Introduction
Melanoma differentiation-associated-7
(mda-7)/interleukin-24 (IL-24), a member of the IL-10 family of
cytokines, is a unique tumor suppressor (1). A number of studies have demonstrated
that IL-24 exhibits broad spectrum antitumor activity without
damaging normal cells (2,3). Forced IL-24 expression and exposure
to IL-24 induces cell-cycle alteration, apoptosis and toxic
autophagy, and consequently elicits growth inhibition in numerous
types of cancer cell (2,3). IL-24 also directly inhibits vascular
endothelial cell differentiation and migration via the
IL-20R2/IL-22R heterodimeric receptor (4) and indirectly represses the production
of the vascular endothelial growth factor and IL-8 proangiogenic
factors (5,6), resulting in the suppression of tumor
angiogenesis. In addition, IL-24 exerts marked immunomodulatory
activity and enhances antitumor immunity through the induction of
robust production of IL-6, tumor necrosis factor-α and interferon-γ
(7). Furthermore, IL-24
efficiently impairs tumor invasion and migration through the
downregulation of phosphatidylinositide 3-kinase (PI3K), focal
adhesion kinase and matrix metalloproteinases (8). Notably, IL-24 exerts potent antitumor
bystander activity via IL-20R1/IL-20R2 and IL-20R2/IL-22R-mediated
autocrine/paracrine signaling (2,9).
Most importantly, IL-24 sensitizes cancer cells to radiation-,
chemotherapy-, monoclonal antibody- and histone deacetylase
inhibitor-induced antitumor effects (2,3,10).
Thus, IL-24 is a multifunctional tumor suppressing cytokine and is
currently regarded as a ‘magic bullet’ for cancer.
Breast cancer is the most common type of malignancy
(23%) among females worldwide and is the second leading cause of
cancer-associated mortality in females (14%) (11). Conventional therapeutic methods for
breast cancer include surgery, chemotherapy, radiation therapy,
hormone therapy and targeted therapy, or a combination of these
treatments (12). In spite of
significant advances in early detection and considerable clinical
therapeutic success, de novo and acquired resistance
currently remains a therapeutic challenge in breast cancer
treatment. Although the primary lesion may be eradicated, the
disease is resistant to the majority of conventional therapeutic
methods, which frequently results in relapse and metastasis, thus
warranting the search for novel therapeutic strategies and agents
for use in breast cancer therapy.
IL-24 has been shown to be substantially
downregulated in breast cancer, which contributes to adverse
pathological features and poor clinical outcomes (13,14).
A preclinical animal study revealed that adenovirus-mediated IL-24
gene therapy (Ad.mda-7) is a promising candidate for the
treatment of breast cancer (15).
The bacteria fusion protein glutathione-S-transferase-IL-24, a
novel therapeutic agent, also exerts cancer-selective killing
activity (16). Furthermore,
bacteria-derived arginine-glycine-aspartic acid-modified IL-24
protein exhibits enhanced antitumor activity against breast cancer
via targeting the αVβ3 and αVβ5 integrin receptors (17). However, to the best of our
knowledge, no side-by-side studies that compare the antitumor
effects of eukaryotically and bacterially expressed recombinant
human IL-24 (rhIL-24) protein have been conducted. In the present
study, the rhIL-24 protein was expressed in BL21 bacteria
transformed with the pET-21a(+)-hIL-24 plasmid and eukaryotic
Chinese hamster ovary (CHO) cells stably transfected with the
pcDNA3-hIL-24 plasmid, and the antitumor efficacy and underlying
mechanisms of the two treatments were directly compared in
vitro using the MDA-MB-231 human triple-negative breast cancer
cell line that expresses the IL-20R1/IL-20R2 receptor (17). The in vivo therapeutic
efficacy of the bacterial rhIL-24 protein and the pcDNA3-hIL-24
naked plasmid were also analyzed using subcutaneously (s.c.)
xenografted MDA-MB-231 breast cancer cell tumors.
Materials and methods
Plasmids, cell lines, reagents and
mice
The pUC19-hIL-24 cloning vector containing
full-length human IL-24 cDNA was constructed in the laboratory of
the Department of Cell and Molecular Biology, Soochow University
(Suzhou, China). The pET21a(+) prokaryotic expression plasmid and
BL21 bacteria were purchased from Novagen (Beijing, China). The
pcDNA3 eukaryotic expression plasmid, Lipofectamine™ 2000 and
neomycin (G418) were obtained from Invitrogen (Shanghai, China).
The MDA-MB-231 human breast cancer cell line and the CHO cell line
were bought from the American Type Culture Collection (Rockville,
MD, USA). The QBI-293A human embryonic kidney cell line was
provided by Professor Jiang Zhong (Department of Microbiology,
College of Life Science, Fudan University, Shanghai, China). The
MDA-MB-231, QBI-293A and CHO cells were cultured in RPMI-1640
medium (Invitrogen) supplemented with 10% fetal bovine serum
(HyClone, Logan, UT, USA). AxyPrep Plasmid Miniprep, AxyPrep DNA
Gel Extraction and AxyPrep DNA Purification kits were purchased
from Axygen Biosciences (Union City, CA, USA). BamHI,
XhoI, KpnI and XbaI restriction endonucleases,
T4 DNA ligase, DL2000 DNA marker and
isopropyl-β-D-1-thiogalactopyranoside (IPTG) were obtained from
Takara Biotechnology Co., Ltd. (Dalian, China). The
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
kit and the mammalian cell lysis kit were purchased from
Sigma-Aldrich (Shanghai, China). The Annexin V-fluorescein
isothiocyanate (FITC)/propidium iodide (PI) apoptosis detection kit
was provided by BD Biosciences (Shanghai, China). The IL-24
antibody and the IL-24 enzyme-lined immunosorbent assay (ELISA) kit
were obtained from R&D Systems (Shanghai, China). The
antibodies specific for B cell lymphoma 2 (Bcl-2), Bcl-2-associated
X protein (Bax), cleaved caspase-3, cluster of differentiation
(CD)34 and β-actin were purchased from Cell Signaling Technology,
Inc. (Boston, MA, USA). The terminal deoxynucleotidyl
transferase-mediated dUTP nick end labeling (TUNEL) apoptosis
detection kit was bought from Beyotime Institute of Biotechnology
(Beijing, China). The UltraSensitive™ SP kit was provided by Fuzhou
Maixin Biotechnology Development Co., Ltd. (Fuzhou, China).
Four-week-old male athymic BALB/c nude mice were purchased from
Shanghai Experimental Animal Center (Shanghai, China) and were
maintained in the animal facility at Soochow University (Suzhou,
China) according to the Soochow University animal research
committee guidelines. The present study was approved by the ethics
committee of Soochow University.
Construction of recombinant vectors
expressing human IL-24
The cDNA fragment encoding full-length mature human
IL-24 protein minus the signal peptide was amplified by polymerase
chain reaction using the pUC19-hIL-24 cloning plasmid as a template
and the following pair of primers specific for mature human IL-24:
Forward: 5′-CGGATCCATGCAGGGCCAAGAATTCCAC-3′ and reverse:
5′-CCTCGAGCTAGAGCTTGTAGAATTTCT-3′. The primers were subsequently
subcloned into the pET-21a(+)prokaryotic expression plasmid at the
BamHI and XhoI sites to generate pET-21a(+)-hIL-24.
The cDNA fragment encoding the full-length human IL-24 protein
containing the signal peptide was directly released from the
pUC19-hIL-24 plasmid using KpnI and XbaI double
digestion, and was subsequently subcloned into the pcDNA3
eukaryotic expression plasmid at the KpnI and XbaI
sites to form pcDNA3-hIL-24.
Expression of rhIL-24
To analyze human IL-24 expression in a prokaryotic
system, BL21 Escherichia coli (E. coli) was transformed with
either the pET-21a(+)-hIL-24 construct or the pET-21a(+) control
plasmid, and the bacteria were treated with IPTG according to the
IPTG kit manufacturer’s instructions. Briefly, the
BL21/pET-21a(+)-hIL-24 and BL21/pET-21a(+) plasmids were incubated
overnight in Luria-Bertani (LB) medium (Sangon Biotech Co., Ltd,
Shanghai, China) containing 100 μg/ml ampicillin (Amp; Sangon
Biotech Co., Ltd). Subsequently, the bacteria were transferred at a
2% dilution into fresh LB medium with 100 μg/ml Amp. When optical
density at 600 nm reached ~0.6, as measured using a SmartSpec 3000
spectrophotometer (Bio-Rad Laboratories, Inc., Hercules, CA, USA),
the bacteria were induced using IPTG at a final concentration of 1
mM and then harvested at different time points (0–5 h). To examine
human IL-24 expression in a eukaryotic system, the QBI-293A and CHO
mammalian cells were transfected with either the pcDNA3-hIL-24
plasmid or the pcDNA3 control plasmid using Lipofectamine 2000
according to the manufacturer’s instructions. After 72 h
transfection, the supernates and the cell lysates from the QBI-293A
transfectants (a high-efficiency transfection cell line) were
collected for the measurement of human IL-24 expression levels. At
48 h after transfection, the CHO transfectants were cultured with 1
mg/ml neomycin for 2–3 weeks, replacing culture medium every 3–4
days. CHO/pcDNA3-hIL-24-positive clones expressing IL-24 were
further selected and amplified.
Purification of bacterial rhIL-24
protein
Following the establishment of optimal conditions
for prokaryotic expression, the rhIL-24 protein was abundantly
induced at the 3 h time point following 1 mM IPTG treatment of
BL21/pET-21a(+)-hIL-24 bacteria. The bacteria were harvested at
1,500 × g and washed with 0.9% NaCl solution. Subsequently, the
samples were sonicated (Shunmayq, Inc., Nanjing, China) at a high
setting (duty time, 30 sec; rest time, 30 sec) at 480 W in an ice
bath and centrifuged at 1,500 × g at 4°C, and the pellets were
resuspended in washing buffer (Sangon Biotech Co., Ltd) containing
20% glycerol (v/v), 2% Triton X-100 (v/v), 2 M urea, 50 mM NaCl, 50
mM ethylenediaminetetraacetic acid (EDTA) and 100 mM Tris-HCl (pH
8.0). Following three cycles of washing, the deposit was denatured
in 7 M urea with 5 mM dithiothreitol for 4 h. The supernate was
collected by centrifugation at 3,600 × g at 4°C, and was placed in
renaturation solution containing 2 M urea, 100 μM PMSF, 50 mM EDTA
and 100 mM Tris-HCl (pH 8.2) at 4°C for 24 h with constant
agitation. Subsequently, the renaturation solution containing the
rhIL-24 protein was dialyzed for 24 h using phosphate-buffered
saline (PBS; Sangon Biotech Co., Ltd), replacing the PBS every 4 h.
The resultant solution containing the renatured rhIL-24 protein was
loaded onto a pre-equilibrated Q-Sepharose column (GE Healthcare,
Beijing, China) according to the manufacturer’s instructions.
Following a further equilibration step, the renatured rhIL-24
protein was eluted and collected.
SDS-PAGE and western blot analysis
The IPTG-induced (1–5 h) and uninduced (0 h)
BL21/pET-21a(+)-hIL-24 or BL21/pET-21a(+), and the purified rhIL-24
protein samples were boiled in SDS sample loading buffer with
β-methasone (Sangon Biotech Co., Ltd) and resolved by 12% SDS-PAGE,
respectively. The prokaryotic rhIL-24 protein present in the gels
was detected with Coomassie brilliant blue R-250 staining. The
IPTG-induced BL21/pET-21a(+)-hIL-24 and purified rhIL-24 protein
were then transferred to a nitrocellulose membrane (Sangon Biotech
Co., Ltd). The membrane was immunoblotted with mouse anti-human
IL-24 monoclonal antibody (1:1,000; Clone 244202, Cat. MAB1965;
R&D Systems) for 1 h at 37°C and washed with Tris-buffered
saline with Tween 20 (Sangon Biotech Co., Ltd) three times,
followed by incubation with horseradish peroxidise-labeled goat
anti-mouse immunoglobulin G (1:3,000; sc-2005; Santa Cruz
Biotechnology, Inc., Dallas, Texas, USA) for another 1 h at 37°C.
Subsequently, the membranes were washed and developed using a
SuperEnhanced chemiluminescence detection kit (Applygen
Technologies Inc., Beijing, China) following the manufacturer’s
instructions. The protein bands were visualized following exposure
of the membrane to Kodak X-ray film (Kodak, Rochester, NY,
USA).
ELISA
The culture supernates derived from the
pcDNA3-hIL-24- and pcDNA3-transfected QBI-293A cells, and the CHO
cells were collected, and the quantities of rhIL-24 in the
supernates were assessed using the human IL-24 ELISA kit according
to the manufacturer’s instructions.
MTT assay
The in vitro cytotoxicity of rhIL-24 to
MDA-MB-231 human breast cancer cells was assessed using an MTT
assay. Briefly, the MDA-MB-231 tumor cells (1×104
cells/well) were dispensed in a 96-well culture plate. At 24 h
after culture, the tumor cells were treated with the purified
prokaryotic rhIL-24 protein at a final concentration of 5 ng/ml for
the indicated time periods (0–5 days). The medium containing PBS
without rhIL-24 served as a control. Another group of tumor cells
were incubated with either medium containing 50% (v/v) of the
supernate with IL-24 derived from the CHO/pcDNA3-hIL-24 stable
transgenic cells (IL-24 final concentration, 5 ng/ml) or the
control supernate from CHO/pcDNA3 cells. Prior to treatment and at
different time points following treatment, the viability of the
MDA-MB-231 tumor cells was determined by an MTT assay according to
the manufacturer’s instructions.
Flow cytometric analysis
The in vitro effect of rhIL-24 treatment on
the apoptosis of MDA-MB-231 human breast cancer cells was evaluated
by flow cytometry. A total of 1×106 MDA-MB-231 tumor
cells were treated with purified prokaryotic rhIL-24 protein (5
ng/ml) or PBS, and 50% (v/v) of the supernate with IL-24 (IL-24
final concentration, 5 ng/ml) or control supernate, respectively.
At 24–72 h after treatment, the MDA-MB-231 tumor cells were
collected and washed in cold PBS. Apoptosis was analyzed by flow
cytometry using Annexin V-FITC (early apoptotic marker) and PI
(late apoptotic marker) double-staining following the
manufacturer’s instructions. Briefly, 1×105 tumor cells
were incubated with 5 μl Annexin V-PE and 5 μl 7-amino-actinomycin
D in 100 μl 1X Annexin V binding buffer at room temperature. After
15 min incubation, 400 μl 1X binding buffer was added and the
numbers of apoptotic cells were determined by flow cytometry.
Animal experiments
The male athymic BALB/c nude mice were s.c.
inoculated in the armpits of the right anterior limbs with
1×106 MDA-MB-231 human breast cancer cells and were then
monitored daily for tumor growth. Tumor volume was measured with a
caliper and was calculated by the following formula: Tumor size =
ab2/2, in which a is the larger and b is the
smaller of the two dimensions. When the MDA-MB-231 s.c. xenografted
tumors had reached a mean tumor volume of ~100 mm3, the
tumor-bearing mice were then subjected to in vivo treatment
experiments. The tumor-bearing mice were randomly divided into four
groups (eight mice in each group) and intratumorally injected with
2 μg prokaryotic rhIL-24 protein or PBS (serving as a control), and
a 1:1 (v/v) mixture of Lipofectamine 2000, and 10 μg pcDNA3-hIL-24
or 10 μg pcDNA3 (control) every other day a total of six times.
Tumor progression and regression were monitored daily. The
tumor-bearing mice were sacrificed three weeks after treatment. The
xenografted tumors were removed, weighted, fixed by 10% neutral
formalin and embedded in paraffin for hematoxylin and eosin
staining, and immunohistochemical analysis.
Immunohistochemical analysis
The in vivo expression levels of Bcl-2, Bax,
cleaved caspase-3, CD34 and IL-24 in the treated and untreated s.c.
xenografted MDA-MB-231 human breast cancer cell tumor sections were
examined by immunohistochemistry using the UltraSensitive™ SP kit
according to the manufacturer’s instructions. The detection of
buffy or brown diaminobenzidine precipitates is indicative of
positive reactivity. The microvessel density (MVD) in the tumor
sections was examined by CD34 immunostaining, as previously
described (18). Any endothelial
cell cluster immunoreactive for CD34 clearly separated from the
adjacent microvessels was considered a single countable vessel. To
analyze the numbers of apoptotic cells in the MDA-MB-231
xenografted tumors, the tumor sections were further examined for
the presence of apoptosis using the TUNEL apoptosis detection kit
according to the manufacturer’s instructions. Each value indicates
the number of immunoreactive cells, microvessels or apoptotic cells
counted at a high-power microscopic view (x200). The mean value
signifies the average number derived from five high-power fields
from each sample.
Statistical analysis
All data are presented as the mean ± standard
deviation. The significance of the difference between groups was
evaluated by Student’s t-test with SPSS 10.0 software (SPSS, Inc.,
Chicago, IL, USA). P<0.05 was considered to indicate a
statistically significant difference.
Results
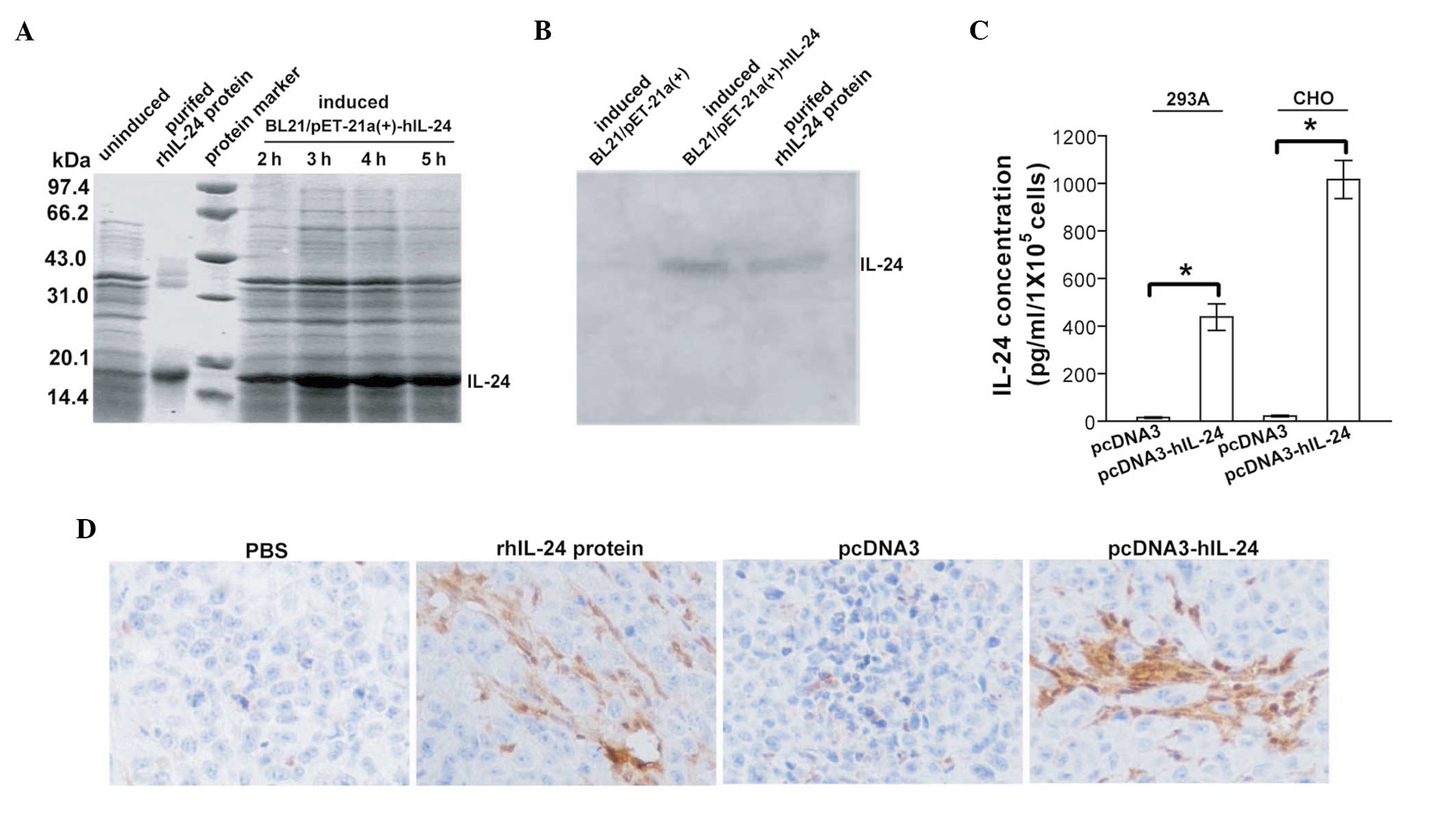
Expression and purification of
rhIL-24
Following IPTG induction, the E. coli
BL21/pET-21a(+)-hIL-24 strain expressed a protein band at ~18.5 kDa
molecular weight, which was particularly evident after 3 h
induction (Fig. 1A). This is
consistent with the theoretical molecular weight of unmodified
mature human IL-24 protein. This band did not appear in the
IPTG-uninduced strain or the IPTG-induced BL21/pET-21a(+) control
strain (data not shown). Western blot analysis (Fig. 1B) further verified that this band
was the human IL-24 protein. To purify the rhIL-24 protein derived
from the prokarytotic expression system, the BL21/pET-21a(+)-hIL-24
stain was abundantly cultured and induced by IPTG for 3 h. The
rhIL-24 insoluble inclusion body was then collected and denatured.
Subsequently, the rhIL-24 soluble protein was renatured and
purified through a Q-Sepharose column. SDS-PAGE (Fig. 1A) and western blot analysis
(Fig. 1B) revealed that the
purified protein was relatively pure rhIL-24 protein. In addition,
the rhIL-24 expression levels in the mammalian cells, QBI-293A
cells transiently transfected with pcDNA3-hIL-24 and CHO cells
stably transfected with pcDNA3-hIL-24, were determined by ELISA
analysis of the respective culture supernates (Fig. 1C). To further analyze the stability
of the purified bacteria rhIL-24 protein and the naked
plasmid-mediated IL-24 expression in vivo, the s.c.
xenografted MDA-MB-231 human breast cancer tumors were removed for
immunohistochemical analysis of IL-24 at three weeks after the
initiation of treatment. In vivo IL-24 expression, following
intratumoral injections of soluble rhIL-24 protein, was
persistently detected, which indicated that the purified rhIL-24
protein was relatively stable and durable (Fig. 1D). Intratumoral injections of the
liposome-coated pcDNA3-hIL-24 naked plasmid were also capable of
efficiently inducing in vivo IL-24 transgene expression
(Fig. 1D).
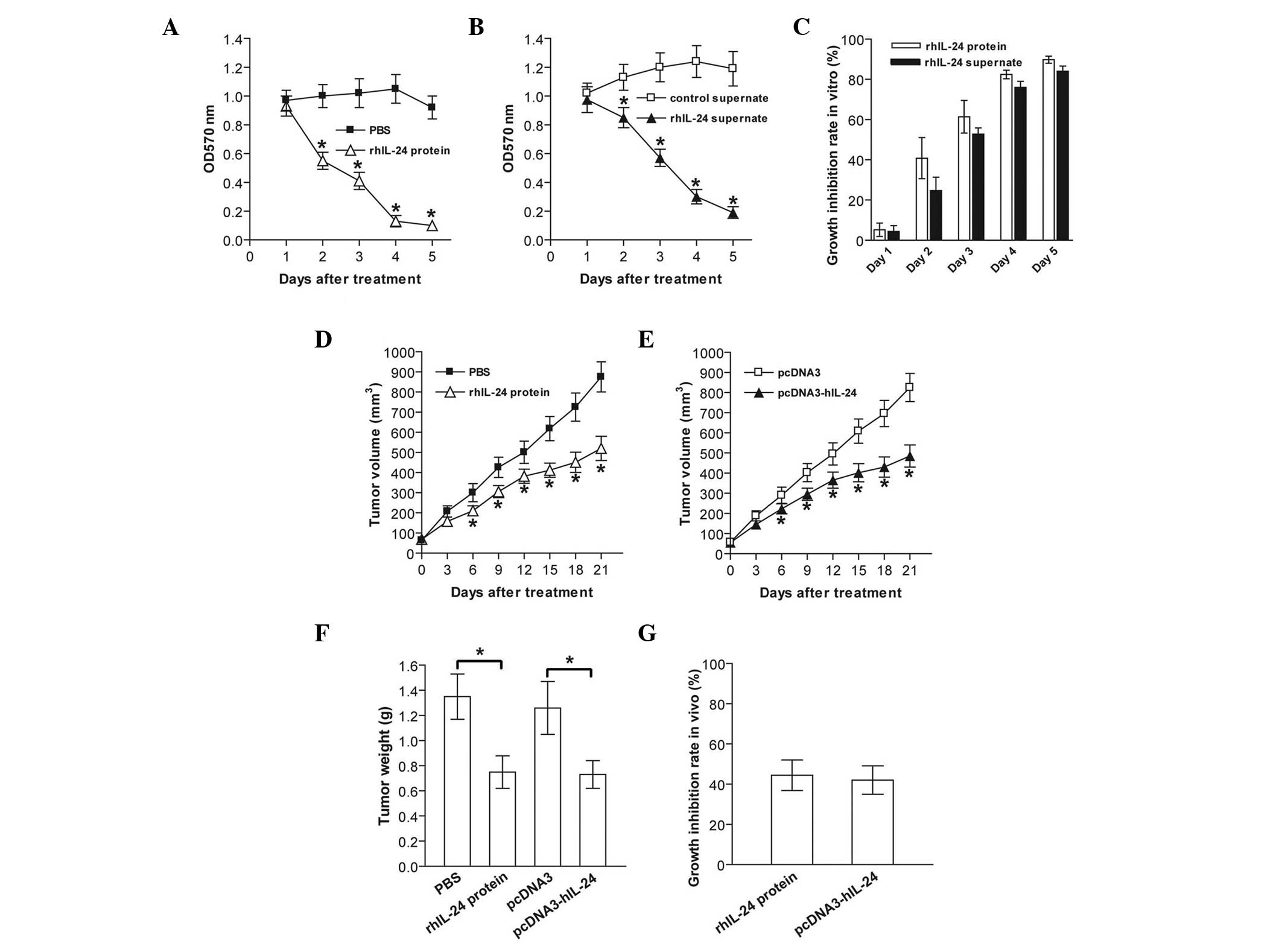
rhIL-24 suppresses breast cancer cell
growth
To examine the cytotoxic activity of prokarytotic
bacteria- and eukaryotic cell-derived rhIL-24 against MDA-MB-231
human breast cancer cells, the cells were treated with purified
rhIL-24 protein from prokarytotic BL21 bacteria and rhIL-24
supernate from the CHO eukaryotic mammalian cells, respectively.
Tumor cell viability was assessed daily for five days using an MTT
assay. The bacterial rhIL-24 protein and eukaryotically expressed
rhIL-24 supernate significantly suppressed in vitro
MDA-MB-231 tumor cell growth in a time-dependent manner (p<0.05;
Fig. 2A–C). Furthermore, the in
vivo growth of s.c. xenografted MDA-MB-231 cell tumors in
athymic nude mice was efficiently retarded when the tumors were
treated with rhIL-24 protein or rhIL-24 supernate (p<0.05;
Fig. 2D–G). These results
indicated that prokarytotic system-derived renatured rhIL-24
protein and eukaryotic system-derived rhIL-24 were capable of
significantly inhibiting MDA-MB-231 tumor growth in vitro
and in vivo.
rhIL-24 induces breast cancer
apoptosis
To analyze the underlying mechanism of
rhIL-24-elicted MDA-MB-231 tumor cell cytotoxicity, the MDA-MB-231
human breast cancer cells were treated with purified rhIL-24
protein or rhIL-24 supernate for 24–72 h. The tumor cells were then
harvested and subjected to apoptotic analysis using Annexin
V-FITC/PI double-staining. Treatment with rhIL-24 protein induced
MDA-MB-231 tumor cell apoptosis in a time-dependent manner
(Fig. 3A). As compared with the
PBS control group, significantly greater numbers [9.5% (24 h),
30.3% (48 h) and 50.2% (72 h)] of late apoptotic cells were
observed in the rhIL-24 protein-treated group (p<0.05; Fig. 3A). Comparable with the
apoptosis-inducing effect of the bacterial rhIL-24 protein, the
eukaryotically expressed rhIL-24 supernate also significantly
induced MDA-MB-231 tumor cell apoptosis (P<0.05; Fig. 3B), in particular at 72 h. To
further verify the in vivo induction of apoptosis in rhIL-24
protein- and rhIL-24 supernate-treated, and untreated control s.c.
xenografted MDA-MB-231 human breast cancer tumors,
immunohistochemical analysis (Fig.
3C) and a TUNEL assay (Fig.
3D) were performed. Consistent with the in vitro flow
cytometric analysis of apoptosis, the two forms of rhIL-24 protein
also significantly induced in vivo apoptosis in MDA-MB-231
tumor cells s.c. implanted in athymic nude mice (p<0.05).
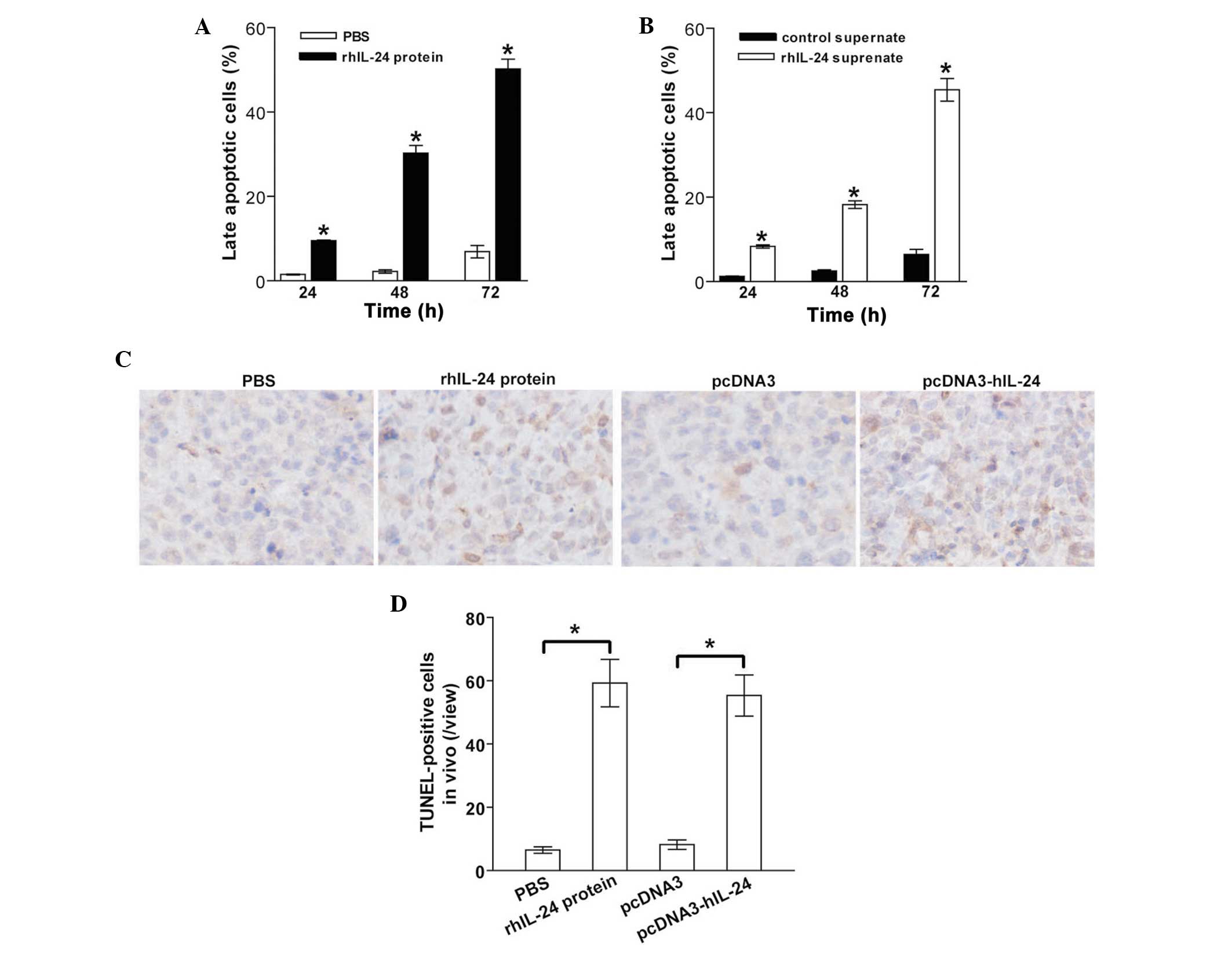 | Figure 3rhIL-24 induces tumor apoptosis in
vitro and in vivo. (A and B) Flow cytometric analysis of
in vitro apoptosis. The MDA-MB-231 human breast cancer cells
were treated with PBS, rhIL-24 protein, control supernate or
rhIL-24 supernate. The tumors were harvested at different time
points (24–72 h) after treatment, stained with Annexin
V-fluorescein isothiocyanate and PI, and then analyzed by flow
cytometry. The double Annexin V- and PI-positive cells in the total
cell population indicated late apoptotic cells. (A) Apoptotic rate
in PBS- and rhIL-24-treated cells. *P<0.05 compared
with the PBS-treated cells. (B) Apoptotic rate in cells treated
with rhIL-24 or control supernate. *P<0.05 compared
with the cells treated with the control supernate. Data were
analyzed by the Student’s t-test; n=3 replicates per condition. (C
and D) TUNEL analysis of in vivo apoptosis in MDA-MB-231
subcutaneously xenografted tumors. (C) Representative images of the
TUNEL assay. (D) TUNEL-positive cells signified in vivo
apoptotic cells. *P<0.05 compared with the PBS and
pcDNA3 cells, respectively. N=8, replicates per condition; n=5,
sections per replicate; n=5, observations per section. Data are
representative of three independent experiments. rhIL-24,
recombinant human interleukin-24; PBS, phosphate-buffered saline;
PI, propidium iodide; TUNEL, terminal deoxynucleotidyl
transferase-mediated dUTP nick end labeling. |
rhIL-24 upregulates Bax/Bcl-2 and
activates caspase-3
To elucidate the underlying molecular mechanism of
the IL-24-mediated antitumor effects, the expression levels of
Bcl-2 family proteins associated with apoptosis, such as Bcl-2 and
Bax, and cleaved caspase-3, in the s.c. xenografted MDA-MB-231
human breast cancer cell tumors following the different treatments
were assessed by immunohistochemistry. The expression levels of the
Bcl-2 antiapoptotic molecule in the rhIL-24 protein and rhIL-24
supernate groups were significantly reduced, whereas the
proapoptotic molecule Bax expression levels in these groups were
significantly increased (Fig. 4A).
Significantly increased cleaved caspase-3 expression levels were
also observed in the rhIL-24 protein and rhIL-24 supernate groups
(Fig. 4A). These results indicated
that bacteria rhIL-24 protein and eukaryotically expressed rhIL-24
supernate treatment efficiently induced MDA-MB-231 breast cancer
apoptosis via upregulation of the Bax/Bcl-2 ratio and activation of
caspase-3. The effect of the rhIL-24 protein and the pcDNA3-hIL-24
naked plasmid expressing human IL-24 on the in vivo
expression levels of Bcl-2, Bax and cleaved caspase-3 in s.c.
xenografted MDA-MB-231 cell tumors was further verified by
immunohistochemical analysis (Fig.
4B–D).
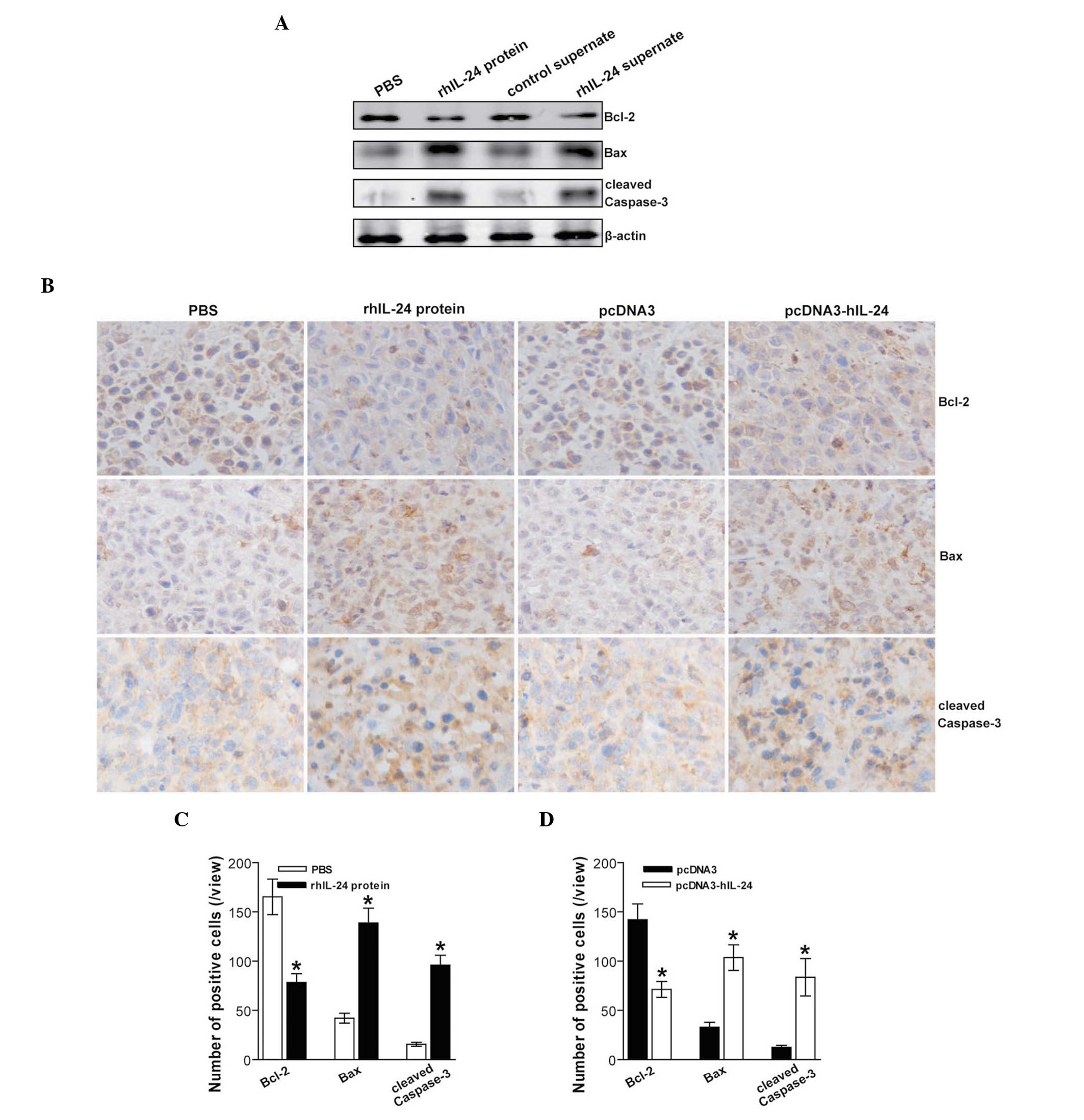 | Figure 4rhIL-24 activates intrinsic apoptosis
through upregulation of Bax/Bcl-2. (A) Western blot analysis of
apoptosis-associated proteins. The total cellular lysates derived
from PBS-, rhIL-24 protein-, control supernate- or rhIL-24
supernate-treated MDA-MB-231 human breast cancer cells were
immunoblotted with a panel of antibodies specific for Bcl-2, Bax,
cleaved caspase-3 and β-actin (serving as an internal control),
respectively. Representative images from western blot analysis are
shown. (B) Representative images of the immunohistochemical
detection of Bcl-2, Bax and cleaved caspase-3 in subcutaneously
xenografted MDA-MB-231 human breast cancer cell tumors. (C and D)
The number of positive cells as determined by immunohistochemical
detection. *P<0.05 compared with the respective
control conditions. Data were analyzed by the Student’s t-test;
n=8, replicates per condition; n=5, sections per replicate; n=5,
observations per section. Data are representative of three
independent experiments. rhIL-24, recombinant human interleukin-24;
PBS, phosphate-buffered saline; Bcl-2, B cell lymphoma 2; Bax,
Bcl-2-associated X protein. |
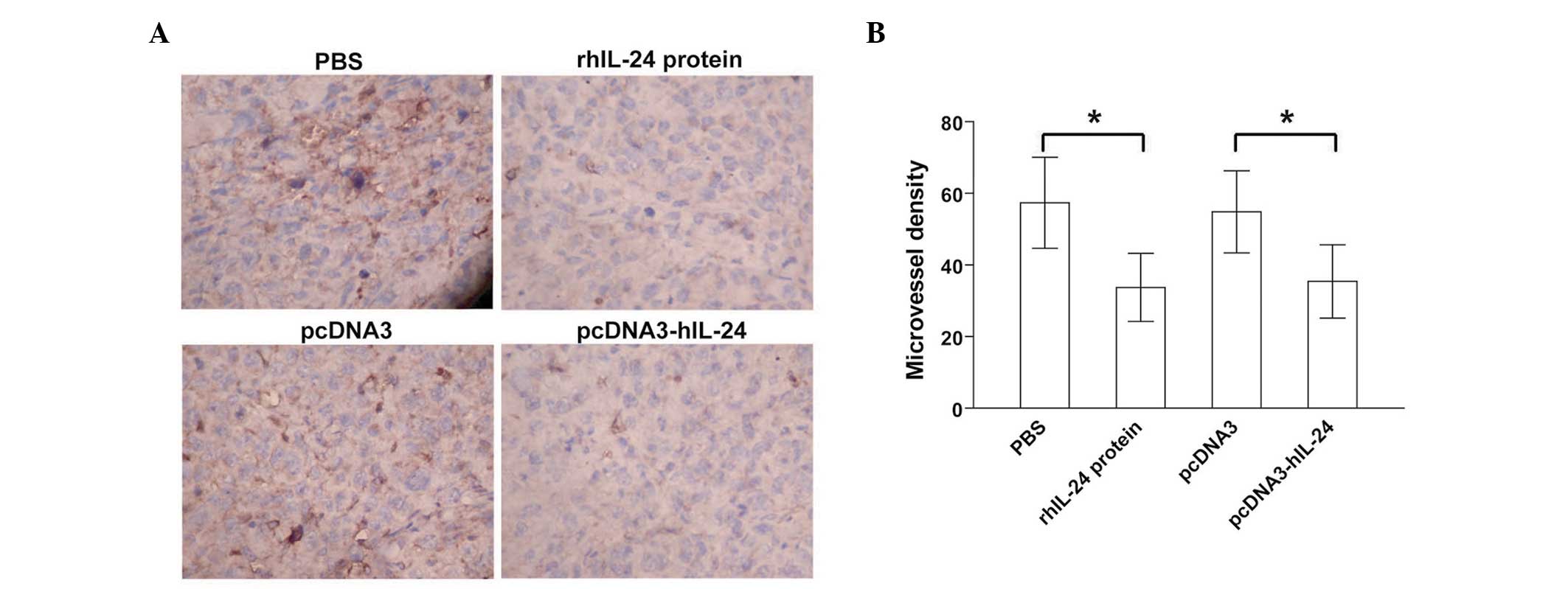
rhIL-24 inhibits tumor angiogenesis
Positive CD34 expression was predominantly evident
as brownish yellow or brownish granules in the vascular endothelial
cells of the s.c. xenografted MDA-MB-231 human breast cancer cell
tumors (Fig. 5A). As compared with
the respective PBS and pcDNA3 control groups, the CD34 expression
levels in tumor vascular endothelial cells in the rhIL-24 protein
and pcDNA3-hIL-24 treated groups were reduced (Fig. 5A). Furthermore, the MVD values
(Fig. 5B) counted in the rhIL-24
protein and pcDNA3-hIL-24 groups were significantly less than those
in the respective PBS and pcDNA3 control groups (p<0.05). These
data indicated that treatment with the bacterial rhIL-24 protein
and the pcDNA3-hIL-24 naked plasmid expressing human IL-24
efficiently suppressed in vivo tumor angiogenesis in
xenografted MDA-MB-231 breast cancer cell tumors.
Discussion
Numerous studies have revealed that cytokine-tumor
suppressor IL-24 inhibits tumor cell growth and induces apoptosis
in a large variety of cancer cell types via the activation of
double-stranded RNA-dependent protein kinase, p38 mitogen-activated
protein kinase, c-Jun NH2-terminal kinase and
endoplasmic reticulum stress-mediated unfolded protein response
signaling pathways, and the inhibition of the β-catenin and PI3K
signaling pathways (3,19). Furthermore, IL-24 as a cytokine may
be processed via class secretory pathways, bind specific cytokine
receptor complexes (IL-20R1/IL-20R2 and IL-20R2/IL-22R), and
consequently activate the Janus kinase/Signal Transducer and
Activator of Transcription signaling pathway (3,19).
The ability of IL-24 to discriminate between normal and tumor
cells, induce apoptosis, suppress tumor angiogenesis, stimulate
immune responses, promote bystander antitumor activity, and
synergize with anticancer drugs and radiation render IL-24 a strong
tumor suppressor in cancer treatment. Adenovirus-mediated IL-24
gene therapy (Ad.mda-7 and INGN 241) is currently undergoing
phase I clinical trials for the treatment of solid tumors (19,20).
Bacterially expressed rhIL-24 protein has also been demonstrated to
exert cancer-specific killing activity, providing an alternate
therapeutic reagent for cancer therapy (16).
rhIL-24 protein has been previously demonstrated to
exert in vitro and in vivo antitumor effects in lung
cancer (21) and gastric cancer
(22). In the present study, the
antitumor activity of bacteria- and CHO mammalian cell-derived
rhIL-24 protein, and pcDNA3-hIL-24 naked plasmid were assessed in
the IL-20R1/IL-20R2-expressing MDA-MB-231 human breast cancer cell
line in vitro and in vivo. The data revealed that the
prokarytotic system-derived rhIL-24 protein and the eukaryotic
system-derived secretory rhIL-24 protein were capable of
efficiently suppressing MDA-MB-231 tumor growth in vitro. In
the animal experiments, the bacterially expressed rhIL-24 protein
and the pcDNA3-hIL-24 naked plasmid exerted significant antitumor
activity on subcutaneously xenografted MDA-MB-231 breast cancer
cell tumors. Flow cytometric analysis and TUNEL assay further
verified that the antitumor efficacy of rhIL-24 in breast cancer
was dependent on the induction of apoptosis. These results were
consistent with those of a previous study that observed that
N-glycosylation of IL-24 was not mandatory for tumor cell-specific
apoptosis or bystander antitumor activity (23).
The ratio of Bax/Bcl-2 pro- to antiapoptotic
molecules constitutes a rheostat that sets the threshold of
susceptibility to caspase-3 activation and apoptosis for the
intrinsic pathway (24). In the
present study, to elucidate the underlying molecular mechanism
responsible for the induction of apoptosis, the Bcl-2 and Bax
apoptosis-associated proteins and cleaved caspase-3 were examined
using western blot analysis and immunohistochemistry. Therefore,
the rhIL-24-elicited upregulation of Bax/Bcl-2 and the activation
of caspase-3 may contribute to IL-24-induced tumor suppression in
breast cancer.
Tumor angiogenesis is also a prerequisite for
successful tumor growth and formation of metastases (25). Antiangiogenic therapy is regarded
as a potential and nontoxic therapeutic strategy in cancer therapy
(26). A great deal of data have
revealed that IL-24 inhibits tumor angiogenesis via direct
interaction with IL-20R2/IL-22R on vascular endothelial cells
(4) and via an indirect reduction
in the production of proangiogenic factor (5,6). In
the present study, CD34 immunohistochemical analysis revealed that
rhIL-24 downregulated CD34 expression and reduced MVD in s.c.
xenografted breast cancer cell tumors, which may be an additional
important mechanism responsible for rhIL-24-mediated in vivo
breast cancer growth suppression in athymic nude mice.
In conclusion, in the present study, prokaryotically
and eukaryotically expressed rhIL-24 were demonstrated to
efficiently suppress MDA-MB-231 tumor growth in vitro.
Similarly, bacteria-derived rhIL-24 protein and pcDNA3-hIL-24 naked
plasmid administration also provided therapeutic benefits for
xenografted MDA-MB-231 cell tumors in vivo. The retarded
breast cancer growth elicited by rhIL-24 was closely associated
with the induction of apoptosis induced by upregulation of the
Bax/Bcl-2 ratio and activation of caspase-3, and the marked
inhibition of tumor angiogenesis. Thus, these results indicate that
prokaryotically expressed rhIL-24 protein may be an alternate and
promising antitumor agent for the treatment of human breast cancer
or other types of cancer.
Acknowledgements
This study was supported by the National Natural
Science Foundation of China (grant no. 81001016).
References
|
1
|
Sauane M, Gopalkrishnan RV, Sarkar D, Su
ZZ, Lebedeva IV, Dent P, et al: MDA-7/IL-24: novel cancer growth
suppressing and apoptosis inducing cytokine. Cytokine Growth Factor
Rev. 14:35–51. 2003. View Article : Google Scholar
|
|
2
|
Fisher PB: Is mda-7/IL-24 a ‘magic bullet’
for cancer? Cancer Res. 65:10128–10138. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Whitaker EL, Filippov VA and
Duerksen-Hughes PJ: Interleukin 24: Mechanisms and therapeutic
potential of an anti-cancer gene. Cytokine Growth Factor Rev.
23:323–331. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ramesh R, Mhashilkar AM, Tanaka F, Saito
Y, Branch CD, Sieger K, et al: Melanoma differentiation-associated
gene 7/interleukin (IL)-24 is a novel ligand that regulates
angiogenesis via the IL-22 receptor. Cancer Res. 63:5105–5113.
2003.PubMed/NCBI
|
|
5
|
Saeki T, Mhashilkar A, Swanson X, Zou-Yang
XH, Sieger K, Kawabe S, et al: Inhibition of human lung cancer
growth following adenovirus-mediated mda-7 gene expression in vivo.
Oncogene. 21:4558–4566. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Nishikawa T, Ramesh R, Munshi A, Chada S
and Meyn RE: Adenovirus-mediated mda-7 (IL24) gene therapy
suppresses angiogenesis and sensitizes NSCLC xenograft tumors to
radiation. Mol Ther. 9:818–828. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Caudell EG, Mumm JB, Poindexter N,
Ekmekcioglu S, Mhashilkar AM, Yang XH, et al: The protein product
of the tumor suppressor gene, melanoma differentiation-associated
gene 7, exhibits immunostimulatory activity and is designated
IL-24. J Immunol. 168:6041–6046. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ramesh R, Ito I, Gopalan B, Saito Y,
Mhashilkar AM and Chada S: Ectopic production of MDA-7/IL-24
inhibits invasion and migration of human lung cancer cells. Mol
Ther. 9:510–518. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Sauane M, Su ZZ, Gupta P, Lebedeva IV,
Dent P, Sarkar D and Fisher PB: Autocrine regulation of mda-7/IL-24
mediates cancer-specific apoptosis. Proc Natl Acad Sci USA.
105:9763–9768. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Hamed HA, Das SK, Sokhi UK, Park MA,
Cruickshanks N, Archer K, et al: Combining histone deacetylase
inhibitors with MDA-7/IL-24 enhances killing of renal carcinoma
cells. Cancer Biol Ther. 14:1039–1049. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Tinoco G, Warsch S, Glück S, Avancha K and
Montero AJ: Treating breast cancer in the 21st century: Emerging
biological therapies. J Cancer. 4:117–132. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Patani N, Douglas-Jones A, Mansel R, Jiang
W and Mokbel K: Tumour suppressor function of MDA-7/IL-24 in human
breast cancer. Cancer Cell Int. 10:292010.PubMed/NCBI
|
|
14
|
Frewer NC, Ye L, Sun PH, Owen S, Ji K,
Frewer KA, et al: Potential implication of IL-24 in
lymphangiogenesis of human breast cancer. Int J Mol Med.
31:1097–1104. 2013.PubMed/NCBI
|
|
15
|
Sarkar D, Su ZZ, Vozhilla N, Park ES,
Gupta P and Fisher PB: Dual cancer-specific targeting strategy
cures primary and distant breast carcinomas in nude mice. Proc Natl
Acad Sci USA. 102:14034–14039. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Sauane M, Gopalkrishnan RV, Choo HT, Gupta
P, Lebedeva IV, Yacoub A, et al: Mechanistic aspects of mda-7/IL-24
cancer cell selectivity analysed via a bacterial fusion protein.
Oncogene. 23:7679–7690. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Pei DS, Yang ZX, Zhang BF, Yin XX, Li LT,
Li HZ and Zheng JN: Enhanced apoptosis-inducing function of
MDA-7/IL-24 RGD mutant via the increased adhesion to tumor cells. J
Interferon Cytokine Res. 32:66–73. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Weidner N: Current pathologic methods for
measuring intratumoral microvessel density within breast carcinoma
and other solid tumors. Breast Cancer Res Treat. 36:169–180. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Lebedeva IV, Emdad L, Su ZZ, Gupta P,
Sauane M, Sarkar D, et al: mda-7/IL-24, novel anticancer cytokine:
Focus on bystander antitumor, radiosensitization and antiangiogenic
properties and overview of the phase I clinical experience
(Review). Int J Oncol. 31:985–1007. 2007.PubMed/NCBI
|
|
20
|
Cunningham CC, Chada S, Merritt JA, Tong
A, Senzer N, Zhang Y, et al: Clinical and local biological effects
of an intratumoral injection of mda-7 (IL24; INGN 241) in patients
with advanced carcinoma: a phase I study. Mol Ther. 11:149–159.
2005. View Article : Google Scholar
|
|
21
|
Xie Y, Sheng W, Xiang J, Ye Z, Zhu Y, Chen
X and Yang J: Recombinant human IL-24 suppresses lung carcinoma
cell growth via induction of cell apoptosis and inhibition of tumor
angiogenesis. Cancer Biother Radiopharm. 23:310–320. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Yan S, Zhang H, Xie Y, Sheng W, Xiang J,
Ye Z, et al: Recombinant human interleukin-24 suppresses gastric
carcinoma cell growth in vitro and in vivo. Cancer Invest.
28:85–93. 2010. View Article : Google Scholar
|
|
23
|
Sauane M, Gupta P, Lebedeva IV, Su ZZ,
Sarkar D, Randolph A, et al: N-glycosylation of MDA-7/IL-24 is
dispensable for tumor cell-specific apoptosis and ‘bystander’
antitumor activity. Cancer Res. 66:11869–11877. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Danial NN and Korsmeyer SJ: Cell death:
Critical control points. Cell. 116:205–219. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Hanahan D and Weinberg RA: Hallmarks of
cancer: The next generation. Cell. 144:646–674. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Welti J, Loges S, Dimmeler S and Carmeliet
P: Recent molecular discoveries in angiogenesis and antiangiogenic
therapies in cancer. J Clin Invest. 123:3190–3200. 2013. View Article : Google Scholar : PubMed/NCBI
|