysis. The results demonstrated
that CoCl2 arrested the cell cycle at S phase and
Endostar pretreatment was able to reverse this effect (Fig. 5). It was observed that treatment
with 200 μM CoCl2 increased the percentage of cells in S
phase from 19.1±0.9 to 27.8±1.5%, but decreased the percentage of
cells in G0/G1 phase from 73.5±1.3 to 62.8±1.1%. However,
pretreatment with 10 μg/ml Endostar completely reversed these
effects, and the percentage of cells in S phase and G0/G1 phase
returned to 16.3±3.5 and 73.9±5.8%, respectively.
Effect of pretreatment with Endostar on
the expression of HIF-1α and VEGF in CoCl2-treated RF/6A
cells
HIF-1α and VEGF mRNA levels were quantified with
RT-PCR, normalized to the internal control GAPDH and compared.
Compared with the control group, 200 μM CoCl2 treatment
induced a 4-fold increase in HIF-1α expression, which was then
attenuated by Endostart in a concetration-dependent manner (0.5–10
μg/ml) (Fig. 6A and B). In
addition, 200 μM CoCl2 increased VEGF mRNA expression by
50%, which was then downregulated in a concentration-dependent
manner following treatment with 0.5–10 μg/ml Endostar (Fig. 6A and C). Furthermore, the amount of
VEGF protein release into the medium was consistent with that of
the mRNA expression of VEGF (Fig.
6D).
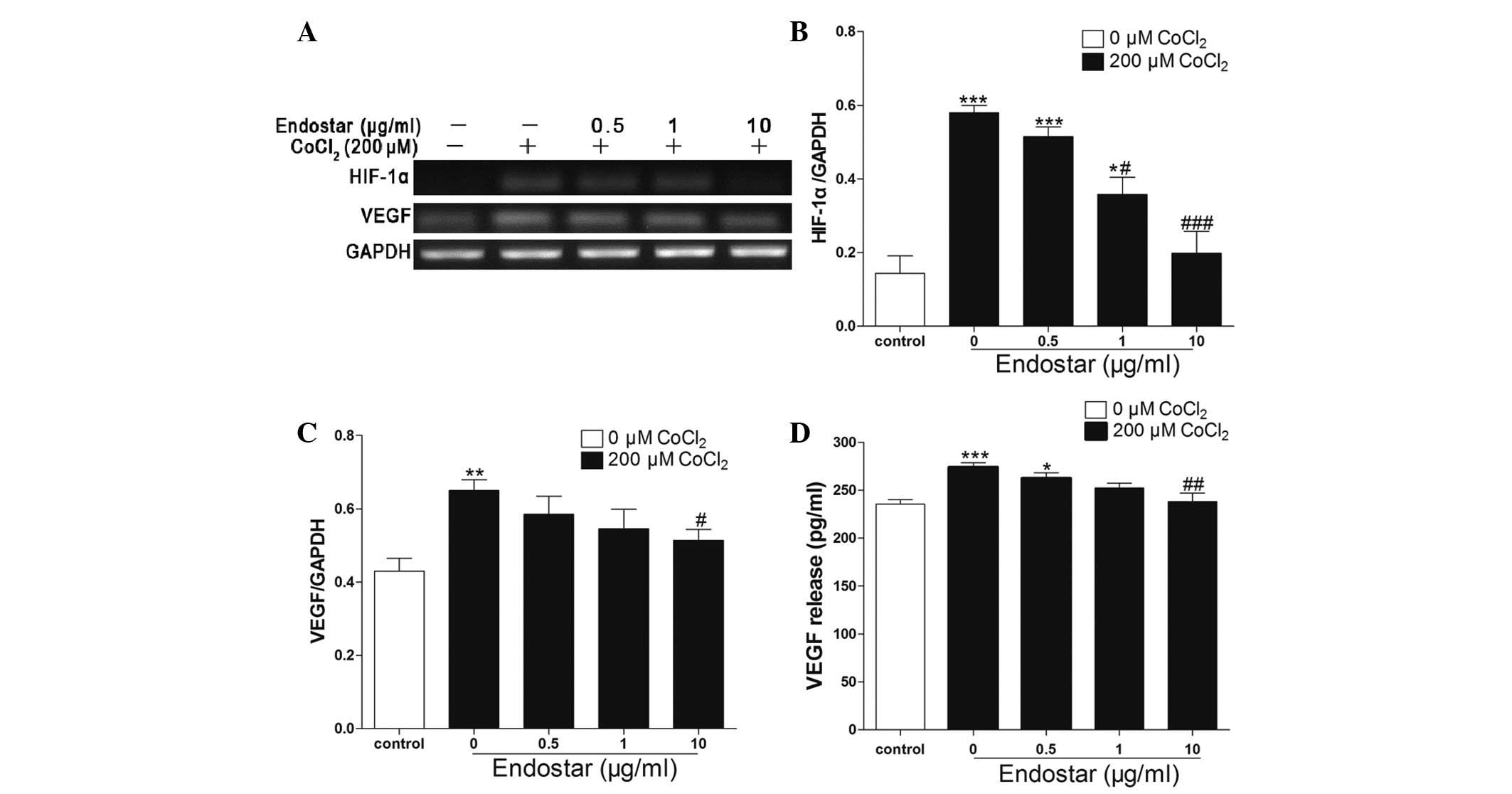 | Figure 6Endostar inhibits HIF-1α and VEGF
expression in hypoxic choroid-retinal endothelial cells (RF/6A).
(A) After the cells were incubated for 24 h with 200 μM
CoCl2 in the absence or presence of 0.5, 1 and 10 μg/ml
Endostar, mRNA levels of HIF-1α and VEGF were detected by RT-PCR.
(B and C) Densitometric analyses of RT-PCR are presented as the
mean ± standard deviation of three independent experiments
performed in triplicate. (D) After the cells were treated with 200
μM CoCl2 in the absence or presence of Endostar (0.5, 1,
and 10 μg/ml), the cell supernatant was used to analyze the
secreted VEGF protein using ELISA. *P<0.05,
**P<0.01 and ***P<0.001 vs. the
control; #P<0.05, ##P<0.01 and
###P<0.001 vs. cells only treated with
CoCl2. HIF-1α, hypoxia-inducible factor 1α; VEGF,
vascular endothelial growth factor; RT-PCR, reverse
transcription-polymerase chain reaction. |
Discussion
Hypoxia/ischemia-associated retinopathy and optic
neuropathy is the major cause of blindness, central/branch retinal
vein occlusion, diabetic retinopathy and retinopathy of
prematurity, which appear to always be accompanied with retinal and
choroidal neovascularization at advanced stages, causing vitreous
hemorrhage, proliferative retinopathy, retinal detachment and
severe visual impairment (14,15).
RF/6A cells were isolated from the choroid retina of a healthy
rhesus fetus and confirmed as endothelial cells by morphology,
growth pattern and immunohistochemistry. RF/6A cells, as a
choroidal endothelial cell line, were used to investigate the
pathogenesis and prevention of CNV-associated diseases and have
been proven to be a reliable in vitro model for the
formation of CNV. CNV formation is a complex process (16,17),
affected by a variety of etiological factors, including hypoxia,
which has an important role in angiogenesis (6). In the present study,
CoCl2, a chemical reagent used to establish a hypoxia
cell model, induced cell proliferation and migration in a certain
dose range. The concentration of 200 μM CoCl2 was
determined to be appropriate for treating RF/6A cells and
simulating CNV formation in vitro.
Endostar, a novel recombinant human endostatin, was
synthesized in China and approved as an anticancer drug by the
State Food and Drug Administration in 2005 (18). Endostar is easier to purify and has
more clinical advantages than Endostatin, demonstrating stable
physicochemical characteristics and higher water solubility
(19). Endostar exhibits
anti-angiogenic effects and has been used to treat numerous types
of cancer, including non-small lung, breast and gastric cancer
(20–22). However, its applications in the
field of ocular disease and the possible underlying molecular
mechanisms of its effects have not been well investigated.
The present study demonstrated that CoCl2
treatment promoted the proliferation and migration of RF/6A cells
and arrested more cells in the S phase, leaving fewer cells in the
G0/G1 phase, while pretreatment with Endostar significantly
reversed all CoCl2-mediated effects in RF/6A cells. This
suggested that the effects of Endostar on the proliferation and
migration of CoCl2-induced RF/6A cells may occur due to
the inhibition of RF/6A cell transition from G0/G1 phase to S
phase.
HIF-1, a DNA binding protein, is an important
transcription factor regulating hypoxia (23). It has been found to be
overexpressed under hypoxic conditions, implying that hypoxia may
increase the content of HIF-1α to regulate the expression of its
downstream genes, including VEGF (7,23–25).
In addition, HIF-1 may directly or indirectly regulate the
expression of numerous genes, such as VEGF, placental growth factor
and TGF-β1 (26), in myocardial
cells, fibroblasts and smooth muscle cells by binding to its
binding site in their promoters (27).
VEGF is a necessary stimulator for retinal and
choroidal neovascularization. Numerous agents that bind VEGF or
block VEGF receptors may suppress retinal and choroidal
neovascularization (28,29). Hypoxia is the main factor leading
to CNV, and the subsequent simultaneous increase in VEGF and HIF-1α
expression (30). These studies
indicated that hypoxia may enhance the expression of HIF-1α, which
subsequently regulates the expression of VEGF. In the present
study, it was identified that CoCl2 enhanced the
expression of HIF-1α and VEGF in RF/6A cells in vitro.
Endostatin has been demonstrated to exert
anti-angiogenenic effects in a HIF-1α-dependent manner (31). In human lung adenocarcinoma cancer
cells, Endostar was able to suppress HIF-1α and VEGF expression and
radiotherapy-induced angiogenesis (32). The present study demonstrated that
Endostar inhibited CoCl2-induced HIF-1α and VEGF
expression in RF/6A cells, suggesting that Endostar may affect cell
proliferation and migration through regulating the HIF-1α/VEGF
pathway.
In conclusion, Endostar, a recently introduced
recombinant human endostatin, is able to inhibit
CoCl2-induced RF/6A cell proliferation and migration
possibly by downregulating HIF-1α and secondarily inhibiting VEGF
expression. These results indicate that Endostar may have an
important role in hypoxia-induced CNV, which highlights its
significant potential for clinical application.
Acknowledgements
This study was supported by the Youth Program of the
National Natural Science Foundation of China (grant no.
11104246/A040414); the Zhejiang Natural Science Foundation (grant
no. Y2100380); the Zhejiang Science and Technology Department
Public Project (grant no. 2010C33085); the Zhejiang Research
Foundation of Integrated Traditional Chinese and Western Medicine
(grant no. 2012LY013); and the Key Lab Fund of Zhejiang Province
(grant no. 2011E10006).
References
|
1
|
Risau W: Mechanisms of angiogenesis.
Nature. 386:671–674. 1997. View
Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ambati J, Ambati BK, Yoo SH, Ianchulev S
and Adamis AP: Age-related macular degeneration: etiology,
pathogenesis, and therapeutic strategies. Surv Ophthalmol.
48:257–293. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Friedman DS, O’Colmain BJ, Munoz B, et al:
Prevalence of age-related macular degeneration in the United
States. Arch Ophthalmol. 122:564–572. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Bhutto I and Lutty G: Understanding
age-related macular degeneration (AMD): relationships between the
photoreceptor/retinal pigment epithelium/Bruch’s
membrane/choriocapillaris complex. Mol Aspects Med. 33:295–317.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Xie P, Zhang W, Yuan S, et al: Suppression
of experimental choroidal neovascularization by curcumin in mice.
PLoS One. 7:e533292012. View Article : Google Scholar
|
|
6
|
Yang XM, Wang YS, Zhang J, et al: Role of
PI3K/Akt and MEK/ERK in mediating hypoxia-induced expression of
HIF-1alpha and VEGF in laser-induced rat choroidal
neovascularization. Invest Ophthalmol Vis Sci. 50:1873–1879. 2009.
View Article : Google Scholar
|
|
7
|
Dong X, Wang YS, Dou GR, et al: Influence
of DII4 via HIF-1alpha-VEGF signaling on the angiogenesis of
choroidal neovascularization under hypoxic conditions. PLoS One.
6:e184812011. View Article : Google Scholar
|
|
8
|
Kang HM and Koh HJ: Intravitreal
anti-vascular endothelial growth factor therapy versus photodynamic
therapy for idiopathic choroidal neovascularization. Am J
Ophthalmol. 155:713–719. 2013. View Article : Google Scholar
|
|
9
|
Kim YM, Jang JW, Lee OH, et al: Endostatin
inhibits endothelial and tumor cellular invasion by blocking the
activation and catalytic activity of matrix metalloproteinase.
Cancer Res. 60:5410–5413. 2000.PubMed/NCBI
|
|
10
|
Abdollahi A, Hlatky L and Huber PE:
Endostatin: the logic of antiangiogenic therapy. Drug Resist Updat.
8:59–74. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Mori K, Ando A, Gehlbach P, et al:
Campochiaro PA. Inhibition of choroidal neovascularization by
intravenous injection of adenoviral vectors expressing secretable
endostatin. Am J Pathol. 159:313–320. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Tatar O, Shinoda K, Kaiserling E, et al:
Early effects of triamcinolone on vascular endothelial growth
factor and endostatin in human choroidal neovascularization. Arch
Ophthalmol. 126:193–199. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Xu W, Ye P, Li Z, Shi J, Wang W and Yao K:
Endostar, a recently introduced recombinant human endostatin,
inhibits proliferation and migration through regulating growth
factors, adhesion factors and inflammatory mediators in
choroid-retinal endothelial cells. Mol Biol. 44:664–670. 2010.
View Article : Google Scholar
|
|
14
|
Neely KA and Gardner TW: Ocular
neovascularization: clarifying complex interactions. Am J Pathol.
153:665–670. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Hay WW Jr and Bell EF: Oxygen therapy,
oxygen toxicity, and the STOP-ROP trial. Pediatrics. 105:424–425.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Plum SM, Vu HA, Mercer B, Fogler WE and
Fortier AH: Generation of a specific immunological response to
FGF-2 does not affect wound healing or reproduction.
Immunopharmacol Immunotoxicol. 26:29–41. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Chen Y, Li XX, Xing NZ and Cao XG:
Quercetin inhibits choroidal and retinal angiogenesis in vitro.
Graefes Arch Clin Exp Ophthalmol. 246:373–378. 2008. View Article : Google Scholar
|
|
18
|
Ling Y, Yang Y, Lu N, et al: Endostar, a
novel recombinant human endostatin, exerts antiangiogenic effect
via blocking VEGF-induced tyrosine phosphorylation of KDR/Flk-1 of
endothelial cells. Biochem Biophys Res Commun. 361:79–84. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Jiang LP, Zou C, Yuan X, Luo W, Wen Y and
Chen Y: N-terminal modification increases the stability of the
recombinant human endostatin in vitro. Biotechnol Appl Biochem.
54:113–120. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Lu N, Ling Y, Gao Y, et al: Endostar
suppresses invasion through downregulating the expression of matrix
metalloproteinase-2/9 in MDA-MB-435 human breast cancer cells. Exp
Biol Med. 233:1013–1020. 2008. View Article : Google Scholar
|
|
21
|
Wen QL, Meng MB, Yang B, et al: Endostar,
a recombined humanized endostatin, enhances the radioresponse for
human nasopharyngeal carcinoma and human lung adenocarcinoma
xenografts in mice. Cancer Sci. 100:1510–1519. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Bong R, Yang S, Li W, Zhang W and Ming Z:
Systematic review and meta-analysis of Endostar (rh-endostatin)
combined with chemotherapy versus chemotherapy alone for treating
advanced non-small cell lung cancer. World J Surg Oncol.
10:1702012. View Article : Google Scholar
|
|
23
|
Covello KL, Simon MC and Keith B: Targeted
replacement of hypoxia-inducible factor-1alpha by a
hypoxia-inducible factor-2alpha knock-in allele promotes tumor
growth. Cancer Res. 65:2277–2286. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Semenza GL: Angiogenesis in ischemic and
neoplastic disorders. Annu Rev Med. 54:17–28. 2003. View Article : Google Scholar
|
|
25
|
Semenza GL: HIF-1: mediator of
physiological and pathophysiological responses to hypoxia. J Appl
Physiol. 88:1474–1480. 2000.PubMed/NCBI
|
|
26
|
Kelly BD, Hackett SF, Hirota K, Oshima Y,
Cai Z, Berg-Dixon S, Rowan A, Yan Z, Campochiaro PA and Semenza GL:
Cell type-specific regulation of angiogenic growth factor gene
expression and induction of angiogenesis in nonischemic tissue by a
constitutively active form of hypoxia-inducible factor 1. Circ Res.
93:1074–1081. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Lukiw WJ, Ottlecz A, Lambrou G, Grueninger
M, Finley J, Thompson HW and Bazan NG: Coordinate activation of
HIF-1 and NF-kappaB DNA binding and COX-2 and VEGF expression in
retinal cells by hypoxia. Invest Ophthalmol Vis Sci. 44:4163–4170.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Berglin L, Sarman S, van der Ploeg I, et
al: Reduced choroidal neovascular membrane formation in matrix
metalloproteinase-2-deficient mice. Invest Ophthalmol Vis Sci.
44:403–408. 2003. View Article : Google Scholar
|
|
29
|
Haas TL: Endothelial cell regulation of
matrix metalloproteinases. Can J Physiol Pharmacol. 83:1–7. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Moses MA: The regulation of
neovascularization of matrix metalloproteinases and their
inhibitors. Stem Cells. 15:S180–S189. 1997. View Article : Google Scholar
|
|
31
|
Jia Y, Liu M, Cao L, et al: Recombinant
human endostatin, Endostar, enhances the effects of
chemo-radiotherapy in a mouse cervical cancer xenograft model. Eur
J Gynaecol Oncol. 32:316–324. 2011.PubMed/NCBI
|
|
32
|
Zhang L, Ge W, Hu K, et al: Endostar
down-regulates HIF-1 and VEGF expression and enhances the
radioresponse to human lung adenocarcinoma cancer cells. Mol Biol
Rep. 39:89–95. 2012. View Article : Google Scholar
|