Introduction
Sepsis, a systemic inflammatory response syndrome
caused by infection, is known to be accompanied by the presence of
bacteria, which may arise from a highly virulent focus of
infection. Sepsis may often cause acute lung injury (ALI) (1,2).
When ALI occurs, cytokines, chemokines, adhesion molecules and
other inflammatory mediators are produced in the endothelial cells
activated within the pulmonary vasculature, which destroys the
integrity of pulmonary vascular endothelial cells. This leads to
increased permeability of capillaries in the lung, and consequent
pulmonary edema (1), which results
in acute respiratory distress syndrome, multiple organ failure,
high mortality and other major problems in intensive care. The
reduction of pulmonary capillary permeability is therefore of
marked clinical importance. Vascular endothelial growth factor
(VEGF) is one of the most important regulatory factors during
vascular formation. Upon activation of inflammation, alveolar
macrophages and neutrophils are able to release a large amount of
VEGF (3,4), which most commonly increases the
permeability of post-capillary venules. In vitro studies
have demonstrated that the effect of VEGF on increasing vascular
permeability is ~20,000 times more potent than that of histamine
(5,6). In the early stage of ALI,
neutrophils, monocytes, macrophages and platelets activated by
inflammation synthesize and release large amounts of VEGF, leading
to high vascular permeability and consequently to pulmonary edema
(6). Melilotus suaveolens
Ledeb (M. suaveolens), a Traditional Tibetan Medicine also
known as Melilotus suavcolen or wild alfalfa, is a bitter,
‘cold-tasting’ herb which has proven to be effective in fever
reduction, detoxification, anti-inflammatory and drying limbs ichor
(7). It is therefore traditionally
applied to treat a range of illnesses, including spleen disease,
twisted intestinal fever, diphtheria and tonsillitis (8). Currently, there are few published
studies on the effects of M. suaveolens. Pharmacological
studies have demonstrated that an ethanolic extract from M.
suaveolens has a powerful anti-inflammatory activity (9), which inhibited formaldehyde and
propylene glycol-induced capillary permeability, and was effective
in the improvement of blood circulation (10). Therefore, downregulating the
expression of VEGF in the lung may be a mechanism by which M.
suaveolens reduces pulmonary capillary permeability. The aim of
the present study was to investigate the mechanism of action by
which M. suaveolens affects CLP-induced pulmonary capillary
permeability in rats, and to establish whether this effect occurs
by regulating VEGF expression.
Materials and methods
Mice
Male Sprague-Dawley mice were purchased from Kunming
Medical University Laboratory Animal Center (Kunming, China). All
of the mice were housed in the Kunming Medical University Animal
Care Facility and were maintained under pathogen-free conditions.
The mice were 8–9 weeks of age at the initiation of the experiment
and were maintained on a standard laboratory diet and water ad
libitum. The experimental procedures were approved by the
Committee of Animal Experimentation of the Kunming Medical
University (Kunming, China).
Reagents
A reverse transcription reaction kit was purchased
from Takara Biotechnology Co. Ltd. (Dalian, China); TRIzol was from
Invitrogen Life Technologies (Carlsbad, CA, USA) and
electrophoresis reagents were from Promag Co. (Ningbo, China); an
RT Reaction kit was obtained from Takara Biotechnology Co. Ltd
(Dailan, China); a PCR Amplification Reagent kit and the 100 bp DNA
ladder marker were obtained from Sangon Biological Engineering Co.
Ltd. (Shanghai, China); GAPDH was obtained from Santa Cruz
Biotechnology, Inc. (Santa Cruz Biotechnology, Inc., Santa Cruz,
CA, USA); rabbit anti-mouse NF-κB and VEGF polyclonal antibodies
were purchased from Wuhan Boster Biological Technology, Ltd.
(Wuhan, China); M. suaveolens Extract Tablets were from
Seiko Eiyo Yakuhin Co. Ltd. (Osaka, Japan); fluorescein
isothiocyanate (FITC)-albumin and hexadecyl-trimethyl-ammonium
bromide were purchased from Sigma-Aldrich (St. Louis, MO, USA) and
SYBR green I was obtained from Biotium (Hayward, CA, USA). The
Oligo (dT18) and primers were synthesized by Shanghai Invitrogen
(Shanghai, China). The dNTP was obtained from Promega Corp.
(Madison, WI, USA).
Animal model of sepsis
All of the studies were performed on rats with an
average weight of 40.4 g. To induce sepsis, the rats were
anesthetized with isoflurane (4% induction, 2% maintenance) and
placed on a warming pad. Following laparotomy, the cecum was
exteriorized, and the membrane between the cecum and the mesentery
was carefully cut to release the cecum. The cecum was ligated 1.5
cm from the tip or just below the ileocecal valve with 4-0 silk.
Two punctures were made with an 18-gauge needle and 1 mm of fecal
material was expressed from the punctures. The incision was sutured
in two layers with 4-0 silk. In the sham animals, the cecum was
located but neither ligated nor punctured. The animals were
resuscitated with 3 ml/100 g body weight normal saline
subcutaneously immediately following surgery.
Grouping and treatment
According to a random number table, 88 rats were
randomly divided into four groups: Normal control group, sham
operation group (sham group), sepsis model group [(untreated)
sepsis group] and M. suaveolens treatment group (treatment
group), with 22 rats in each group. The model group and treatment
group were induced by cecal ligation and puncture (CLP) and, 2 h
prior to surgery, were administered the M. suaveolens
extract via tube, at a dose of 25 mg/kg every 8 h. The normal
control group, sham group and (untreated) sepsis group were subject
to treatment with the same volume of normal saline. A total of 22
rats in each group were anesthetized using ether at each of the
following time-points 24 h post-surgery. Subsequently, the right
internal carotid artery was isolated. Blood was collected via the
orbital sinus. EDTA was used as an anti-coagulant, and the plasma
was isolated by centrifugation at 10,000 × g for 5 min. All the
animals were sacrificed 24 h following surgery via anesthesia with
ether and lung tissues were collected, washed with saline solution,
dried with filter paper and weighed. The plasma and tissues were
stored at −20°C for subsequent experiments.
RNA isolation and quantitative polymerase
chain reaction (qPCR)
The left lung tissues were homogenized in TRIzol
reagent using a Mixer Mill 301 (Tianjin Tian Chang Technology Co.,
Ltd., Tianjin, China). The total RNA was extracted using TRIzol
reagent according to the manufacturer’s instructions and quantified
spectrophotometrically. A total of 2 μg RNA from each sample was
added to a total volume of 25 μl reaction mixture containing 2.5 μM
oligo (dT) primer (Promega Corp.; cat. no. C110A), and 200 U Molony
murine leukemia virus reverse transcriptase (M-MLV; Promega
Corporation; cat. no. M5314). The reaction was initiated by
incubating the reaction mixture for 1 h at 42°C for reverse
transcription and stopped by heating for 10 min at 70°C. An aliquot
(0.5 μl) of each reverse transcription product was added to 20 μl
reaction mixture containing LightCycler-FastStart DNA Master SYBR
Green I, 0.5 μM of each primer corresponding to mouse NF-κB and
VEGF or GAPDH, and 4 mM MgCl2 to amplify the genes in a
LightCycler (Roche, Mannheim, Germany). For reverse transcription
PCR, 1 μg of total RNA from each sample was resuspended in 25 μl
final volume of reaction buffer. GAPDH was used as an internal
control. The following primers were used for PCR: VEGF forward
primer, 5′-GCTCTCTTGGGTGCACTGGA-3′ and reverse primer,
5′-CACGCCTTGGCTTGTCACCA-3′; NF-κB forward primer,
5′-GCACGGATGACAGAGGCGTGTATAAGG-3′ and reverse primer,
5′-GGCGGATGATCTCCTTCTCTCTGTCTG-3′; and GAPDH forward primer, 5′-AAT
GCA TCC TGC ACC ACC AA-3′ and reverse primer, 5′-GTA GCC ATA TTC
ATT GTC ATA-3′. Following pre-incubation at 95°C for 10 min, the
PCR was performed as follows: 35 cycles of denaturation at 95°C for
15 sec, annealing at 60°C for 5 sec and elongation at 72°C for 12
sec. The 2−[ΔΔCt] method was used to compare the mRNA
expression levels of genes in the experimental groups with the
control groups.
Western blot analysis
The lung tissues were snap-frozen in liquid
nitrogen, pulverized and resuspended in ice-cold lysis buffer
(Solarbio Science & Technology, Beijing, China). Protein
concentrations were determined with the Bradford method. Lysates
were allowed to solubilize on ice for 30 min and particulate mass
was removed by centrifugation (15,000 × g) for 15 min at 4°C. The
supernatants were analyzed by SDS-PAGE. Primary antibodies used
included rabbit anti-VEGF monoclonal antibody (1:400), rabbit
anti-NF-κB65 monoclonal antibody (1:400) (Boster Biological
Technology, Ltd) and mouse anti- GAPDH monoclonal antibody (1:400)
(Santa Cruz Biotechnology, Inc). The secondary antibodies were
horseradish peroxidase-labeled antibodies (Pierce; Thermo
Scientific, Rockford, IL, USA). The blots were processed for
enhanced chemifluorescence using a Pierce ECL Western blotting
substrate (Pierce; Thermo Scientific).
Immunohistochemistry
Immunostaining was performed on the lung sections
following antigen retrieval using Retrievagen A (Zymed
Laboratories, Inc., San Francisco, CA, USA) at 100°C for 20 min,
and quenching endogenous peroxidases with 3%
H2O2. The sections were blocked with 2%
bovine serum albumin (BSA) in phosphate-buffered saline (PBS)
followed by staining with primary anti-VEGF-α and anti-NF-κBp65 (BD
Pharmingen, San Jose, CA, USA) at room temperature for 1 h. The
sections were washed and following application of secondary
antibody (R&D Systems, Minneapolis, MN, USA), tissues were
developed using Vectastain ABC (Vector Laboratories, Inc.,
Burlingame, CA, USA) and 3,3′-diaminobenzidine (Vector
Laboratories, Inc.). Following staining, five high-power fields
(x200) were randomly selected in each slide, and the average
proportion of positive expression in each field was counted using
the true color multi-functional cell image analysis management
system (Image-Pro Plus; Media Cybernetics, Rockville, MD, USA), and
expressed as positive unit (PU).
Cytokine and VEGF measurements in
bronchoalveolar lavage (BAL) and plasma
Mice were sacrificed after 24 h of treatment, and
BAL was performed via the tracheal catheter in the right lung lobes
using 0.8 ml PBS; the withdrawn fluid was centrifuged, and the
supernatant was snap frozen and stored at −80°C for further use.
Aliquots of BAL fluid and plasma were detected in duplicate by
ELISA (ELISA kit offered by Glory Science Co., Ltd., Del Rio, TX,
USA) kits for tumor necrosis factor-α (TNF-α), interleukin (IL)-1β,
IL-6 and IL-10 according to the manufacturer’s instructions.
Pulmonary vascular permeability
assays
Two hours prior to sacrification, FITC-labeled
albumin (5 mg/kg body weight) was administered via tail-vein
injection at 6 and 24 h. Immediately following sacrification, the
lungs were lavaged three times with PBS (0.5 ml per lavage) and the
samples combined. Fluid recovery was ~95%. The BAL samples were
centrifuged at 3,000 × g for 10 min. FITC fluorescence in the BAL
fluid was measured using a 960CRT computer controlled fluorescence
spectrophotometer (Shanghai Tiancheng Technology Co., Ltd.,
Shanghai, China) with excitation at 484 nm and emission at 510
nm.
Wet/dry (W/D) lung weight ratio and water
content
W/D weight ratio was used as an index of tissue
water content. 24 h following administration of M.
suaveolens extract, the animals were anesthetized using
ketamine (80 mg/Kg i.p.) and xylazine (20 mg/Kg i.p.), sacrificed
and lungs were excised en bloc. The lung lobes were cut, blot dried
and placed on pre-weighed glass plates. The wet weight of the
tissue was determined immediately. The tray with the tissue was
then baked in an oven at 55°C for 72 h to obtain a constant weight.
After the dry weight of the tissue was determined, the wet/dry
(W/D) lung weight ratio was calculated. The lung water content was
calculated as the wet weight minus dry weight and wet weight ratio
of lung tissue multiplied by 100%.
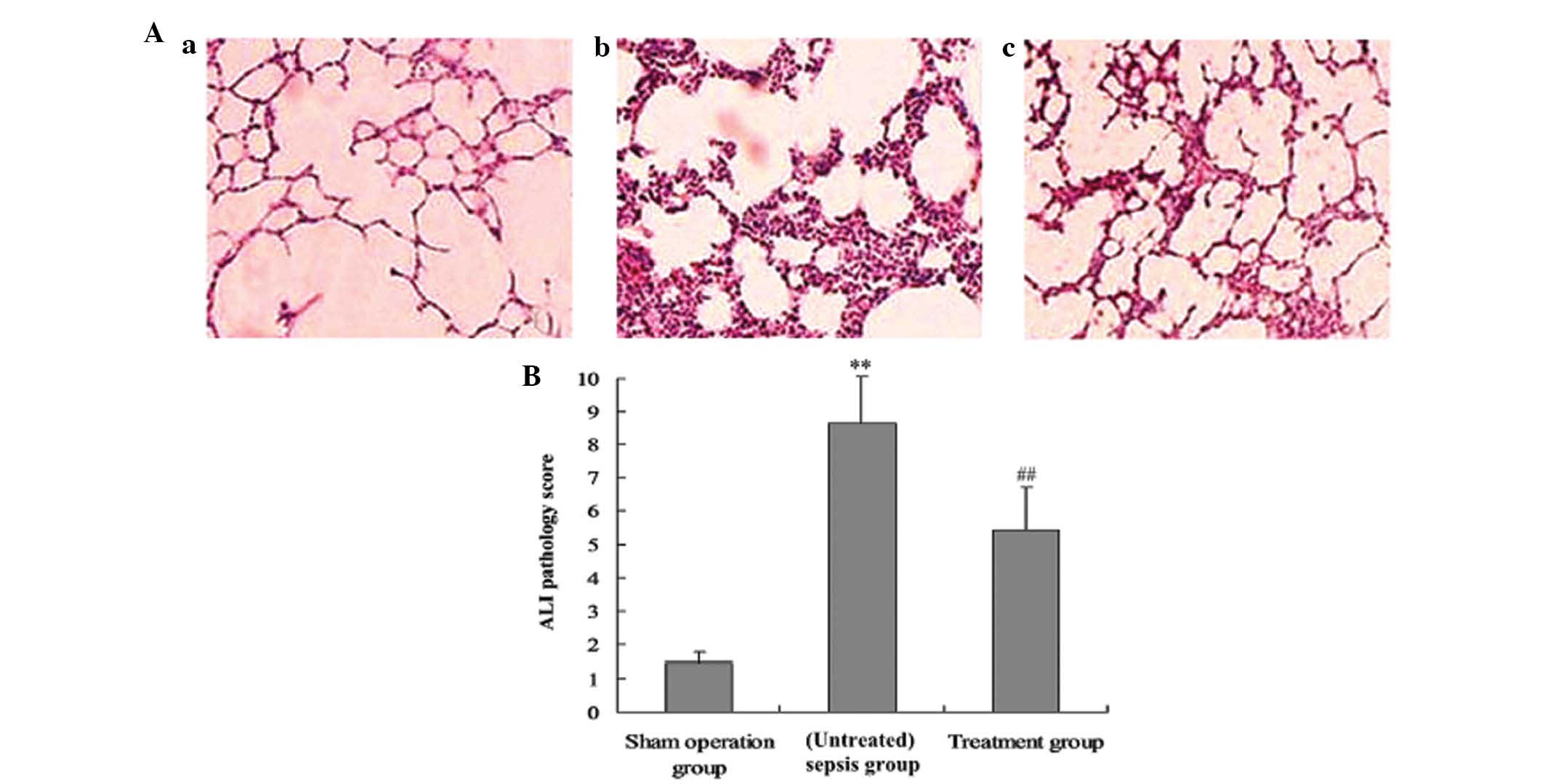
Pathological observation of lung
tissues
The middle lobe of the right lung was fixed by
infusing 10% formaldehyde solution in the same pressure, and the
inflation of the lung was kept uniform. The tissue was then
embedded in paraffin wax, cut into sections and stained with
hematoxylin-eosin (H&E). Pathological changes in the tissues
were observed using optical microscopy. Lung injury, based on
infiltration of inflammatory cells, pulmonary interstitial and
alveolar edema, damage to alveolar structure and degree of fibrosis
were assessed using the grading system reported by Szapiel et
al (11). ALI was scored as
follows (12): i) Alveolar
congestion; ii) hemorrhage; iii) infiltration or aggregation of
neutrophils in airspace or vessel wall, and iv) thickness of
alveolar wall/hyaline membrane formation. Each item was scored on a
five-point scale as follows: 0, minimal damage; 1, mild damage; 2,
moderate damage; 3, severe damage; and 4, maximal damage.
Repeatedly measured data were statistically analyzed using analysis
of variance (ANOVA).
Statistical analysis
Statistical analysis was performed with the SPSS
version 15.0 (SPSS, Inc., Chicago, IL, USA). Data were analyzed for
normality using the Kolmogorov-Smirnov method, and the normally
distributed data were expressed as the mean ± standard deviation.
To compare the normally distributed data between each group,
one-way ANOVA followed by the Student-Newman-Keul’s post-hoc test
was employed. P<0.05 was considered to indicate a statistically
significant difference.
Results
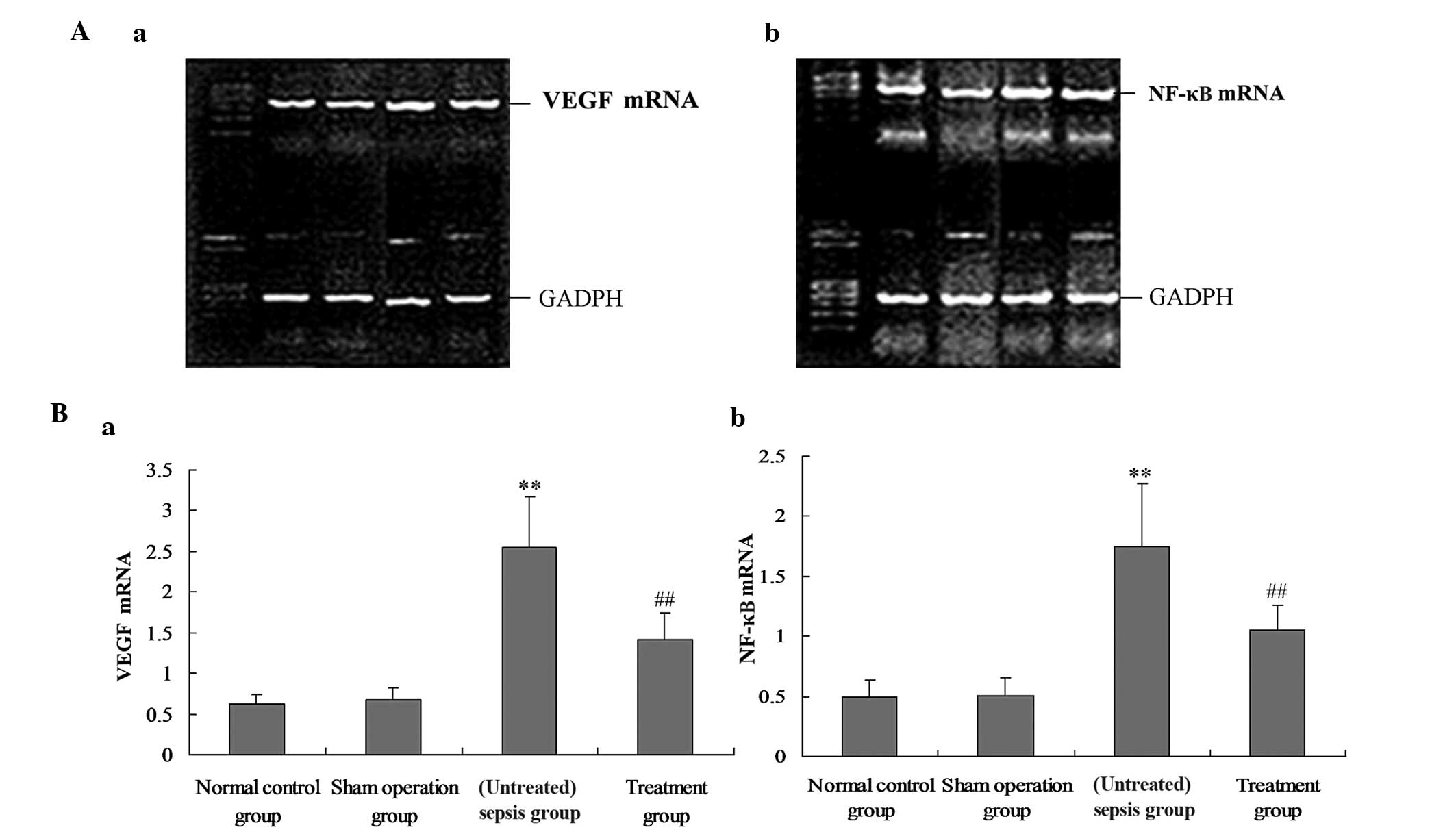
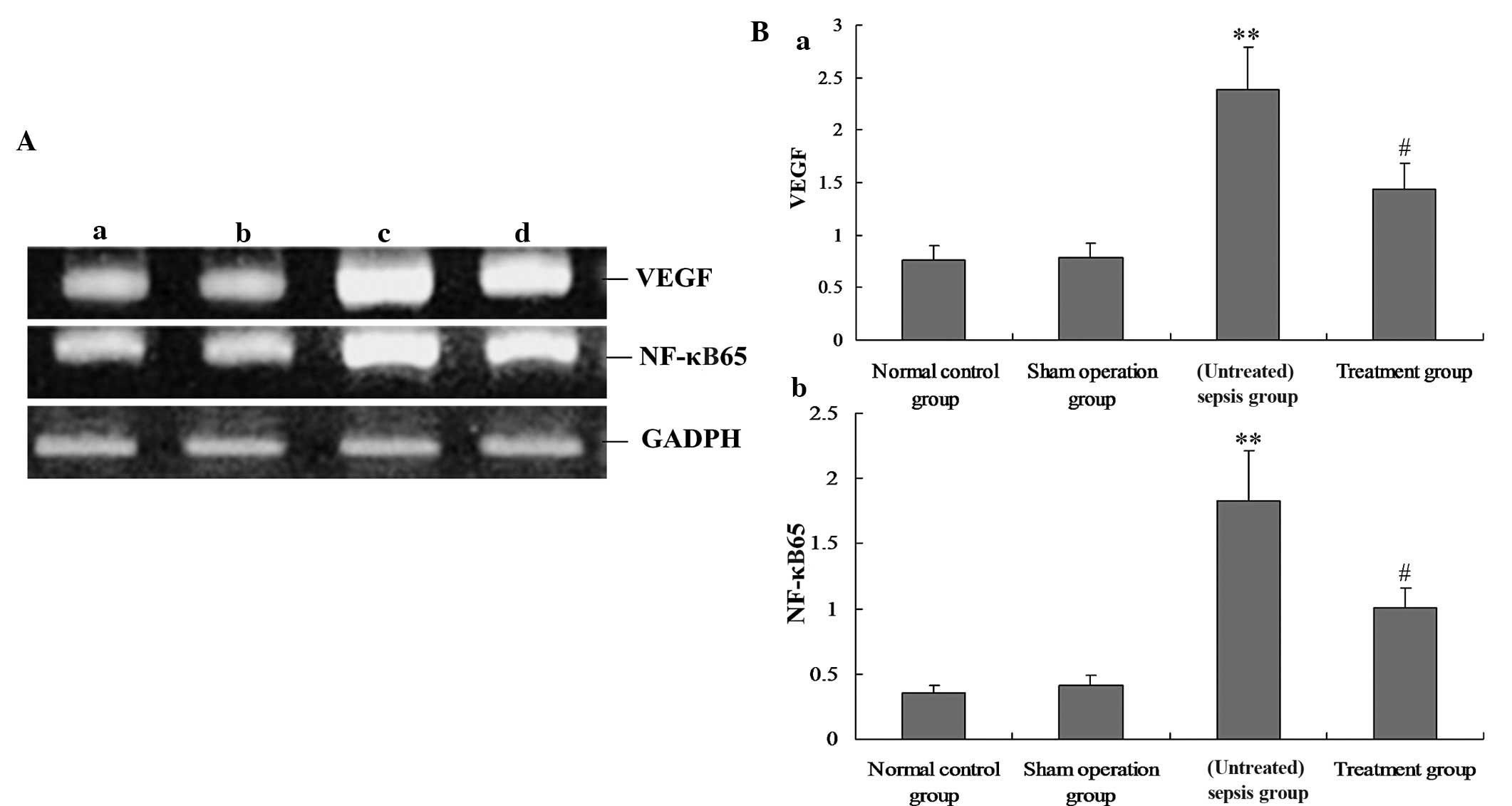
M. suaveolens extract downregulates the
expression of VEGF and NF-κB in lung tissue
To investigate the underlying mechanism of the
effect of M. suaveolens extract on CLP, the mRNA and protein
expression of VEGF and NF-κB were measured by qPCR and western blot
analysis, respectively. In vivo, the mRNA and protein
expression levels of VEGF and NF-κB in the rat lung demonstrated
significant increases during CLP-induced ALI (P<0.05; Figs. 1 and 2); however, these were significantly
decreased in response to the administration of M. suaveolens
extract (P<0.05; Figs. 1 and
2). A positive correlation was
evident between the levels of VEGF and NF-κB mRNA (r=0.852,
P<0.05) and between VEGF and NF-κB protein expression (r=0.794,
P<0.01).
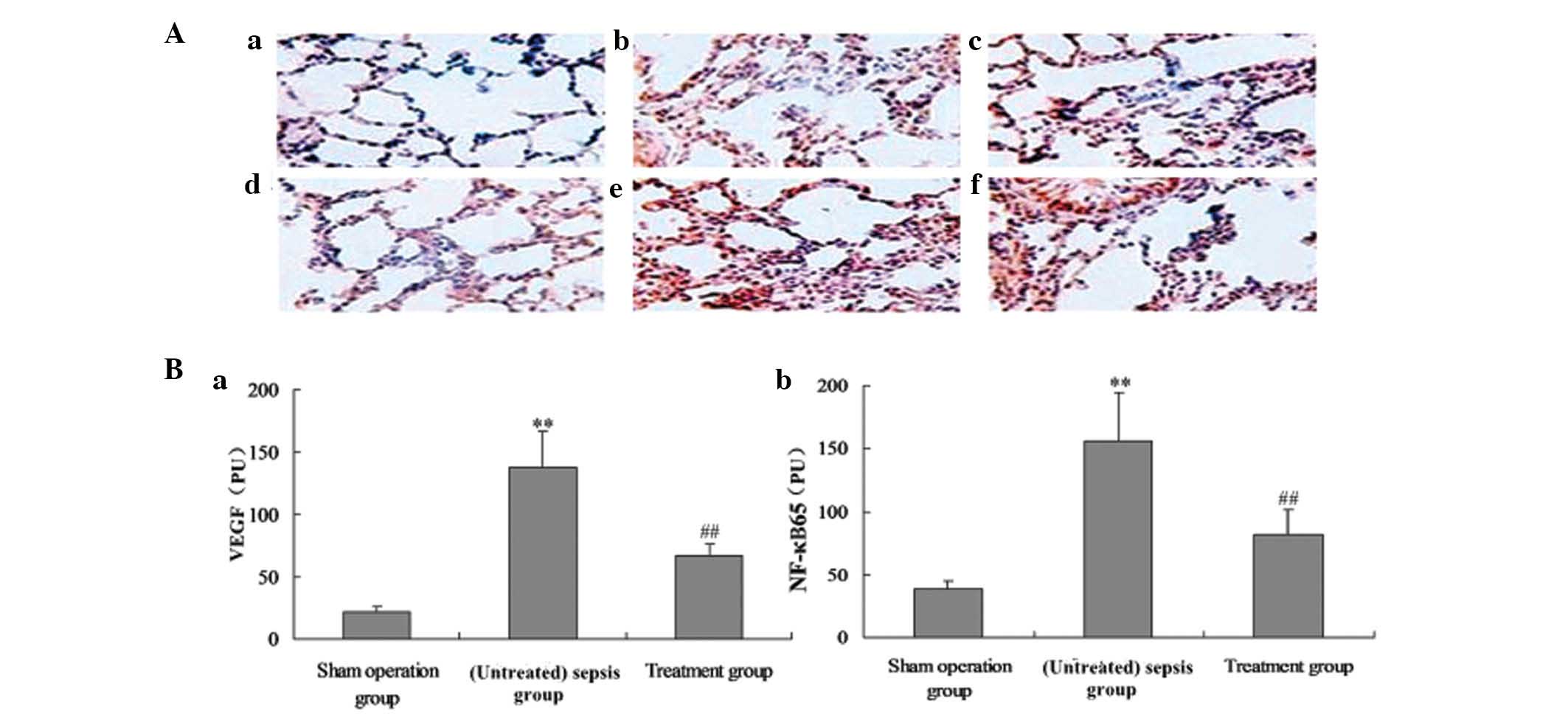
Effect of M. suaveolens extract on lung
localization of VEGF and NF-κB65 in CLP-induced ALI
Immunohistochemical analysis was used to determine
the distribution of VEGF and NF-κB65 in rat lung 24 h following CLP
or saline treatment. Positively immunostained cells appeared brown
(Fig. 3A). VEGF and NF-κB65 were
localized to the alveolar epithelium. The number of cells
expressing VEGF and NF-κB65 was significantly increased in
CLP-induced ACI, and this was significantly reduced by treatment
with M. suaveolens extract (Fig. 3A and B).
M. suaveolens extract decreases
pro-inflammatory cytokines and VEGF production in CLP-induced
rats
Serum and BAL were collected 24 h after the animals
received CLP to evaluate the levels of VEGF, TNF-α, IL-1β, IL-6,
IL-4 and IL-10. CLP caused a significant acute systemic
inflammatory response and pulmonary capillary permeability, as
demonstrated by the increased serum and BAL concentrations of the
pro-inflammatory mediators TNF-α, IL-1β, IL-6 and VEGF. The
presence of M. suaveolens extract reduced the increase of
all of these pro-inflammatory mediators. CLP also caused an
increase in the serum and BAL concentration of the
anti-inflammatory cytokines IL-10 and IL-4. This change in IL-10
and IL-4 concentration was enhanced by the administration of M.
suaveolens extract (Fig. 4E and
F; Fig. 5E and F).
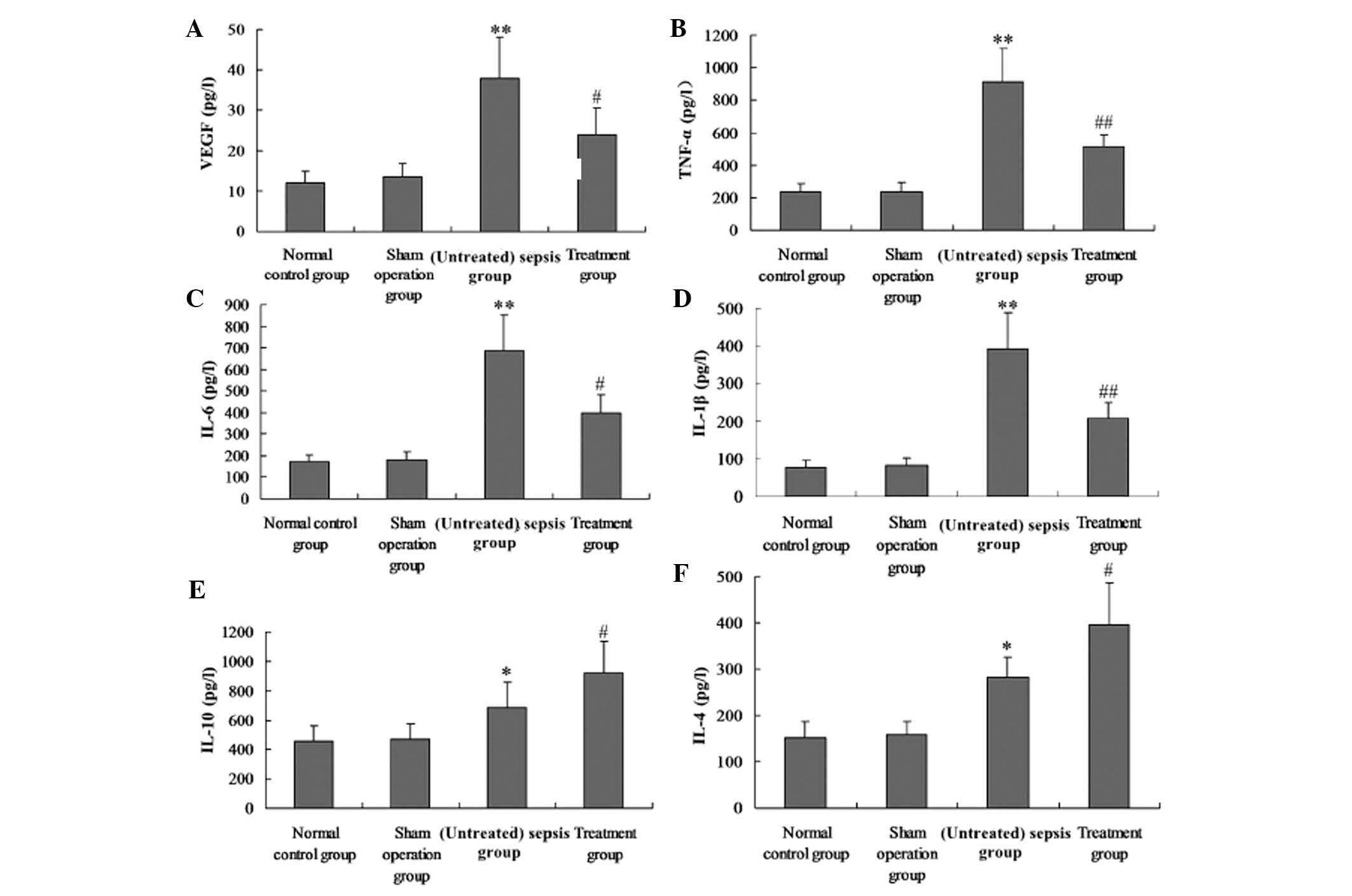 | Figure 4Effect of M. suaveolens
extract on plasma levels of VEGF, TNF-α, IL-6, IL-1β, IL-4 and
IL-10 levels in plasma. Groups of mice were challenged with cecal
ligation and puncture, and treated with M. suaveolens
extract 24 h later. (A) VEGF, (B) TNF-α, (C) IL-6, (D) IL-1β, (E)
IL-10 and (F) IL-4 levels in plasma were determined by ELISA. Data
are presented as the mean ± standard deviation of one experiment
consisting of three replicates. *P<0.05,
**P<0.01 vs. the sham operation group and normal
control group; #P<0.05, ##P<0.01 vs.
(untreated) sepsis group. VEGF, vascular endothelial growth factor;
NF-κB, nuclear factor kappa B; TNF-α, tumor necrosis factor-α; IL,
interleukin. |
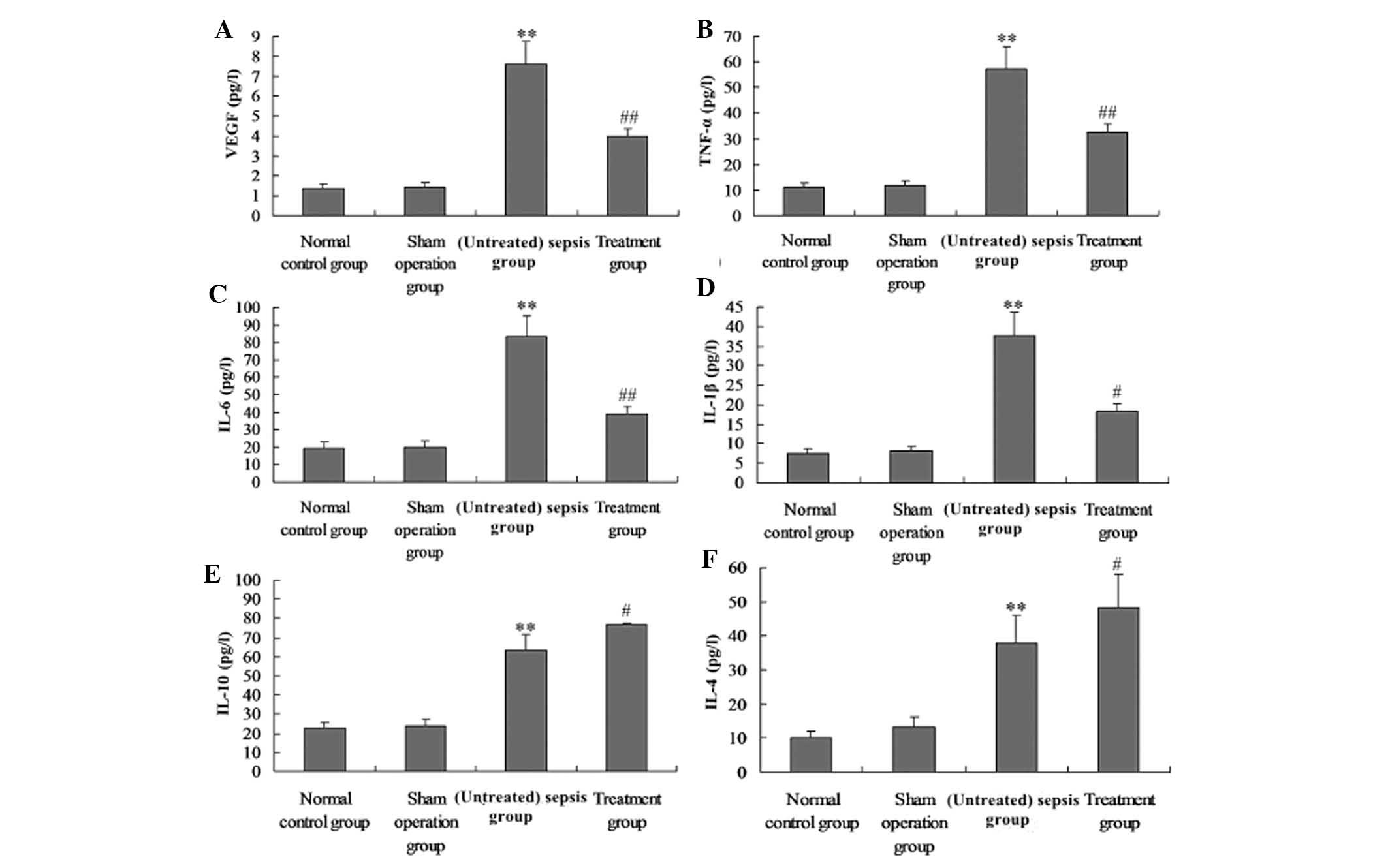 | Figure 5Administration of M.
suaveolens extract attenuated lipopolysaccharide-induced
pulmonary inflammation. Groups of mice were challenged with cecal
ligation and puncture, and treated with M. suaveolens
extract 24 h later. (A) VEGF, (B) TNF-α, (C) IL-6, (D) IL-1β, (E)
IL-10 and (F) IL-4 levels in bronchoalveolar lavage were determined
by ELISA. Data are presented as the mean ± standard deviation of
one experiment consisting of three replicates.
**P<0.01 vs. the sham operation group and normal
control group; #P<0.05, ##P<0.01 vs.
(untreated) sepsis group. VEGF, vascular endothelial growth factor;
NF-κB, nuclear factor kappa B; TNF-α, tumor necrosis factor-α; IL,
interleukin. |
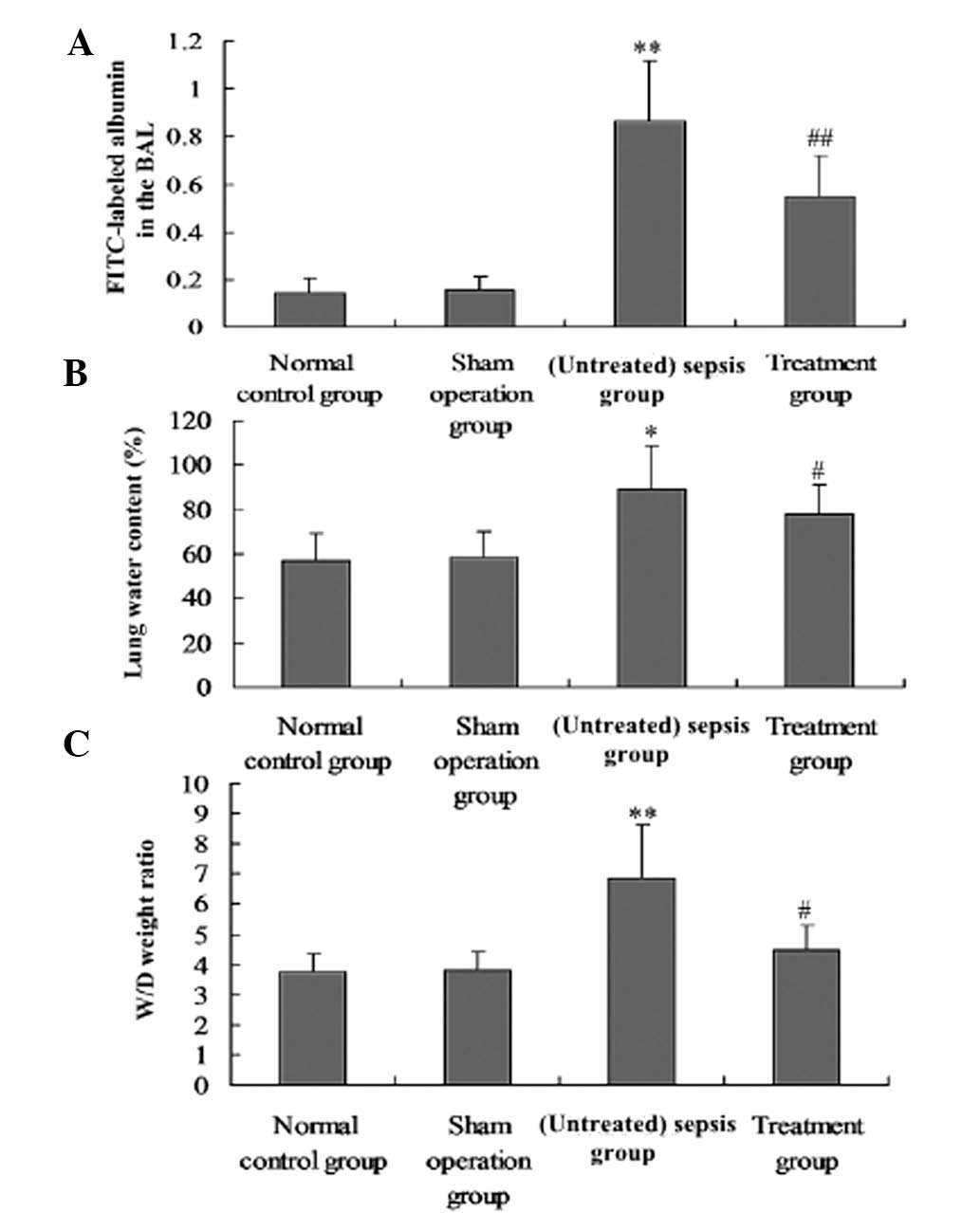
Effect of M. suaveolens extract on
pulmonary vascular permeability
M. suaveolens extract significantly reduced
the CLP-induced increases in i.v. administered FITC-labeled albumin
in BAL, as well as the W/D lung weight ratio and the water content
of the lung tissue (P<0.05; Fig.
6A–C). The effects of CLP on pulmonary vascular permeability
were also significantly suppressed by M. suaveolens extract
(P<0.05; Fig. 6A–C).
Effect of M. suaveolens extract on
histological parameters of ACI
The lung tissue was significantly injured as
indicated by the presence of intra-alveolar exudate, edema and
inflammatory cell infiltration in the control group (Fig. 7A), and an increase in lung injury
score (P<0.05, Fig. 7B). M.
suaveolens extract significantly attenuated CLP-induced
pathologic changes, demonstrating a significant decrease in lung
injury score.
Discussion
Numerous studies have demonstrated that the animal
model of sepsis induced by the CLP method is highly stable and
reproducible, and is applicable as a model of human sepsis
(13). It is therefore currently
regarded as the ‘gold standard’ for the study of sepsis. These
animals demonstrate a hyperdynamic circulation and high metabolism
in the early stage and a low dynamic circulation state at the later
stage resembling the human condition; therefore, this is regarded
as the sepsis model with the strongest clinical relevance (13). As a result, this CLP animal model
of sepsis was adopted for the present study.
When sepsis occurs, a large number of inflammatory
mediators are released(1,14–17);
endothelial cells are impaired and contract, and the distance
between endothelial cells is enlarged (14,15),
all of which result in protein-rich fluid entering the mesenchymal
cells from the blood vessels. This leads to a drop in the vascular
endothelial colloidal osmotic pressure and an increase of colloidal
osmotic pressure for tissue clearance, resulting in the presence of
more water molecules among the organelles (16,17),
and thus the development of interstitial edema. The diffusion
distance for oxygen molecules from the blood capillary to tissue
organs is increased, further aggregating hypoxia and leading to
organ dysfunction (15,16). Furthermore, hypoxemia and hypoxia
form a vicious cycle, resulting in further hypoxia-induced injury
within the endothelial cells, leading to further capillary leakage
(14). In the present study, CLP
markedly enhanced the expression of pro-inflammatory mediators,
including TNF-α and IL-6, and anti-inflammatory mediators,
including IL-10, and increased lung capillary leakage. However, the
administration of M. suaveolens extract significantly
inhibited the expression of pro-inflammatory mediators, elevated
the levels of anti-inflammatory mediators and reduced lung
capillary leakage. Inhibition of inflammatory mediators by M.
suaveolens therefore reduced the permeability of the lung
capillary wall and reduced CLP-induced lung injury.
VEGF, a protein first obtained by isolation from
in vitro cultures of bovine pituitary follicles in 1989, has
specific mitogenic effects on vascular endothelial cells which
promote endothelial cell proliferation, increase microvascular
permeability and promote the growth of endothelial cells within the
blood and lymphatic vessels (18).
VEGF is therefore a survival factor existing in endothelial cells.
VEGF has been used in numerous fields since its discovery,
including as a marker for clinical tumor prognosis, early diagnosis
of acute myocardial ischemia and evaluation of bronchial asthma
(19). In recent years, its role
in sepsis blood capillary leakage has drawn increasing attention
(5,19,20).
VEGF has a significant role among the known microvascular
permeability inducers, and effects ~50,000-fold of those of
histamine have been observed, with activities at concentrations
<1 nmol/l. Such effects are not inhibited by antihistamines,
platelet-activating factor inhibitors or other inhibitors of
inflammation (5). An excessive
increase of vascular permeability may cause blood flow into the
tissue, leading to poor blood supply for visceral function,
eventually resulting in organ dysfunction. van der Flier et al
(19) suggested that an increase
in capillary permeability is a key factor in the occurrence and
development of sepsis, while VEGF is the key molecule for
controlling vascular permeability, and is therefore a potential
factor that leads to inflammation-associated capillary
permeability. For this reason, VEGF was used as a measurement index
in the present study. The results indicated that increased
expression of NF-κB promotes the expression and production of VEGF,
and significantly aggravates pathological lung damage in rats with
sepsis.
It has been demonstrated that through its
involvement in the transcription of a variety of cytokine genes,
NF-κB has a complex and important role in regulating the
inflammatory network (21).
Activated NF-κB may increase the transcription of numerous
cytokines, including TNF-α and IL-1, thus rapidly increasing the
quantity of inflammatory factors synthetized by inflammatory cells
(22,23). Therefore, blocking the activation
of NF-κB may facilitate controlling the blood capillary
permeability of lung tissue, and thus attenuate lung microvascular
leakage and ALI in rats with sepsis. It is observed from this study
that inhibition of NF-κB activity by M. suaveolens
significantly reduced lung inflammatory responses, decreased the
expression of VEGF and alleviated capillary permeability in rats
with sepsis.
Vascular leakage in multiple organs is a
characteristic pathological change in sepsis (24). In the present study, the W/D ratio
of the lung was firstly examined. It was identified that
salidroside treatment attenuates the development of pulmonary
edema, as demonstrated by a significant decrease in lung W/D ratio.
Another index of ALI by CLP is the total protein concentration in
the BAL fluid which indicated epithelial permeability and pulmonary
edema. FITC-labeled albumin, a macromolecular marker, is widely
used to evaluate pulmonary microvascular permeability (25), and FITC-labeled albumin was
therefore injected and assessed in the BAL in the present study. As
expected, CLP was identified to cause a significant increase in BAL
fluid protein concentration, lung W/D ratio and FITC-labeled
albumin. CLP-induced increases in total protein in the BAL fluid,
lung W/D ratio and FITC-labeled albumin were inhibited by M.
suaveolens. In the present study, it was also identified that
M. suaveolens significantly reduced the accumulation and
sequestration of activated macrophages, suppressed formation of
pulmonary edema, prevented an increase in septa thickness and
ameliorated the lung capillary leakage in rats with sepsis.
Capillary leakage and endothelial dysfunction are
increasingly recognized to significantly contribute to organ
failure and death in sepsis and systemic inflammation (26–28).
Therapeutic targeting of capillary leakage in sepsis and systemic
inflammation is therefore considered a highly relevant clinical
approach. The pharmacological effects of M. suaveolens
(8,29–34)
indicate that one of the active components, coumarin, is able to
reduce capillary permeability and vascular resistance (8,29),
increase vein tension and improve circulation (30,31),
so that the open arteriovenous anastomosis tubes are closed to
reduce local congestion, therefore reducing soft tissue bleeding.
Coumarin may also inhibit the release of a variety of vascular
active substances (32),
suppressing adenosine diphosphate and collagen-induced platelet
aggregation as well as the release of 5-hydroxytryptamine, platelet
factor 4, thromboxane A2 and platelet-derived growth factor from
the platelet (33,34). These effects prevent the loss of
serum, maintain normal colloid osmotic pressure and have anti-edema
effects, therefore reducing post-traumatic swelling. The tannic
acid component of M. suaveolens inhibits the synthesis of
prostaglandins and other inflammatory mediators, as well as
reducing pain and the inflammatory reaction, reducing vascular
permeability and leakage from tissue clearance. Other effects
include increasing the formation of newborn granulation tissue,
promoting wound healing (closing the wound and preventing secondary
wound exudate), expanding lymphatic vessels, increasing lymph flow,
accelerating lymph circulation and reducing soft tissue swelling.
Medicinal chemistry study results have identified that M.
suaveolens contains coumarin, flavonoids, phenolic acids,
saponins and other substances that have effective anti-inflammatory
and antibacterial activities (31). Although its effect of reducing the
blood capillary leakage has been reported, studies of how this
occurs at the molecular level remain limited. The present study
revealed that M. suaveolens is able to inhibit the
activation and expression of NF-κB in rats with sepsis, reduce the
generation of inflammatory mediators, including TNF-α and IL-6
(with consequent reduction of VEGF expression), therefore
decreasing lung capillary permeability and having a protective role
in ALI.
Although an ability of M. suaveolens to
reduce blood capillary leakage has been reported in clinical
practice, the molecular biological mechanisms mediating this effect
have not been well investigated. The results of the present study
suggest a novel compensatory mechanism for the action of M.
suaveolens in the maintenance of lung vascular permeability
under pathological inflammatory conditions, through downregulating
VEGF expression. To the best of our knowledge, the present study
demonstrated for the first time the role of M. suaveolens in
mediating anti-inflammatory and barrier protective effects of lung
capillary permeability in a model of ALI. Therefore, the beneficial
effects of in vivo administration of M. suaveolens on
the parameters of capillary permeability in lung injury described
in this study may represent a novel therapeutic modality for the
treatment of CLP-induced lung capillary permeability and ALI.
Acknowledgements
The authors are grateful to Professor Mei-xian Sun
and Professor Lan-fang Qin for their technical assistance.
Abbreviations:
|
VEGF
|
vascular endothelial growth factor
|
|
NF-κB
|
nuclear factor kappa B
|
|
IL-6
|
interleukin-6
|
|
TNF-α
|
tumor necrosis factor-α
|
|
LPS
|
lipopolysaccharide
|
|
PCR
|
polymerase chain reaction
|
|
SIRS
|
systemic inflammatory response
syndrome
|
|
CLP
|
cecal ligation and puncture
|
References
|
1
|
Park SJ, Pai KS, Kim JH and Shin JI: What
dose of intravenous immunoglobulin should be administered in
Kawasaki disease with suspected systemic capillary leak syndrome?
Comment on: shock: an unusual presentation of Kawasaki disease (Eur
J Pediatr 2011 Jul; 170(7):941–3). Eur J Pediatr. 17:203–204. 2012.
View Article : Google Scholar
|
|
2
|
Zhao L, Tao JY, Zhang SL, et al: N-butanol
extract from Melilotus Suaveolens Ledeb affects pro- and
anti-inflammatory cytokines and mediators. Evid Based Complement
Alternat Med. 7:97–106. 2010. View Article : Google Scholar :
|
|
3
|
van Meurs M, Castro P, Shapiro NI, et al:
Adiponectin diminishes organ-specific microvascular endothelial
cell activation associated with sepsis. Shock. 37:392–398. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ueda T, Takeyama Y, Yasuda T, et al:
Vascular endothelial growth factor increases in serum and protects
against the organ injuries in severe acute pancreatitis. J Surg
Res. 134:223–230. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Mura M, dos Santos CC, Stewart D and Liu
M: Vaseular endothelial growth factor and related moleeules in
acute lung injury. J Appl Physiol 1985. 97:1605–1167. 2004.
View Article : Google Scholar
|
|
6
|
Abadie Y, Bregeon F, Papazian L, et al:
Decreased VEGF concentration in lung tissue and vascular injury
during ARDS. Eur Respir J. 25:139–146. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Földi M and Zoltán OT: Unconditioned
reflex activity in experimental lymphostatic encephalopathy and the
therapeutic action of coumarin from Melilotus officinalis.
Arzneimittel-Forschung. 20:1623–1624. 1970.
|
|
8
|
Liu MW, Su MX, Wang YH, Wei W, Qin LF, Liu
X, Tian ML and Qian CY: Effect of melilotus extract on lung injury
by upregulating the expression of cannabinoid CB2 receptors in
septic rats. BMC Complement Altern Med. 14:942014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Sonkodi S: Decrease of spontaneous
motility in experimental lymphogenic encephallopathy and the
protective activity of coumarin from Melilotus officinalis.
Arzneimittel-Forschung. 20(Suppl 11a): 16171970.(In German).
|
|
10
|
Macias FA, Simonet AM, Galindo JCG,
Pacheco PC and Sanchez JA: Bioactive polar triterpenoids from
Melilotus messanensis. Phytochemistry. 49:709–717. 1998. View Article : Google Scholar
|
|
11
|
Szapiel SV, Elson NA, Fulmer JD,
Hunninghake GW and Crystal RG: Bleomycin-induced interstitial
pulmonary disease in the nude, athymic mouse. Am Rev Respir Dis.
120:893–899. 1979.PubMed/NCBI
|
|
12
|
Mikawa K, Nishina K, Takao Y and Obara HA:
ONO-1714, a nitric oxide synthase inhibitor, attenuates
endotoxin-induced acute lung injury in rabbits. Anesth Analg.
97:1751–1755. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Seely KA, Holthoff JH, Burns ST, et al:
Hemodynamic changes in the kidney in a pediatric rat model of
sepsis-induced acute kidney injury. Am J Physiol Renal Physiol.
301:F209–F217. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Mancuso P, Whelan J, DeMichele SJ, Snider
CC, Guszcza JA, Claycombe KJ, Smith GT, Gregory TJ and Karlstad MD:
Effects of eicosapentaenoic and gamma-linolenic acid on lung
permeability and alveolar macrophage eicosanoid synthesis in
endotoxic rats. Crit Care Med. 25:523–532. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Liu MW, Wang YH, Qian CY and Li H:
Xuebijing exerts protective effects on lung permeability leakage
and lung injury by upregulating Toll-interacting protein expression
in rats with sepsis. Int J Mol Med. 34:1492–504. 2014.PubMed/NCBI
|
|
16
|
Castanares-Zapatero D, Bouleti C, et al:
Connection between cardiac vascular permeability, myocardial edema,
and inflammation duringsepsis: role of the α1AMP-activated protein
kinase isoform. Crit Care Med. 41:e411–e422. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Fisher BJ, Kraskauskas D, Martin EJ, et
al: Mechanisms of attenuation of abdominal sepsis induced acute
lung injury by ascorbic acid. Am J Physiol Lung Cell Mol Physiol.
303:L20–L32. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Skóra J, Barć P, Pupka A, et al:
Transplantation of autologous bone marrow mononuclear cells with
VEGF gene improves diabetic critical limb ischaemia. Endokrynol
Pol. 64:129–138. 2013.PubMed/NCBI
|
|
19
|
van der Flier M, van Leeuwen HJ, van
Kessel KP, et al: Plasma vascular endothelial growth factor in
severe sepsis. Shock. 23:35–38. 2005. View Article : Google Scholar
|
|
20
|
Yano K, Liaw PC, Mullington JM, et al:
Vascular endothelial growth factor is an important determinalt of
sepsis morbidity and mortality. J Exp Med. 203:1447–1458. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wei J, Huang X, Zhang Z, et al: MyD88 as a
target of microRNA-203 in regulation of lipopolysaccharide or
Bacille Calmette-Guerin induced inflammatory response of macrophage
RAW264.7 cells. Mol Immunol. 55:303–309. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Huang Y, Nikolic D, Pendland S, Locklear
TD and Mahady GB: Effects of cranberry extracts and ursolic acid
derivatives on P-fimbriated Escherichia coli, COX-2 activity,
pro-inflammatory cytokine release and the NF-κB transcriptional
response in vitro. Pharm Biol. 47:18–25. 2009. View Article : Google Scholar
|
|
23
|
Ghose R, Guo T, Vallejo JG and Gandhi A:
Differential role of Toll-interleukin 1 receptor domain-containing
adaptor protein in Toll-like receptor 2-mediated regulation of gene
expression of hepatic cytokines and drug-metabolizing enzymes. Drug
Metab Dispos. 39:874–881. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Oltean S, Neal CR, Mavrou A, et al:
VEGF165b overexpression restores normal glomerular water
permeability in VEGF164-overexpressing adult mice. J Am Soc
Nephrol. 21:F1026–36. 2012.
|
|
25
|
Lucas R, Sridhar S, Rick FG, et al:
Agonist of growth hormone-releasing hormone reduces
pneumolysin-induced pulmonary permeability edema. Proc Natl Acad
Sci USA. 109:2084–2089. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Russell JA: Management of sepsis. Minerva
Med. 99:431–458. 2008.
|
|
27
|
Schick M, Isbary T, Schlegel N, et al: The
impact of crystalloid and colloid infusion on the kidney in rodent
sepsis. Intensive Care Med. 36:541–548. 2010. View Article : Google Scholar
|
|
28
|
Schlegel N, Baumer Y, Drenckhahn D and
Waschke J: Lipopolysaccharide-induced endothelial barrier
breakdownis cAMP dependent in vivo and in vitro. Crit Care Med.
37:1735–1743. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kaphle K, Wu LS, Yang NY and Lin JH:
Herbal medicine research in Taiwan. Evid Based Complement Alternat
Med. 3:149–155. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Trouillas P, Calliste CA, Allais DP, et
al: Antioxidant, anti-inflammatory and antiproliferative properties
of sixteen water plant extracts used in the Limousin countryside as
herbal teas. Food Chemistry. 80:399–407. 2003. View Article : Google Scholar
|
|
31
|
Zhao L, Tao JY, Zhang SL, et al: Inner
anti-inflammatory mechanisms of petroleum ether extract from
Melilotus suaveolens Ledeb. Inflammation. 30:213–223. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Gebre-Mariam T, Asres K, Getie M, et al:
In vitro availability of kaempferolglycosidesfrom cream
formulationsof methanolic extractof the leaves of Melilotus
elegans. Eur J Pharm Biopharm. 60:31–38. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Asres K, Gibbons S, Hana E and Bucar F:
Anti-inflammatory activity of extracts and a saponin isolated from
Melilotus elegans. Pharmazie. 60:310–312. 2005.PubMed/NCBI
|
|
34
|
Guo X, Pan Y, Xiao C, et al: Fractalkine
stimulates cell growth and increases its expression via NF-κB
pathway in RA-FLS. Int J Rheum Dis. 15:322–329. 2012. View Article : Google Scholar : PubMed/NCBI
|