Introduction
Gallbladder cancer (GBC) is a relatively rare type
of neoplasm, but is particularly life-threatening. It is the most
common biliary tract tumor and the seventh most common type of
malignancy of the digestive tract worldwide (1). As the clinical symptoms are subtle,
the majority of patients are diagnosed at an advanced stage. The 5
year mortality rate is up to 90% (2), while the median survival prognosis
for patients is 4–6 months. Due to the advanced stage at
presentation, only a third of patients are potential candidates for
surgery (3). GBC is highly
invasive and spreads to regional lymph nodes at an early stage. In
addition, it has a high rate of recurrence (4). Treatment with adjuvant therapy has
been considered, however, no previous studies have provided
conclusive evidence supporting the benefit of adjuvant treatment
for GBC (5). Thus, the majority of
patients present with metastasis at the time of diagnosis.
GBC is suspected in patients with a long history of
chronic cholecystitis secondary to cholelithiasis who demonstrate a
change in symptoms. Interleukin-6 (IL-6) is a pleiotropic cytokine
involved in acute inflammation, hematopoiesis (6,7) and
the proliferation of cancer cells (8). Increased expression of IL-6 has been
detected and associated with an unfavorable prognosis and
metastasis in patients with cancer (9). Therefore, targeting IL-6-mediated
pathways can offer an effective treatment modality (10). Several studies have suggested the
role of IL-6 in modulating the tumor microenvironment, which is
triggered by inducing epithelial-to mesenchymal transition (EMT)
followed by downregulation the expression of E-cadherin and
upregulation the expression levels of Vimentin, N-cadherin, Snail
and Twist (11,12). EMT is an important mechanism in
tumor invasion and metastasis (13) and Twist is important in promoting
EMT (14). However, the exact
effect of the expression of IL-6 remains to be elucidated.
The present study examined the association between
IL-6, Twist, EMT and GBC. The progression, invasion and metastasis
of GBC was analyzed by investigating the expression of the
epithelial marker E-cadherin and interstitial marker Vimentin. The
results may improve understanding of GBC prognosis and targeted
therapy.
Materials and methods
Clinical specimens
Human GBC tissues were obtained with informed
consent from the Eastern Hepatic Biliary Hospital affiliated with
the Second Military Medicine University (Shanghai, China) and the
procedures used in the present study were approved by the
Protection of Human Subjects Committee of the Eastern Hepatic
Biliary Hospital affiliated with the Second Military Medicine
University. A total of 20 GBC specimens and their surrounding
tissues were obtained from patients who underwent cholecystectomy.
Immediately following surgical removal, half of the tissues were
snap-frozen in liquid nitrogen for storage and the remaining half
were fixed in 4% paraformaldehyde (DingGuo Biotech Co., Ltd,
Shanghai, China) and embedded in paraffin (DingGuo Biotech Co.,
Ltd).
Immunohistochemical staining
The samples, prepared from the paraffin-embedded
block, were rehydrated and then incubated in 3% hydrogen peroxide
for 15 min to block endogenous peroxidase. For antigen retrieval,
the samples were boiled in a pressure cooker for 10 min.
Nonspecific binding was inhibited with 10% normal goat serum
(Boshide Biological Engineering, Co., Ltd, Wuhan, China) for 20 min
at 37°C. The samples were then incubated at 4°C overnight with the
following primary antibodies: Rabbit anti-mouse polyclonal IL-6
(1:50; cat. no. ab6672; Abcam, Cambridge, MA, USA), mouse anti-goat
polyclonal Twist (1:50; cat. no. ab50887; Abcam), rabbit anti-mouse
polyclonal E-cadherin (1:100; sc-7870; Santa Cruz Biotechnology,
Inc., Santa Cruz, CA, USA) and rabbit anti-mouse polyclonal
Vimentin (1:100; cat. no. sc-5565; Santa Cruz Biotechnology, Inc.).
The sections were treated with goat anti-rabbit/anti-mouse
polyclonal secondary antibodies conjugated to horseradish
peroxidase (Abcam, Cambridge, MA, USA) for 30 min at room
temperature and stained with diaminobenzidine (Beyotime Institute
of Biotechnology, Shanghai, China) until brown granules appeared.
The sections were then counterstained with hematoxylin (DingGuo
Biotech Co., Ltd) for 2 min at room temperature.
Evaluation of immunohistochemical
staining
The sections were evaluated by two pathologists in a
blinded-manner using a light microscope (DMI3000B; Leica
Microsystems AG, Solms, Germany). A semi-quantitative scoring
criterion for immunohistochemistry was used, in which expression
was determined based on the percentage of positive cells and
staining intensity. The scores were interpreted as shown in
Table I.
 | Table IImmunohistochemical staining score
system. |
Table I
Immunohistochemical staining score
system.
| Score | Description |
|---|
| 0 | ≤5% of positive cells
or negative staining |
| 1 | 5–25% of positive
cells or weak staining |
| 2 | 25–50% of positive
cells or moderate staining |
| 3 | 50% of positive cells
or strong staining |
The final score was determined by the expression
rate and intensity of proteins, graded as ‘−’ for 0 point, ‘+’ for
1–2 points, ‘++’ for 3–4 points and ‘+++’ for 5–6 points.
Immunoreactivity ‘±’ was used to denote overexpression and ‘++/+++’
was used to denote underexpression for statistical analysis.
Reverse transcription quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was extracted from 100 mg GBC tissues and
the surrounding tissues. The tissues were added to 1 ml TRIzol
reagent (Takara Bio, Inc., Tokyo, Japan) and homogenized according
to the manufacturer’s instructions. The first strand of cDNA was
synthesized from 500 ng total RNA using PrimeScript®
Reverse Transcriptase (Takara Bio, Inc.). The qPCR was performed in
a reaction volume of 20 μl, including 2 μl cDNA. The primer
sequences used are shown in Table
II.
 | Table IIPrimer sequences. |
Table II
Primer sequences.
| Gene | Forward/Reverse | Sequence (5′-3′) |
|---|
| β-actin | Forward |
CTGGGACGACATGGAGAAAA |
| Reverse |
AAGGAAGGCTGGAAGAGTGC |
| Interleukin-6 | Forward |
CCACACAGACAGCCACTCAC |
| Reverse |
GATGATTTTCACCAGGCAAGTC |
| Twist | Forward |
AGTCCGCAGTCTTACGAGGAG |
| Reverse |
GACCTGGTAGAGGAAGTCGATG |
| E-cadherin | Forward |
GTCTCTCTCACCACCTCCACAG |
| Reverse |
CTCGGACACTTCCACTCTCTTT |
| Vimentin | Forward |
GAAGAGAACTTTGCCGTTGAAG |
| Reverse |
GAAGGTGACGAGCCATTTC |
The PCR conditions were as follows: 95°C for 30 sec,
followed by 40 cycles of 95°C for 5 sec and 60°C for 34 sec. The
relative quantification of genes was analyzed using the comparative
threshold cycle (Ct) method. To ensure that only a
specific band was produced, melting curve analysis was performed at
the end of each PCR experiment. The term −ΔCt was used
to describe the expression level of mRNA. The expression was
subsequently divided into lower expression and higher expression
groups, based on whether the mRNA levels were above or below the
mean value.
Western blot analysis
Western blot analysis was performed, as previously
described (15). For the total
protein extraction, 100 mg of frozen tissue samples, previously
stored in liquid nitrogen, were ground and homogenized using
radioimmunoprecipitation assay lysis buffer (Beyotime Institute of
Technology). The total protein concentrations of the cell extracts
were determined using a bicinchoninic acid assay system (Beyotime
Institute of Biotechnology) with bovine serum albumin as the
standard. For electrophoresis, 80 μg of the total protein was added
to each lane on SDS-PAGE gels (8, 10 and 15%; Beyotime Institute of
Biotechnology) and the protein was then blotted onto a
polyvinylidene difluoride membrane (DingGuo Biotech Co., Ltd) by
wet transfer (200 mA, 1–2 h). The membranes were inhibited with 5%
skimmed milk and incubated with anti-IL-6 (cat. no. ab6672; Abcam,
US), anti-Twist (cat. no. ab50887; Abcam), anti-E-cadherin (cat.
no. sc-7870; Santa Cruz Biotechnology, Inc.), anti-Vimentin (cat.
no. sc-5565; Santa Cruz Biotechnology, Inc.) and anti-β-actin
antibodies (cat. no. #4970; Cell Signaling Technology, Inc.),
respectively, at 4°C overnight. This was followed by incubation
with goat anti-rabbit/anti-mouse secondary antibody conjugated to
horseradish peroxidase (1:1,000). The stain was visualized using an
enhanced chemiluminescent (ECL) detection reagent from Millpore
(Rockford, IL, USA). Images were captured and the optical densities
of the bands were quantified using a Gel Doc 2000 system (BioRad,
Hercules, CA, USA).
Statistical analysis
The results of all the assays are expressed as the
mean ± standard deviation. All the assays were performed
independently in triplicate. The data were analyzed using Prism 5.0
software (GraphPad Software, Inc., La Jolla, CA, USA). The survival
curves were generated using the Kaplan-Meier method. The
significance of the observed differences were determined using
Student’s t-test or χ2 test. Associations among the
IL-6, Twist, E-cadherin and Vimentin mRNA were analyzed by
correlation coefficients and linear regression analysis. P<0.05
was considered to indicate a statistically significant
difference.
Results
Expression of IL-6 and EMT-associated
protein in the GBC tissues
The expression levels of IL-6 and the Twist,
E-cadherin and Vimentin EMT-associated proteins were determined in
20 human GBC tissues and 20 surrounding tissues using
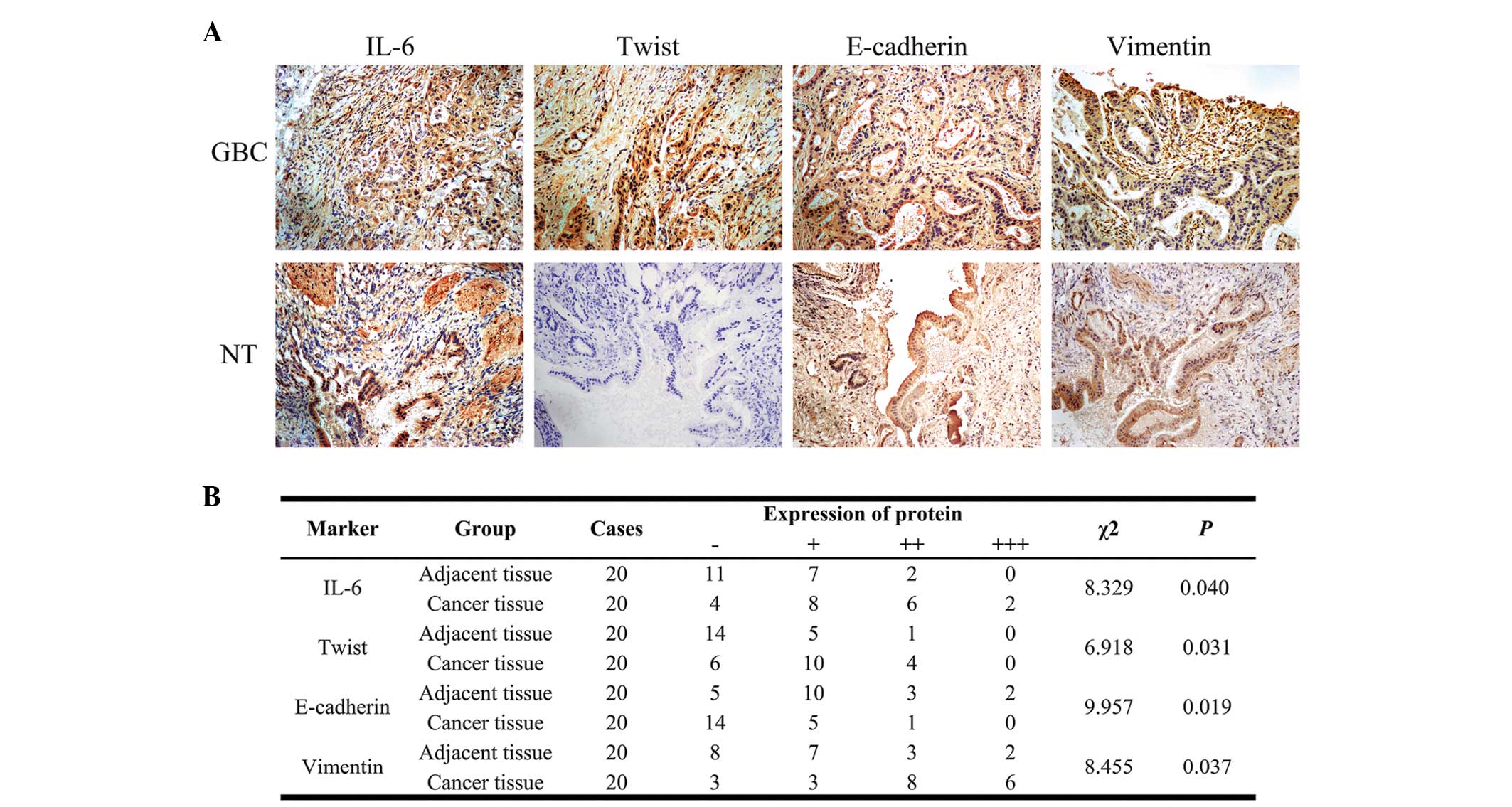
immunohistochemistry (Fig. 1A).
The results revealed overexpression of IL-6, Twist and Vimentin and
underexpression of E-cadherin in the GBC tissues compared with the
adjacent tissues (Fig. 1A). The
results also revealed that the IL-6, Twist and Vimentin proteins
were overexpressed in 8, 4 and 14 (40, 20 and 70%) of the GBC
samples, respectively. The underexpression of E-cadherin was
observed in a single GBC sample (5%). By contrast, the expression
levels of IL-6, Twist, Vimentin in the GBC tissues were
significantly higher compared with those in the surrounding tissues
(χ2=8.329, P=0.040; χ2=6.918, P=0.031 and
χ2=8.455, P=0.037, respectively). However, the
expression of E-cadherin was significantly lower in the GBC tissues
(χ2=9.957, P=0.019).
Western blot analysis also revealed the
overexpression of IL-6, Twist and Vimentin and underexpression of
E-cadherin in the GBC tissues compared with the normal adjacent
tissues (P<0.05; Fig. 2Aa and
b). The decrease in the differentiation level of the GBC tissue
(Fig. 2Ac and d), increase in
tumor-node-metastasis (TNM) stage (Fig. 2Ae and f) and positive local
invasion (Fig. 2A, g and h) were
correlated with increased expression levels of IL-6, Twist and
Vimentin (P<0.05). The expression of E-cadherin in the GBC
tissues was significantly lower compared with that in the
surrounding tissues (P<0.05) and was lower in patients with GBC
exhibiting high grade differentiation, local invasion and a high
TNM stage (P<0.05; Fig.
2A).
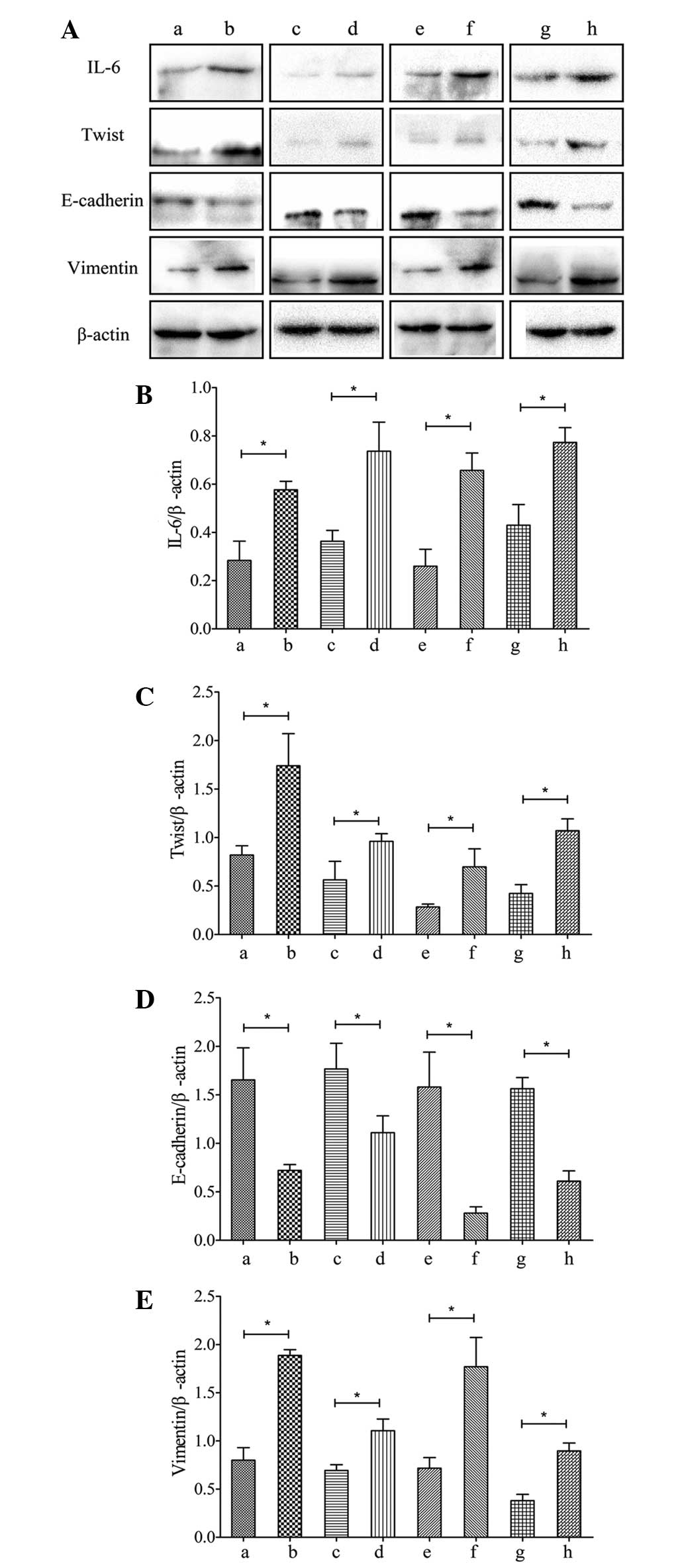 | Figure 2(A) Protein expression levels of IL-6,
Twist, E-cadherin and Vimentin in gallbladder cancer tissues. (B–E)
Relative protein expression levels of IL-6, Twist, E-cadherin and
Vimentin in gallbladder cancer tissues. a, adjacent tissue; b,
gallbladder cancer tissue; c, well and moderately differentiated;
d, poorly differentiated; e, TNM stage I–II; f, TNM stage III–IV;
g, without local invasion; h, local invasion. Values are presented
as the mean ± standard deviation. *P<0.05. IL-6,
interleukin-6; TNM, tumor-node-metastasis. |
IL-6 and EMT-associated mRNA expression
in gastric cancer tissues is associated with advanced clinical
stage, lymph node metastasis and poor patient prognosis
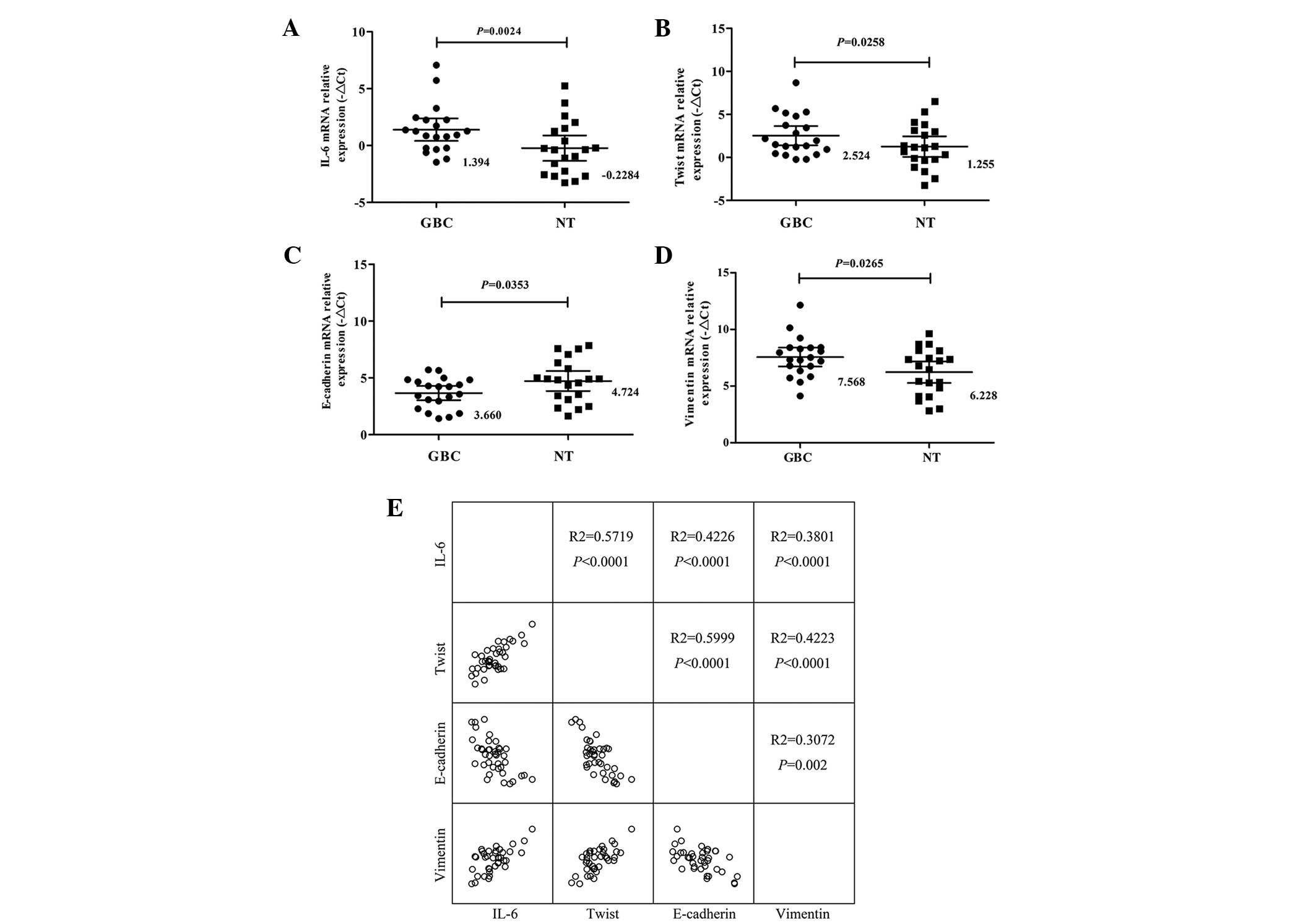
Consistent with the above data, the results
confirmed that the expression levels of IL-6, Twist and Vimentin in
the GBC tissues were significantly higher (P<0.05; Fig. 3A, B and D). However, the mRNA
expression of E-cadherin was significantly lower in the GBC tissues
(P=0.0265; Fig. 3C). The
correlations between the IL-6 and EMT-associated mRNA expression
and the clinicopathologic characteristics of GBC are summarized in
Table III. The differentiation,
local invasion, lymph node status and clinical stages were
correlated with the expression of IL-6. The median expression level
of IL-6 was 2.41±2.21 in the 20 cases with advanced stage (stage
III and IV) and 0.15±1.20 (P=0.014) in cases with early-stage
(stage I and II) disease. In the 20 cases of GBC with either local
invasion or lymph node metastasis, the median expression levels of
IL-6 were 2.70±2.36 and 2.66±2.58, respectively. This was
significantly higher compared with the expression levels in the 20
adjacent tissues (0.32±1.14; P=0.009 and 0.55±1.27; P=0.025,
respectively). The expression of IL-6 in the GBC patients did not
correlate with gender, age or tumor size. A statistically
significant correlation was observed between the degree of
differentiation, local invasion, lymph node metastasis, clinical
stage and Twist/E-cadherin expression. However, no statistically
significant correlation was observed between the expression of
Vimentin and the clinicopathologic characteristics. In addition,
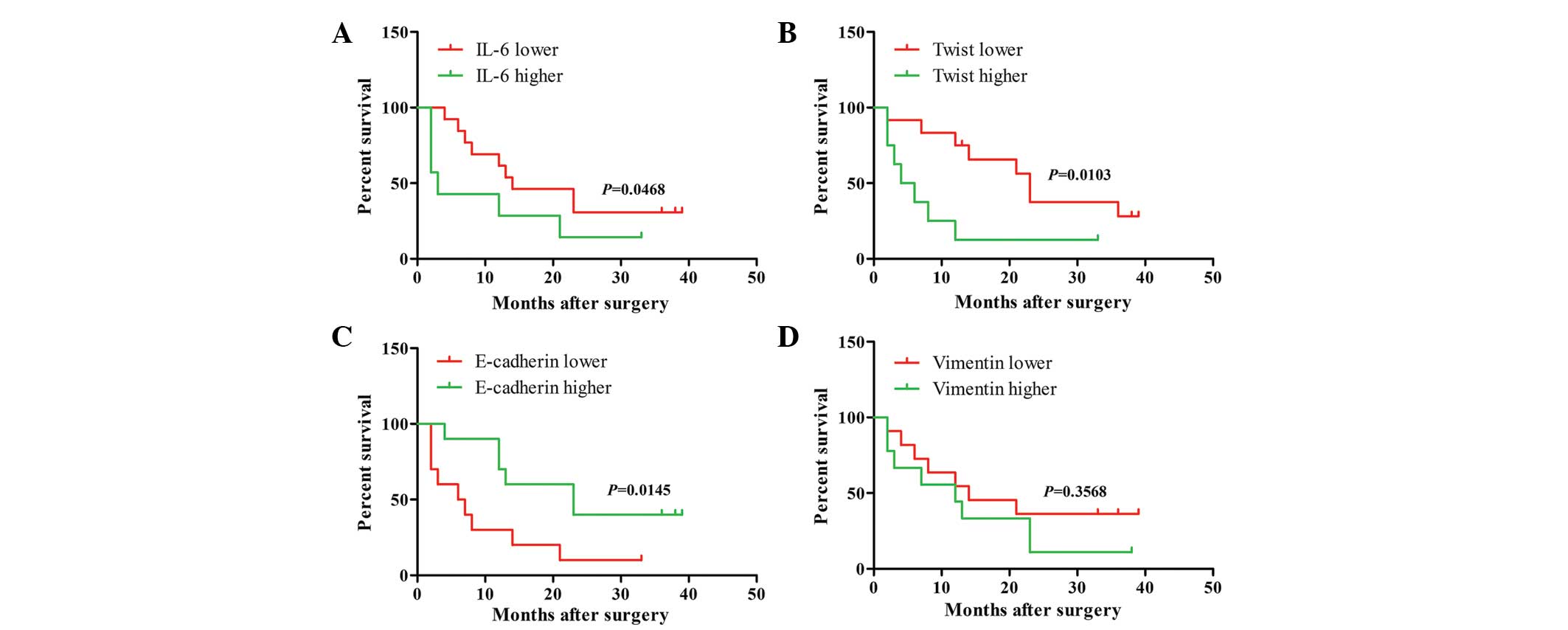
the present study examined whether the mRNA expression levels of
Il-6, Twist, E-cadherin and Vimentin were associated with survival
rate in patients with GBC. Based on the mean expression level of
IL-6 (1.394), Twist (2.524), E-cadherin (3.660) and Vimentin
(7.568), as shown in Fig. 3A–D,
the GBC specimens were divided into a higher and a lower expression
group. Kaplan-Meier survival analyis revealed that patients whose
tumors exhibited increased expression of IL-6 or Twist compared
with that of the lower expression group or reduced expression of
E-cadherin compared with that of the higher expression group had a
shorter median survival rates at 10.71±12.19, 8.75±10.38 and
9.8±10.20 months, respectively (P=0.0486, P=0.0103 and P=0.0145;
Fig. 4A–D).
 | Table IIImRNA expression levels of IL-6,
Twist, E-cadherin and Vimentin and the association with the
clinical pathological features of gallbladder cancer. |
Table III
mRNA expression levels of IL-6,
Twist, E-cadherin and Vimentin and the association with the
clinical pathological features of gallbladder cancer.
| Variable | Cases | % | Mean ±SD expression
of IL-6 | P-value | Mean ± SD
expression of Twist | P-value | Mean ±SD expression
of E-cadherin | P-value | Mean ±SD expression
of Vimentin | P-value |
|---|
| Normal tissue | 20 | | 1.39±2.12 | 0.002 | 2.52±2.39 | 0.026 | 3.66±1.34 | 0.035 | 7.57±1.77 | 0.026 |
| Carcinoma
tissue | 20 | | −0.23±2.37 | | 1.26±2.55 | | 4.72±1.89 | | 6.23±2.03 | |
| Gender | | | | 0.558 | | 0.569 | | 0.725 | | 0.903 |
| Male | 6 | 30 | 0.95±1.18 | | 2.04±2.50 | | 3.49±1.40 | | 7.65±0.61 | |
| Female | 14 | 70 | 1.58±2.43 | | 2.73±2.40 | | 3.73±1.36 | | 7.54±2.11 | |
| Age (years) | | | | 0.992 | | 0.368 | | 0.901 | | 0.872 |
| ≤60 | 13 | 65 | 1.39±2.54 | | 2.89±2.35 | | 3.69±1.14 | | 7.52±2.15 | |
| >60 | 7 | 35 | 1.40±1.16 | | 1.85±2.49 | | 3.61±1.76 | | 7.66±0.85 | |
| Tumor size
(cm) | | | | 0.806 | | 0.314 | | 0.939 | | 0.889 |
| ≤5 | 11 | 55 | 1.50±1.85 | | 2.02±2.18 | | 3.68±1.33 | | 7.62±1.30 | |
| >5 | 9 | 45 | 1.26±2.53 | | 3.13±2.61 | | 3.63±1.44 | | 7.50±2.31 | |
| Degree of
differentiation | | | | 0.021 | | 0.026 | | 0.012 | | 0.062 |
| Well and
moderately differentiated | 18 | 90 | 0.70±1.09 | | 1.77±1.60 | | 4.13±1.08 | | 7.09±1.40 | |
| Poorly
differentiated | 2 | 10 | 3.02±3.08 | | 4.29±3.1 | | 2.56±1.33 | | 8.69±2.16 | |
| Local invasion | | | | 0.009 | | 0.007 | | 0.001 | | 0.18 |
| Positive | 7 | 35 | 2.70±2.36 | | 4.03±2.84 | | 2.69±1.25 | | 8.16±1.82 | |
| Negative | 13 | 65 | 0.32±1.14 | | 1.29±0.81 | | 4.45±0.81 | | 7.08±1.66 | |
| Lymph-node
metastasis | | | | 0.025 | | 0.003 | | 0.006 | | 0.064 |
| Positive | 12 | 60 | 2.66±2.58 | | 4.32±2.45 | | 2.71±1.36 | | 8.46±1.96 | |
| Negative | 8 | 40 | 0.55±1.27 | | 1.33±1.46 | | 4.29±0.92 | | 6.97±1.42 | |
| TNM stage | | | | 0.014 | | 0.025 | | 0.003 | | 0.144 |
| I–II | 4 | 20 | 0.15±1.20 | | 1.24±0.62 | | 4.58±0.82 | | 6.92±1.66 | |
| III–IV | 16 | 80 | 2.41±2.21 | | 3.58±2.79 | | 2.91±1.23 | | 8.10±1.76 | |
IL-6 is associated with the expression of
twist and can accurately discriminate between GBC and adjacent
tissues
Line regression results produced R2
values to compare the mRNA expression levels of IL-6, Twist,
E-cadherin and Vimentin, respectively, in the GBC and adjacent
tissues. Significant correlations were observed among these four
mRNAs (P<0.05;Fig. 3E).
Discussion
The present study demonstrated theat IL-6 protein
and mRNA were overexpressed in GBC tissues (Figs. 1–3) and IL-6, Twist and E-cadherin were
associated with local invasion, lymph node metastasis, poor
differentiation and poor clinical prognosis in GBC. This is the
first study, to the best of our knowledge, to examine the
correlation between IL-6 and EMT and prognosis.
Although the mRNA expression of IL-6 was increased
in the 20 GBC tissues, its source remains to be elucidated. Several
cancer patients exhibit increased serum levels of IL-6, which can
originate from a number of sources, including tumor cells and
macrophages (16). If cancer cells
increasingly secrete IL-6, it may act in an autocrine manner to
enhance the metastatic ability (17) and resistance of the tumor to
treatment (18). The increased
levels of IL-6 have been correlated with poor prognosis and
survival rate in a variety of types of cancer (19–22).
Previous studies on solid tumors, including gastric, renal cell,
colorectal, prostate, non-small cell lung, melanoma and head and
neck cancer and hematologic malignancies, including myeloma and
non-Hodgkin’s lymphoma, have indicated the potential prognostic
significance of IL-6 levels (19–22).
IL-6 signaling activates STAT3 (23), which is required for malignant
transformation. It also has multiple protumorigenic functions,
including the promotion of tumor cell proliferation, survival,
invasion, metastasis and angiogenesis (24,25).
IL-6 induces EMT changes in tumor cells via activation of the STAT3
signaling pathway and STAT3-knockdown reverses these changes
(26). The key activity of EMT
(27) is hypothesized to be the
reduction of cell-to-cell adhesion and induction of cell motility
through downregulation of E-cadherin and may be associated with the
expression of Twist and Vimentin (14).
Consistent with the data of the present study,
certain studies have identified that IL-6 induces an EMT phenotype
(11,12). Additionally, increased expression
of Twist and reduced expression of E-cadherin are correlated with
poor differentiation and local invasion. Twist and E-cadherin are
statistically significant prognostic factors in several types of
cancer (28–32).
In the present study, linear correlation analysis
revealed that the mRNA expression of IL-6 was positively correlated
with the EMT-associated markers (Fig.
3E). These results suggested that the four genes have
synergistic effects in tumorgenesis and metastasis in GBC. It also
indicated that the malignant transformation process of GBC is
accompanied by EMT.
In conclusion, the expression of IL-6 correlated
with EMT-associated mRNA and protein expression, local invasion,
lymph node metastasis, shorter survival time, poor clinical stage
and differentiation. GBC is often diagnosed at an advanced stage
and is associated with poor prognosis. It is possible to
downregulate the expression of IL-6 through adjuvant therapy and
several clinical studies have supported the use of IL-6 as a
therapeutic target (33,34). However, further studies are
required to fully define the association between IL-6 and EMT in
GBC.
Acknowledgements
This study was supported by the Foundation of
Shanghai Jiaotong University School of Medicine (no. 12XJ22004) the
Shanghai Science and Technology Bureau Introductory Project (no.
124119a0600) and the National Natural Science Foundation of China
(no. 81272747).
References
|
1
|
Ferlay J, Shin HR, Bray F, Forman D,
Mathers C and Parkin DM: GLOBOCAN 2008v2.0: Cancer incidence and
mortality worldwide. IARC CancerBase No. 10 Lyon, France:
International Agency for Research on Cancer; 2010, http://globocan.iorc.fr.
Accessed August 2012
|
|
2
|
Alvi AR, Siddiqui NA and Zafar H: Risk
factors of gallbladder cancer in Karachi-a case-control study.
World J Surg Oncol. 9:1642011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Reid KM, Ramos-De la Medina A and Donohue
JH: Diagnosis and surgical management of gallbladder cancer: a
review. J Gastrointest Surg. 11:671–681. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hundal R and Shaffer EA: Gallbladder
cancer: epidemiology and outcome. Clin Epidemiol. 6:99–109.
2014.PubMed/NCBI
|
|
5
|
Jayaraman S and Jarnagin WR: Management of
gallbladder cancer. Gastroenterol Clin North Am. 39:331–342. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kishimoto T: Interleukin-6: discovery of a
pleiotropic cytokine. Arthritis Res Ther. 8(Suppl 2): 22006.
View Article : Google Scholar
|
|
7
|
Qi J, Chen N, Wang J and Siu CH:
Transendothelial migration of melanoma cells involves
N-cadherin-mediated adhesion and activation of the beta-catenin
signaling pathway. Mol Biol Cell. 16:4386–4397. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Di GH, Liu Y, Lu Y, Liu J, Wu C and Duan
HF: IL-6 secreted from senescent mesenchymal stem cells promotes
proliferation and migration of breast cancer cells. PLoS One.
9:e1135722014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Soubrane C, Rixe O, Meric JB, Khayat D and
Mouawad R: Pretreatment serum interleukin-6 concentration as a
prognostic factor of overall survival in metastatic malignant
melanoma patients treated with biochemotherapy: a retrospective
study. Melanoma Res. 15:199–204. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ataie-Kachoie P, Pourgholami MH and Morris
DL: Inhibition of the IL-6 signaling pathway: a strategy to combat
chronic inflammatory diseases and cancer. Cytokine Growth Factor
Rev. 24:163–173. 2013. View Article : Google Scholar
|
|
11
|
Sullivan NJ, Sasser AK, Axel AE, et al:
Interleukin-6 induces an epithelial-mesenchymal transition
phenotype in human breast cancer cells. Oncogene. 28:2940–2947.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Na YR, Lee JS, Lee SJ and Seok SH:
Interleukin-6-induced Twist and N-cadherin enhance melanoma cell
metastasis. Melanoma Res. 23:434–443. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Thiery JP: Epithelial-mesencymal
transitions in tumour progression. Nat Rev Cancer. 2:442–454. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Liu AN, Zhu ZH, Chang SJ and Hang XS:
Twist expression associated with the epithelial-mesenchymal
transition in gastric cancer. Mol Cell Biochem. 367:195–203. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Qin Y, Zhang S, Gong W, Li J, Jia J and
Quan Z: Adenovirus-mediated gene transfer of tissue factor pathway
inhibitor-2 inhibits gallbladder carcinoma growth in vitro and in
vivo. Cancer Sci. 103:723–730. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Diehl S, Anguita J, Hoffmeyer A, et al:
Inhibition of Th1 differentiation by IL-6 is mediated by SOCS1.
Immunity. 13:805–815. 2000. View Article : Google Scholar
|
|
17
|
Luo F, Xu Y, Ling M, et al: Arsenite
evokes IL-6 secretion, autocrine regulation of STAT3 signaling and
miR-21 expression, processes involved in the EMT and malignant
transformation of human bronchial epithelial cells. Toxicol Appl
Pharmacol. 273:27–34. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wang Y, Qu Y, Zhang XL, et al: Autocrine
production of interleukin-6 confers ovarian cancer cells resistance
to tamoxifen via ER isoforms and SRC-1. Mol Cell Endocrinol.
2013.
|
|
19
|
Yeh KY, Li YY, Hsieh LL, et al: Analysis
of the effect of serum interleukin-6 (IL-6) and soluble IL-6
receptor levels on survival of patients with colorectal cancer. Jpn
J Clin Oncol. 40:580–587. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Duffy SA, Taylor JM, Terrell JE, et al:
Interleukin-6 predicts recurrence and survival among head and neck
cancer patients. Cancer. 113:750–757. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Suh SY, Choi YS, Yeom CH, et al:
Interleukin-6 but not tumour necrosis factor-alpha predicts
survival in patients with advanced cancer. Support Care Cancer.
21:3071–3077. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Chang CH, Hsiao CF, Yeh YM, et al:
Circulating interleukin-6 level is a prognostic marker for survival
in advanced nonsmall cell lung cancer patients treated with
chemotherapy. Int J Cancer. 132:1977–1985. 2013. View Article : Google Scholar
|
|
23
|
Yu H, Kortylewski M and Pardoll D:
Crosstalk between cancer and immune cells: role of STAT3 in the
tumour microenvironment. Nat Rev Immunol. 7:41–51. 2007. View Article : Google Scholar
|
|
24
|
Ho PL, Lay EJ, Jian W, Parra D and Chan
KS: Stat3 activation in urothelial stem cells leads to direct
progression to invasive bladder cancer. Cancer Res. 72:3135–3142.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Rathinavelu A, Narasimhan M and Muthumani
P: A novel regulation of VEGF expression by HIF-1α and STAT3 in
HDM2 transfected prostate cancer cells. J Cell Mol Med.
16:1750–1757. 2012. View Article : Google Scholar
|
|
26
|
Yadav A, Kumar B, Datta J, Teknos TN and
Kumar P: IL-6 promotes head and neck tumor metastasis by inducing
epithelial-mesenchymal transition via the JAK-STAT3-SNAIL signaling
pathway. Mol Cancer Res. 9:1658–1667. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
de Freitas Silva BS, Yamamoto-Silva FP,
Pontes HA and Pinto Júnior DS: E-cadherin downregulation and Twist
overexpression since early stages of oral carcinogenesis. J Oral
Pathol Med. 43:125–31. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Yuen HF, Chua CW, Chan YP, Wong YC, Wang X
and Chan KW: Significance of TWIST and E-cadherin expression in the
metastatic progression of prostatic cancer. Histopathology.
50:648–658. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Shibata K, Kajiyama H, Ino K, et al: Twist
expression in patients with cervical cancer is associated with poor
disease outcome. Ann Oncol. 19:81–85. 2008. View Article : Google Scholar
|
|
30
|
Zhao Z, Ge J, Sun Y, et al: Is E-cadherin
immunoexpression a prognostic factor for head and neck squamous
cell carcinoma (HNSCC)? A systematic review and meta-analysis. Oral
Oncol. 48:761–767. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Bačić B, Haller H, Mrklić I, Košta V,
Čarić A and Tomić S: Prognostic role of E-cadherin in patients with
advanced serous ovarian cancer. Arch Gynecol Obstet. 287:1219–1224.
2013. View Article : Google Scholar
|
|
32
|
Aamodt R, Bondi J, Andersen SN, Bakka A,
Bukholm G and Bukholm IR: The prognostic impact of protein
expression of E-cadherin-catenin complexes differs between rectal
and colon carcinoma. Gastroenterol Res Pract. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Shinriki S, Jono H, Ota K, et al:
Humanized anti-interleukin-6 receptor antibody suppresses tumor
angiogenesis and in vivo growth of human oral squamous cell
carcinoma. Clin Cancer Res. 15:5426–5434. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Guo Y, Xu F, Lu T, Duan Z and Zhang Z:
Interleukin-6 signaling pathway in targeted therapy for cancer.
Cancer Treat Rev. 38:904–10. 2012. View Article : Google Scholar : PubMed/NCBI
|