Introduction
Orexin A and B are neuropeptides derived from the
proteolytic cleavage of a common 130-amino acid precursor peptide
prepro-orexin. In humans their amino acids are 46% identical
(1). These peptides act via two
closely associated G-protein coupled receptors, the orexin
receptors 1 (OX1R) and 2 (OX2R) for orexins (1,2).
OX1R appears to be highly selective for orexin A, whereas OX2R
binds orexin A and B with similar affinity (3). Orexins are involved in the regulation
of numerous body functions, including food intake (4), the sleep-wake cycle (4), breathing (5), the reward system (4,6) and
drug addiction (6,7). Orexin-producing neurons are present
in the dorsal and lateral hypothalamus, where they project to and
excite numerous structures of the brain (8). It has been reported that orexins are
not restricted to the hypothalamus, but may also be expressed in
peripheral tissues, including adrenals, gastrointestinal tract and
endocrine pancreas (9).
It has been reported that orexins may also have a
role in the proliferation of certain types of cancer cells
(10). For example, orexins
induced apoptosis in human colon cancer cell lines, which greatly
reduced cell growth. This effect was observed in human
neuroblastoma cells (10) and rat
pancreatic tumor cells (11).
However, orexin A stimulated cell proliferation in adrenal gland
tumor cells, the effects of which were more remarkable in cultured
adenomatous cells than in normal adrenocortical cells (12). In addition, orexin A had no effect
on proliferation of rat C6 glioma cells (13). These studies added a novel
dimension to the biological functions of orexins. However, to the
best of our knowledge, the effects of orexin A on proliferation and
apoptosis have not been demonstrated in gastric cancer cells.
Numerous studies have demonstrated that disorders of
the phosphatidylinositol 3-kinase (PI3K)/AKT/mechanistic target of
rapamycin (mTOR) signaling pathway are associated with the process
of proliferation and apoptosis in various tumor cells (14–18).
Phosphorylation of AKT occurs primarily in gastric cancer cells and
activation of these cells can prolong their survival and increase
proliferation, thereby promoting tumor development and angiogenesis
(19). Moreover, phosphorylated
AKT exerts anti-apoptotic effects through the phosphorylation of
B-cell lymphoma-associated death promoter (Bad) (20) and caspase-9 (21). Therefore, AKT may be involved in
the regulation of gastric cancer cell survival and apoptosis.
In the present study, BGC-823 gastric cancer cell
apoptosis and proliferation assays were performed to evaluate the
effect of orexin A on gastric cancer cell growth. Furthermore, cell
death levels and caspase-3 activation were examined in order to
determine the protective effect of orexin A against apoptosis. In
addition, in order to verify the involvement of the PKB/AKT
pathway, the activation of phosphorylated AKT and total AKT were
examined following the treatment of cells with a series of
concentrations of exogenous orexin A and associated inhibitors. The
results of the present study provided evidence for the functional
role of orexin A in gastric cancer cells via OX1R-stimulated AKT
signaling pathway.
Materials and methods
Reagents
The orexin A and caspase-3 assay kits were obtained
from Sigma (St. Louis, MO, USA). RPMI 1640 medium and fetal bovine
serum (FBS) were purchased from Gibco-BRL (Grand Island, NY, USA).
The AKT inhibitor PF-04691502 was purchased from Selleck (Houston,
TX, USA). OX1R-specific antagonist SB334867 was obtained from
Tocris (Minneapolis, MA, USA). The Cell Death Detection ELISA kit
and Cell Proliferation ELISA bromodeoxyuridine (BrdU) colorimetric
kit were purchased from Roche Diagnostics (Penzberg, Germany).
Total/phospho-AKT (s473) polyclonal antibodies, β-actin (c4):
sc-47778 mouse monoclonal antibody was purchased from Santa Cruz
Biotechnology, Inc. (Santa Cruz, CA, USA). Total/phospho-AKT (s473)
rabbit polyclonal antibodies and the OX1R rabbit polyclonal
antibody were obtained from Abcam (Cambridge, UK).
Cell culture
Human BGC-823 gastric cancer cells were obtained
from the American Type Culture Collection (Manassas, VA, USA) and
maintained in RPMI 1640 medium supplemented with 10% (wt/vol) FBS,
L-glutamine (2 mM), penicillin (50 μg/ml) and streptomycin (100
μg/ml) (Xianfeng, Shanghai, China). The cells were grown in a
humidified atmosphere containing 5% CO2 at 37°C. Prior
to an experiment, cells were grown in petri dishes in serum-free
medium for 24 h. The next day, cells were treated with different
concentrations of orexin A (0, 10−10, 10−8
and 10−6 M), or 10−8 M orexin A with either
SB334867, PF-04691502 or their combination for 20 min.
Cell proliferation assays
BGC-823 gastric cancer cells were seeded
(2×103 cells/well) in 96-well plates and cultured for 24
h. To synchronize cell cycles, cells were serum-deprived for 24 h
and then treated with various concentrations (0, 10−10,
10−8 and 10−6M) of orexin A or
10−8M orexin A along with 10−6M OX1R
antagonist SB334867 for a further 24 h. BrdU solution
(10−6 M) was then added and cells were incubated for 2.5
h. BrdU incorporation into DNA was measured using the Cell
Proliferation ELISA BrdU colorimetric kit (Roche Diagnostics).
Cell viability
BGC-823 gastric cancer cells were seeded
(2×103 cells/well) in 96-well plates and cultured for 24
h. Following incubation in a serum free RPMI 1640 medium
supplemented with test agents for 48 h, MTT (Sigma) solution (0.5
mg/ml) was added. After 3 h, the culture medium was removed and the
formazan crystals that formed were dissolved in 100 μl dimethyl
sulfoxide (Merck KGaA, Darmstadt, Germany). Optical density was
measured using a plate reader (SpectraMax Plus384 microplate
reader; Molecular Devices, Ismaning, Germany) at 570 and 650 nm
(reference wave/length).
Annexin V/propidium iodide (PI) assays
for apoptosis
Apoptotic cells were quantified using the Annexin
V/PI Apoptosis Detection kit and evaluated for apoptosis by BD
Accuri™ C6 Flow Cytometer according to the manufacturer’s
instructions (BD Pharmingen, San Diego, CA, USA). Cells were
treated with different concentrations of orexin A in the absence of
serum for 48 h. 1×105 cells were briefly washed twice
with phosphate-buffered saline, then stained with 5 μl Annexin
V-fluorescein isothiocyanate (FITC) and 10 μl PI in 500 μl binding
buffer for 15 min at room temperature in the dark. Apoptosis was
determined by counting the number of cells stained by FITC-labeled
Annexin V by fluorescence-activated cell sorting analysis. Early
apoptotic cells were identified as PI-negative and FITC-Annexin V
positive; cells that were in late apoptosis or already dead were
positive for PI as well as FITC-Annexin V.
Activity of caspase-3 in BGC-823
cells
BGC-823 cells were cultured in serum-free medium
using six-well plates (1.5×105 cells/well). Culture
medium was then replaced with fresh culture medium with or without
orexins. After 24 h, caspase-3 activity was assessed using a
Caspase-3 Colorimetric Assay kit following the manufacturer’s
instructions (Sigma).
Polymerase chain reaction (PCR)
Total RNA was extracted from BGC-823 cells using
TRIzol reagent (Invitrogen, Carlsbad, CA, USA). The expression of
OX1R and OX2R messenger RNA (mRNA) was detected using PCR and
TaqMan reagents (Takara, Otsu, Japan). The following specific
primers were used: OX1R forward, 5′-TGC GGC CAA CCC TAT CAT CTA-3′
and reverse, 5′-ACC GGC TCT GCA AGG ACA A-3′); OX2Rforward, 5′-ATC
GCA GGG TAT ATC ATC GTG TTC-3′ and reverse, 5′-TGA CTG TCC TCA TGT
GGT GGT TC-3′. As an internal control for reverse transcription
(RT) and reaction efficiency, amplification of GAPDH mRNA was
performed in parallel for each sample. The following specific
primers were used: GAPDH forward, 5′-GGC ACA GTC AAG GCT GAG AAT
G-3′ and reverse, 5′-ATG GTG GTG AAG ACG CCA GTA-3′. The PCR
reactions were performed using the following conditions: 95°C for
30 sec, then 40 cycles of 95°C for 5 sec, 60°C for 30 sec and 95°C
for 15 sec. All primers and TaqMan probes specific to OX1R, OX2R
and GAPDH were designed using Primer Premier 5.0 software (Premier
Biosoft International, Palo Alto, CA, USA).
Protein preparations and western blot
analysis
BGC-823 cells were washed with cold PBS and
harvested in radioimmunoprecipitation assay buffer (Beyotime
Biotechnology, Jiangsu, China) containing protease inhibitors like
phenylmethylsulfonyl fluoride (Beijing, Jiangsu, China) and
phosphatase inhibitors (KeyGEN Biotech Co., Ltd., Nanjing, China).
Cell lysates were incubated on ice for 30 min, then collected and
centrifuged at 12,000 × g for 10 min at 4°C. The supernatants were
collected, mixed with 5× loading buffer and then denatured by
boiling for 10 min. Samples were separated by SDS-PAGE and
transferred to polyvinylidene fluoride membranes at 60 V for 2.5 h
in a transfer buffer containing 20 mM Tris (bioWORLD, Dublin, OH,
USA), 150 mM glycine (Solarbio, Beijing, China) and 20% methanol
(Xinxing, Liaoning, China). Membranes were then incubated with a
primary antibody against OX1R at a 1:250 dilution or
phospho/total-AKT at a 1:1,000 dilution in TBST overnight at 4°C.
The membranes were washed and incubated with horseradish
peroxidase-conjugated anti-species secondary antibody for 1.5 h at
room temperature, and then washed three times with TBST for 30 min.
Protein were visualized by BeyoECL (Beyotime Biotechnology,
Jiangsu, China). Band densities were measured using Quantity-One
V4.6.2 software (Bio-Rad, Hercules, CA, USA).
Statistical analysis
Values are expressed as the mean ± standard error of
the mean and differences between the means were analyzed by one-way
analysis of variance. P<0.05 was considered to indicate a
statistically significant difference between values. Statistical
analysis was performed using the SPSS 15.0 software package (SPSS
Inc., Chicago, IL, USA).
Results
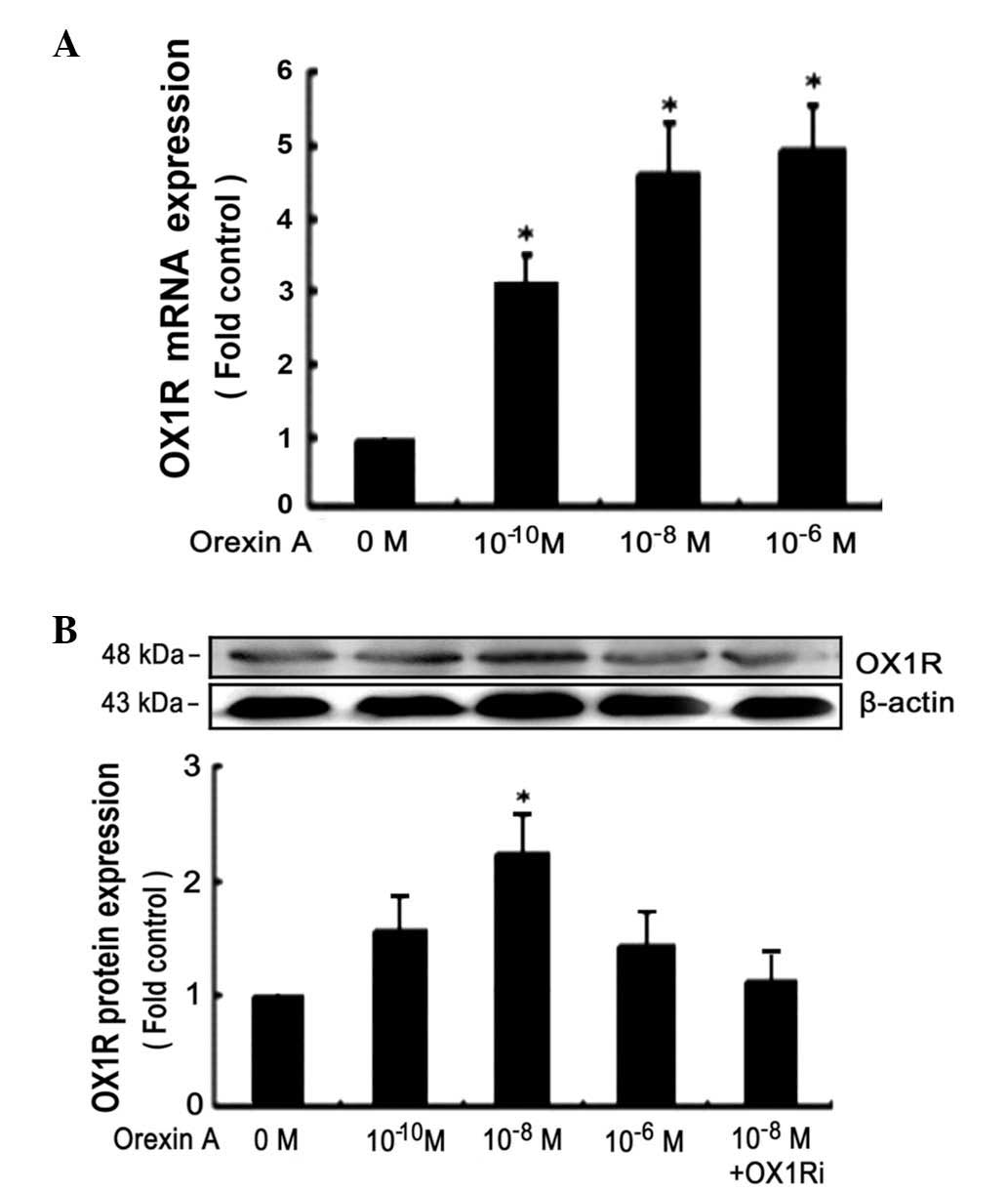
Orexin A stimulates OX1R protein
expression in BGC-823 cells
BGC-823 cells were cultured for 24 h at 37°C and
treated with orexin A at concentrations of 0, 10−10,
10−8 and 10−6 M, for 24 h. PCR assays
demonstrated that OX1R mRNA was expressed in BGC-823 cells
(Fig. 1A). However, OX2R mRNA was
not detected under identical conditions (data not shown). Treatment
with Orexin A caused a dose-dependent increase of OX1R protein
expression in BGC-823 cells with 10−6 M being the most
potent concentration of orexin A (~2.1-fold increase) (Fig. 1B). Orexin A (10−8 M) in
the presence of 10−6 M SB334867, a high-affinity
OX1R-specific non-peptide antagonist, significantly inhibited the
expression of OX1R protein (Fig.
1B).
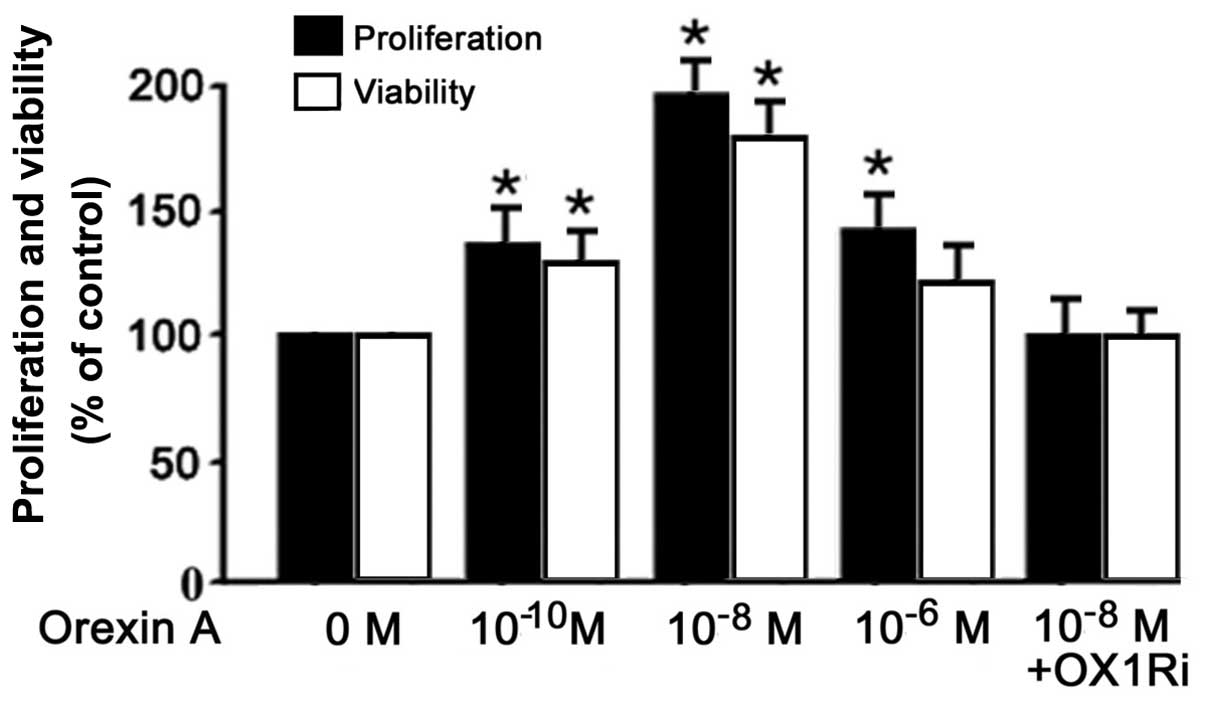
Effects of orexin A on proliferation and
viability of BGC-823 cells
To determine the effects of orexin A on cell
survival, BGC-823 cells were stimulated with orexin A at
concentrations (0, 10−6, 10−8 and
10−10 M) in combination with OX1R antagonist SB334867
(10−6 M). The cells were serum-starved for 24 h prior to
exposure to the tested compounds in order to avoid interactions
with growth factors and other mediators present in serum. An MTT
assay showed that orexin A, at all the tested concentrations,
significantly promoted the proliferation and viability of XR0416R
cells (P<0.05; Fig. 2). The
present study determined that treatment with 10−8 M
orexin A increased cell proliferation and viability by 1.5-fold
compared with that of the control. The proliferation and viability
of the group of cells treated with orexin A (10−8 M) and
OX1R-specific antagonist SB334867 were not significantly higher
than those of the control. This therefore indicated that SB334867
significantly inhibited the proliferation and viability of BGC-823
cells in comparison with the cells exposed to orexin A
(10−8 M) at an identical concentration (Fig. 2).
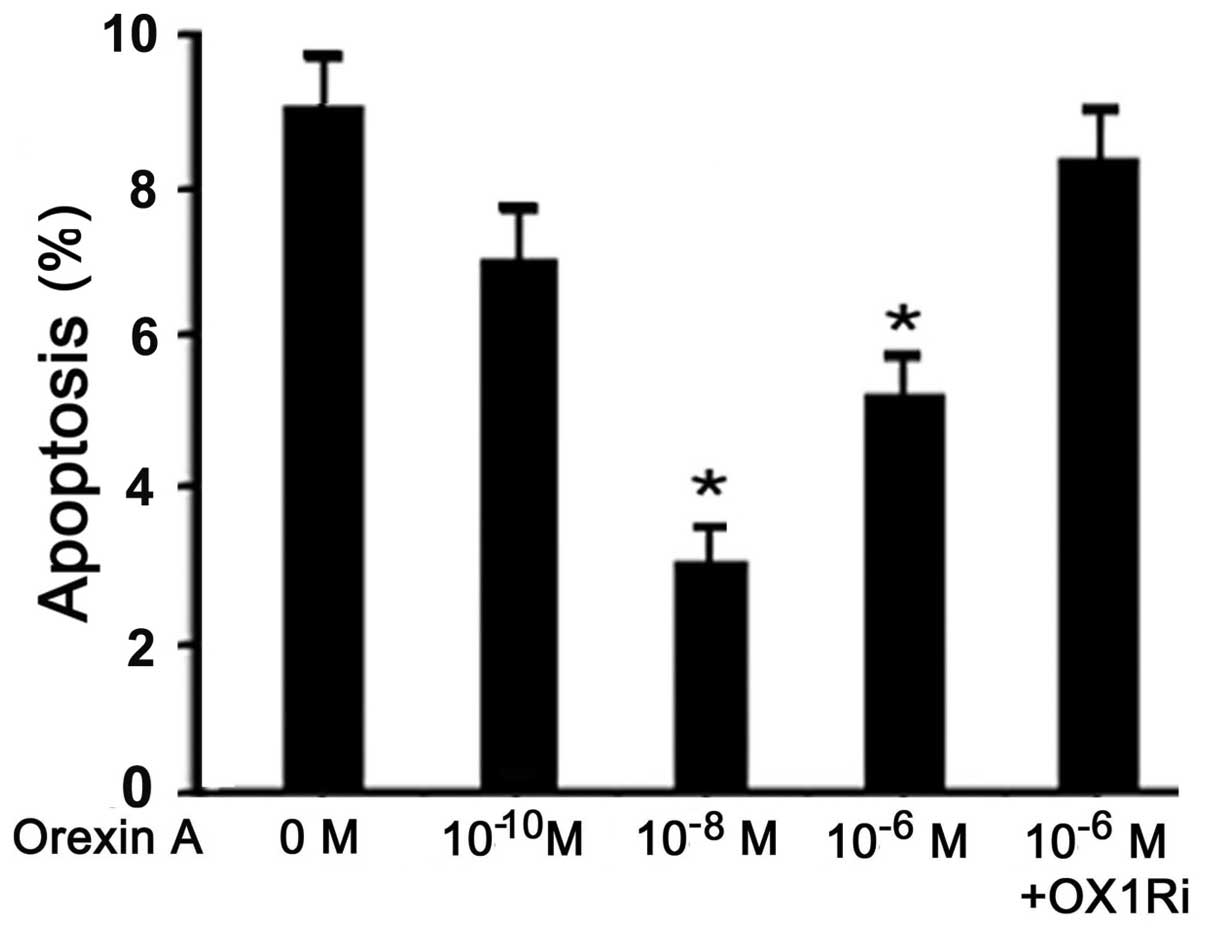
Orexin A protects BGC-823 cells from
apoptosis
Orexin A treatment (10−10,
10−8 and 10−6 M) resulted in a decreased
apoptotic index, measured using the Cell Death Detection ELISA kit.
Concentrations of 10−8 and 10−6 M orexin A
resulted in a significant decrease in the apoptotic rate of H295R
cells compared to that of the control (P<0.05) (Fig. 3); however, orexin A
(10−6M) failed to protect cells against apoptosis in the
presence of 10−6 M SB334867 (Fig. 3).
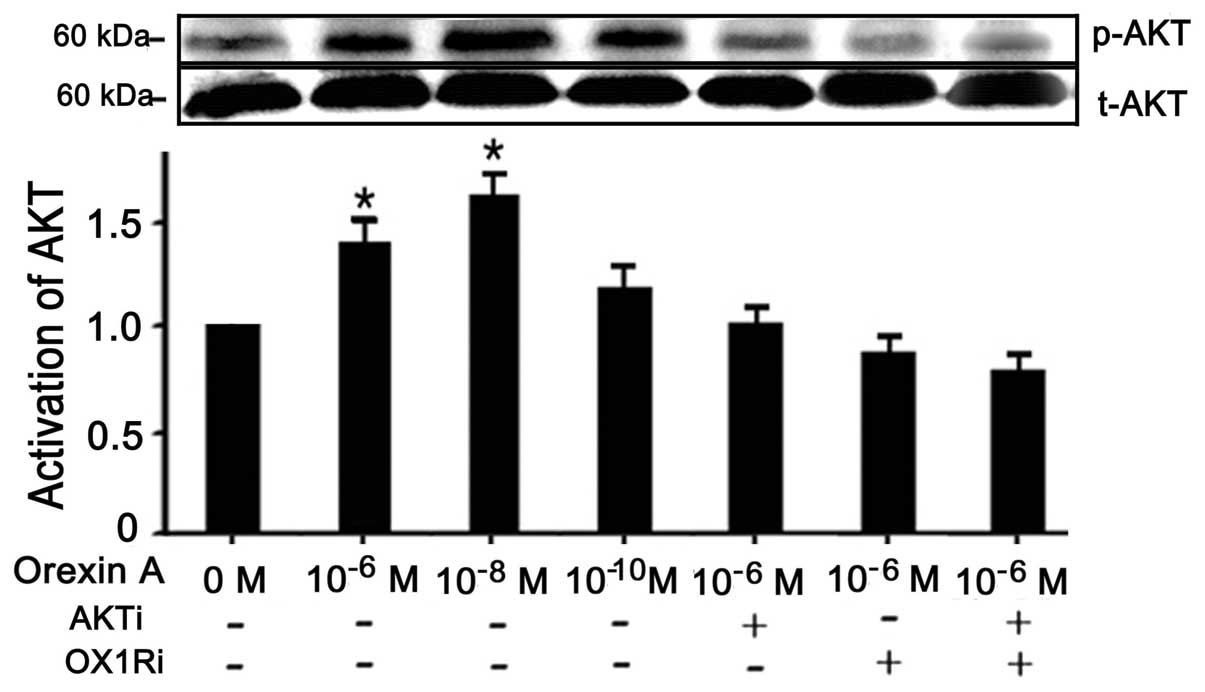
Orexin A enhances proliferation of
BGC-823 cells via OX1R-evoked AKT signaling pathway
It is known that the PI3K/AKT signaling pathway is
involved in cell survival and apoptotic signaling; therefore, the
present studies investigated whether orexin A-stimulation of
BGC-823 cells induced activation of AKT (22,23).
The data confirmed a 1.5-fold increase of p-AKT protein in BGC-823
cells treated with 10−8 M orexin A, compared to that of
the untreated control cells (P<0.05) (Fig. 4). However, the total AKT levels
remained unaffected by the aforementioned treatment. Moreover,
10−6 M AKT antagonist PF-04691502 and 10−6 M
OX1R antagonist SB334867 abolished the relative increase in AKT
activation in response to orexin A, independently and in
combination (Fig. 4).
Effects of orexin A on proliferation and
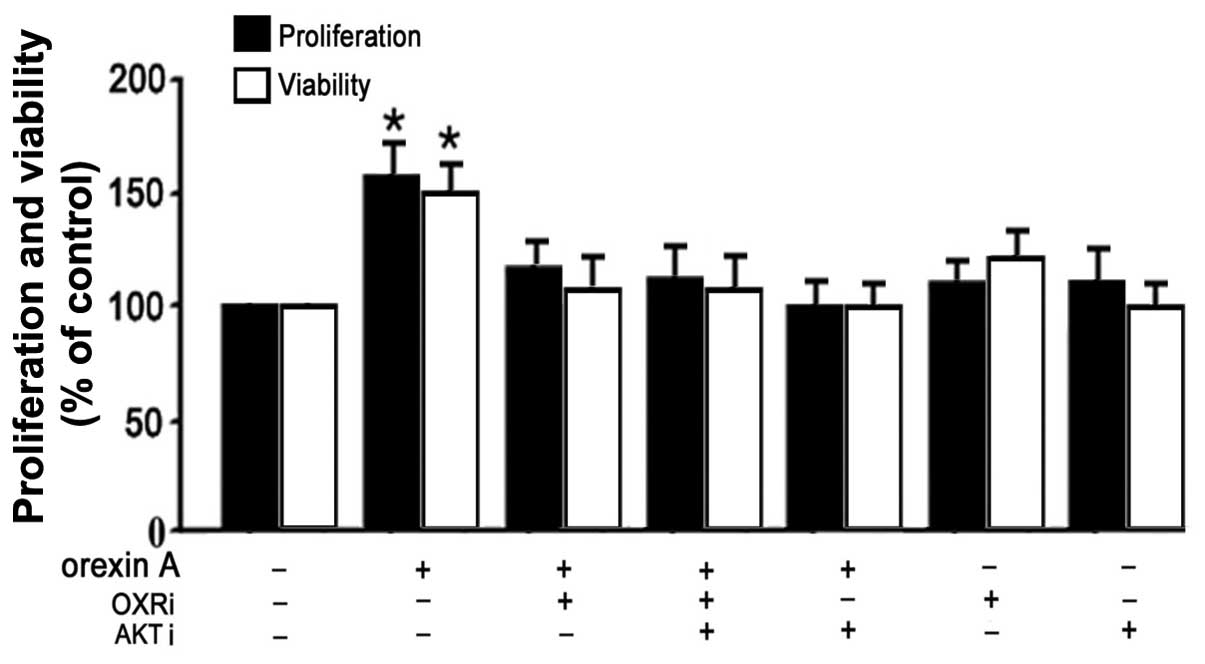
viability of BGC-823 cells through the AKT signaling pathway
In order to confirm the involvement of the AKT
signaling pathway in orexin A-mediated proliferation and viability
in BGC-823 cells, cell survival rates were determined using BrdU
and MTT analysis. 10−8 M orexin A significantly promoted
the proliferation and viability of BGC-823 cells (P<0.05)
(Fig. 5). However, these effects
were blocked with AKT antagonist (PF-04691502), OX1R antagonist
(SB334867) or their combination (Fig.
5). Moreover, the proliferation and viability were not changed
significantly when treated with AKT antagonist or OX1R antagonist
in the absence of orexin A co-treatment (Fig. 5). The data suggested that AKT
participated in orexin A-induced stimulation of proliferation and
viability of BGC-823 cells.
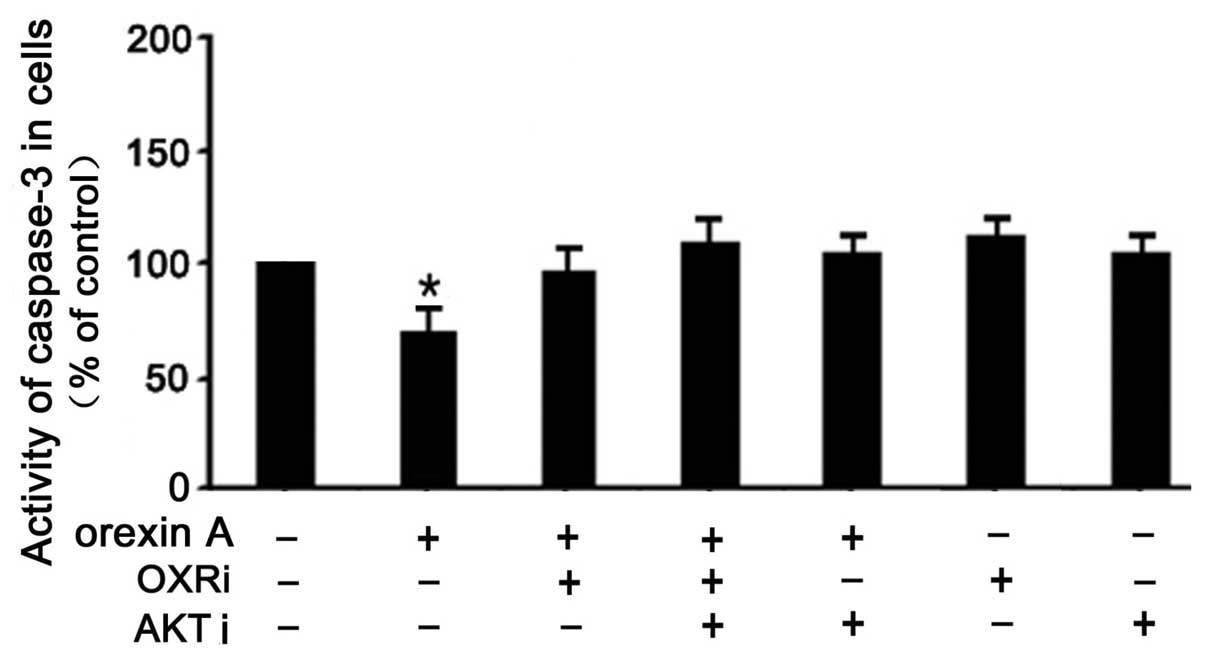
Effects of orexin A on caspase-3
activation in BGC-823 cells
To determine whether the activation of the caspase
pathway was affected by orexin A, leading to the protection of
BGC-823 cells from apoptosis, caspase-3 activity was measured.
Treatment of BGC-823 cells with 10−8 M orexin A
significantly decreased caspase-3 activity (30% below that of the
control) (Fig. 6). This effect was
reversed in the presence of PF-04691502 (10−6 M),
SB334867 (10−6 M) and a combination of the inhibitors
(Fig. 6). These results indicated
that apoptosis induced by orexin A was mediated, at least in part,
through caspase-3.
Discussion
The present study demonstrated, for the first time,
to the best of our knowledege, that OX1R was expressed at mRNA and
protein levels in BGC-823 gastric cancer cells. In order to explore
the potential role of orexins in BGC-823 gastric cancer cells, the
effects of orexin A on BGC-823 cell proliferation and apoptosis
were examined. Orexin A stimulated the proliferation and viability
of BGC-823 gastric cancer cells and protected them from apoptosis
via the AKT signaling pathway.
Evidence suggested that the effects of orexin A on
proliferation and apoptosis may vary dependent on the type of
cancer cells (10–13). For example, orexin A suppressed
cell growth by inducing apoptosis in human colon cancer,
neuroblastoma cells and rat pancreatic tumor cells (10,11).
However, orexin A has also been reported to stimulate cell
proliferation in adrenal gland tumor cells; the effects were more
marked in cultured adenomatous than those in normal adrenocortical
cells (12). In addition, orexin A
had no effect on proliferation of rat C6 glioma cells, as assessed
using a (3H) thymidine incorporation assay (13). PI3K/PKB activators stimulated cell
proliferation and viability in 3T3-L1 cells (24,25).
However, a more recent study showed that PI3K/PKB was not essential
for orexin A-induced stimulation of proliferation and viability in
3T3-L1 preadipocytes (26). The
mechanisms by which orexin A has opposing effects on apoptosis in
different cancer cell types remains to be elucidated. One possible
explanation may be that cells have different intrinsic
sensitivities to cytochrome c. The differential sensitivity
to cytochrome c may be due to high levels of apoptotic protease
activating factor 1 (Apaf-1) in tumor tissues, such as
neuroblastoma, in comparison with low levels of Apaf-1 in the
adjacent brain tissue (27).
Cytochrome c, once released from the mitochondria, binds to Apaf-1,
leading to the formation of the apoptosome and the recruitment of
procaspase-9. Activated caspase-9 activates caspase-3 and
caspase-7, thereby promoting cell death (28). Another possible explanation of what
determines the influence of orexins on cell apoptosis may be the
activation of mitogen-activated protein kinase (MAPK) signaling
pathways. Studies have reported the expression of stable OX1R in
human embryonic kidney-293 and Chinese hamster ovary cells. These
studies have shown that orexins can exert converse effects on cell
apoptosis through activation of the classical MAPK signaling
pathways (29,30). The extracellular signal-regulated
kinase 1/2 pathway was shown to protect against apoptosis, whereas
p38 was a key promoter of cell death (29,30).
In addition, it has also been reported that activation of OX1R
resulted in mobilization of intracellular calcium through a
Gq-dependent mechanism (31).
Although increases of cytosolic calcium are well known to occur
during cell apoptosis, this does not provide sufficient evidence to
explain the proapoptotic effect of orexins (32). Numerous GPCRs in human colon cancer
cells are known to promote intracellular Ca2+
mobilization (33–35). These receptors include NT1
receptors for neurotensin (33),
protease-activated receptors 1 for thrombin (34), protease-activated receptors 2 for
trypsin (35) or muscarinic M3
receptors for acetylcholine (36).
These receptors do not trigger apoptosis but conversely stimulate
cell proliferation.
In the present study, orexin A, at all tested
concentrations, significantly promoted the proliferation and
viability of BGC-823 gastric cancer cells. In addition, the effects
of orexin A on proliferation and apoptosis in BGC-823 gastric
cancer cells were blocked by AKT-specific inhibitors. This
suggested the involvement of the activated PI3K/AKT signaling
pathway in gastric cell proliferation and apoptosis induced by
orexin A. The PI3K pathway has an important role in cell growth,
proliferation, survival and apoptosis. Abnormal cell signaling via
this pathway occurs in diverse types of cancer (37,38).
PI3K is activated by both receptor tyrosine kinases and Ras, and in
turn activates multiple downstream signaling pathways. The AKT
family, a multifunctional serine-threonine protein kinase, is a
major downstream signaling molecule of PI3K and a critical target
of PI3K in human cancer. PI3K/AKT signaling has been shown to be
activated in various types of cancer. Activated AKT phosphorylates
Bad and caspase-9 and the activated nuclear factor
kappa-light-chain-enhancer of activated B cells pathway may promote
resistance to apoptosis in cancer cells (39–43).
In the present study, it was demonstrated that BGC-823 gastric
cancer cells treated with orexin A had lower rates of apoptosis
than the control treated cells. Orexin A treatment caused a
significant decrease in caspase-3 activity in BGC-823 cells.
Caspase-3 is a key molecule involved in the execution of apoptosis
and acts downstream in the apoptotic cascade (44). Although not documented in the
present study, other caspase pathways can be studied in the future.
It is necessary to investigate whether caspases are involved in the
extrinsic (receptor-mediated) pathway of apoptosis. Furthermore, by
employing the AKT-specific inhibitor PF-04691502, the present study
demonstrated that orexin A inhibited apoptosis and regulated
apoptosis-associated proteins in BGC-823 gastric cancer cells via
AKT signaling pathways. Overall, the results of the present study
suggested that orexin A promoted the activation of the PI3K/AKT
pathway to inhibit the apoptosis of gastric cancer cells. However,
further studies are required to clarify the mechanism by which
orexin A modulates activation of PI3K/AKT and other crucial
signaling pathways for cancer cell survival and
chemoresistance.
In conclusion, the present study provided the first
evidence for the presence of orexin receptors in BGC-823 gastric
cancer cells. Furthermore, the study showed that orexin A regulated
BGC-823 gastric cancer cell proliferation and survival, reduced
pro-apoptotic caspase-3 activity, and protected against apoptotic
death via OX1R through the AKT signaling pathway.
Acknowledgements
The authors would like to thank The China Medical
University Affiliated Hospital Laboratory Center for kindly
providing the equipment. This study was supported by the National
Natural Science Foundation of China (grant nos. 30872724, 81071460
and 81271996) and the Natural Science Foundation of Liaoning
Province (grant no. 201202292).
References
|
1
|
Sakurai T, Amemiya A, Ishii M, Matsuzaki
I, Chemelli RM, Tanaka H, Williams SC, Richardson JA, Kozlowski GP,
Wilson S, et al: Orexins and orexin receptors: a family of
hypothalamic neuropeptides and G protein-coupled receptors that
regulate feeding behavior. Cell. 92:573–585. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kukkonen JP, Holmqvist T, Ammoun S and
Akerman KE: Functions of the orexinergic/hypocretinergic system. Am
J Physiol Cell Physiol. 283:C1567–C1591. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
de Lecea L, Kilduff TS, Peyron C, Gao X,
Foye PE, Danielson PE, Fukuhara C, Battenberg EL, Gautvik VT,
Bartlett FS II, et al: The hypocretins: hypothalamus-specific
peptides with neuroexcitatory activity. Proc Natl Acad Sci USA.
95:322–327. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Matsuki T and Sakurai T: Orexins and
orexin receptors: from molecules to integrative physiology. Results
Probl Cell Differ. 46:27–55. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Gestreau C, Bévengut M and Dutschmann M:
The dual role of the orexin/hypocretin system in modulating
wakefulness and respiratory drive. Curr Opin Pulm Med. 14:512–518.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Aston-Jones G, Smith RJ, Sartor GC,
Moorman DE, Massi L, Tahsili-Fahadan P and Richardson KA: Lateral
hypothalamic orexin/hypocretin neurons: a role in reward-seeking
and addiction. Brain Res. 1314:74–90. 2010. View Article : Google Scholar
|
|
7
|
Kodadek T and Cai D: Chemistry and biology
of orexin signaling. Mol Biosyst. 6:1366–1375. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Nambu T, Sakurai T, Mizukami K, Hosoya Y,
Yanagisawa M and Goto K: Distribution of orexin neurons in the
adult rat brain. Brain Res. 827:243–260. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Voisin T, Rouet-Benzineb P, Reuter N and
Laburthe M: Orexins and their receptors: structural aspects and
role in peripheral tissues. Cell Mol Life Sci. 60:72–87. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Rouet-Benzineb P, Rouyer-Fessard C, Jarry
A, Avondo V, Pouzet C, Yanagisawa M, Laboisse C, Laburthe M and
Voisin T: Orexins acting at native OX(1) receptor in colon cancer
and neuroblastoma cells or at recombinant OX(1) receptor suppress
cell growth by inducing apoptosis. J Biol Chem. 279:45875–45886.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Voisin T, Firar AE, Avondo V and Laburthe
M: Orexin-induced apoptosis: the key role of the
seven-transmembrane domain orexin type 2 receptor. Endocrinology.
147:4977–4984. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Spinazzi R, Rucinski M, Neri G,
Malendowicz LK and Nussdorfer GG: Prepro Orexin and orexin
receptors are expressed in cortisol-secreting adrenocortical
adenomas, and orexins stimulate in vitro cortisol secretion and
growth of tumor cells. J Clin Endocrinol Metab. 90:3544–3549. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Biegańska K, Sokołowska P, Jöhren O and
Zawilska JB: Orexin A suppresses the growth of rat C6 glioma cells
via a caspase-dependent mechanism. J Mol Neurosci. 48:706–712.
2012. View Article : Google Scholar
|
|
14
|
Martin JL and Baxter RC: Expression of
insulin-like growth factor binding protein-2 by MCF-7 breast cancer
cells is regulated through the phosphatidylinositol
3-kinase/AKT/mammalian target of rapamycin pathway. Endocrinology.
148:2532–2541. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Chow S, Minden MD and Hedley DW:
Constitutive phosphorylation of the S6 ribosomal protein via mTOR
and ERK signaling in the peripheral blasts of acute leukemia
patients. Exp Hematol. 34:1183–1191. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Bessard A, Frémin C, Ezan F, Coutant A and
Baffet G: MEK/ERK-dependent uPAR expression is required for
motility via phosphorylation of P70S6K in human hepatocarcinoma
cells. J Cell Physiol. 212:526–536. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Papadimitrakopoulou V and Adjei AA: The
Akt/mTOR and mitogen-activated protein kinase pathways in lung
cancer therapy. J Thorac Oncol. 1:749–751. 2006. View Article : Google Scholar
|
|
18
|
Chan SM, Weng AP, Tibshirani R, Aster JC
and Utz PJ: Notch signals positively regulate activity of the mTOR
pathway in T-cell acute lymphoblastic leukemia. Blood. 110:278–286.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Han Z, Wu K, Shen H, Li C, Han S, Hong L,
Shi Y, Liu N, Guo C, Xue Y, et al: Akt1/protein kinase B alpha is
involved in gastric cancer progression and cell proliferation. Dig
Dis Sci. 53:1801–1810. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Downward J: Mechanisms and consequences of
activation of protein kinase B/Akt. Curr Opin Cell Biol.
10:262–267. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kim JH, Go HY, Jin DH, Kim HP, Hong MH,
Chung WY, Park JH, Jang JB, Jung H, Shin YC, et al: Inhibition of
the PI3K-Akt/PKB survival pathway enhanced an ethanol extract of
Rhus verniciflua Stokes-induced apoptosis via a mitochondrial
pathway in AGS gastric cancer cell lines. Cancer Lett. 265:197–205.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Liu M, Li CM, Chen ZF, Ji R, Guo QH, Li Q,
Zhang HL and Zhou YN: Celecoxib regulates apoptosis and autophagy
via the PI3K/Akt signaling pathway in SGC-7901 gastric cancer
cells. Int J Mol Med. 33:1451–14588. 2014.PubMed/NCBI
|
|
23
|
Walczak K, Turski WA and Rajtar G:
Kynurenic acid inhibits colon cancer proliferation in vitro:
effects on signaling pathways. Amino Acids. 46:2393–2401. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kim MS, Yoon CY, Jang PG, Park YJ, Shin
CS, Park HS, Ryu JW, Pak YK, Park JY, Lee KU, et al: The mitogenic
and antiapoptotic actions of ghrelin in 3T3-L1 adipocytes. Mol
Endocrinol. 18:2291–2301. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Gagnon A, Dods P, Roustan-Delatour N, Chen
CS and Sorisky A: Phosphatidylinositol-3,4,5-trisphosphate is
required for insulin-like growth factor 1-mediated survival of
3T3-L1 preadipocytes. Endocrinology. 142:205–212. 2001.PubMed/NCBI
|
|
26
|
Skrzypski M, Kaczmarek P, Le TT,
Wojciechowicz T, Pruszyńska-Oszmalek E, Szczepankiewicz D, Sassek
M, Arafat A, Wiedenmann B, Nowak KW and Strowski MZ: Effects of
orexin A on proliferation, survival, apoptosis and differentiation
of 3T3-L1 preadipocytes into mature adipocytes. FEBS Lett.
586:4157–4164. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Johnson CE, Huang YY, Parrish AB, Smith
MI, Vaughn AE, Zhang Q, Wright KM, Van Dyke T, Wechsler-Reya RJ,
Kornbluth S and Deshmukh M: Differential Apaf-1 levels allow
cytochrome c to induce apoptosis in brain tumors but not in normal
neural tissues. Proc Natl Acad Sci USA. 104:20820–20825. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Danial NN and Korsmeyer SJ: Cell death:
critical control points. Cell. 116:205–219. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Ammoun S, Lindholm D, Woolz H, Akerman KE
and Kukkonen JP: G-protein-coupled OX1 orexin/hcrtr-1 hypocretin
receptors induce caspase-dependent and -independent cell death
through p38-/stress-activated protein kinase. J Biol Chem.
281:834–842. 2006. View Article : Google Scholar
|
|
30
|
Tang J, Chen J, Ramanjaneya M, Punn A,
Conner AC and Randeva HS: The signalling profile of recombinant
human orexin-2 receptor. Cell Signal. 20:1651–1661. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Darker JG, Porter RA, Eggleston DS, Smart
D, Brough SJ, Sabido-David C and Jerman JC: Structure-activity
analysis of truncated orexin-A analogues at the orexin-1 receptor.
Bioorg Med Chem Lett. 11:737–740. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Rizzuto R, Pinton P, Ferrari D, Chami M,
Szabadkai G, Magalhães PJ, Di Virgilio F and Pozzan T: Calcium and
apoptosis: facts and hypotheses. Oncogene. 22:8619–8627. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Maoret JJ, Anini Y, Rouyer-Fessard C,
Gully D and Laburthe M: Neurotensin and a non-peptide neurotensin
receptor antagonist control human colon cancer cell growth in cell
culture and in cells xenografted into nude mice. Int J Cancer.
80:448–454. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Darmoul D, Gratio V, Devaud H, Lehy T and
Laburthe M: Aberrant expression and activation of the thrombin
receptor protease-activated receptor-1 induces cell proliferation
and motility in human colon cancer cells. Am J Pathol.
162:1503–1513. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Darmoul D, Gratio V, Devaud H and Laburthe
M: Protease-activated receptor 2 in colon cancer: trypsin-induced
MAPK phosphorylation and cell proliferation are mediated by
epidermal growth factor receptor transactivation. J Biol Chem.
279:20927–20934. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Keely SJ, Uribe JM and Barrett KE:
Carbachol stimulates transactivation of epidermal growth factor
receptor and mitogen-activated protein kinase in T84 cells.
Implications for carbachol-stimulated chloride secretion. J Biol
Chem. 273:27111–27117. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Cantley LC: The phosphoinositide 3-kinase
pathway. Science. 296:1655–1657. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Shaw RJ and Cantley LC: Ras, PI(3)K and
mTOR signalling controls tumour cell growth. Nature. 441:424–430.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Li Y, Liu J, Liu X, Xing K, Wang Y, Li F
and Yao L: Resveratrol-induced cell inhibition of growth and
apoptosis in MCF7 human breast cancer cells are associated with
modulation of phosphorylated Akt and caspase-9. Appl Biochem
Biotechnol. 135:181–192. 2006. View Article : Google Scholar
|
|
40
|
Zhang G, Li M, Zhu X, Bai Y and Yang C:
Knockdown of akt sensitizes osteosarcoma cells to apoptosis induced
by Cisplatin treatment. Int J Mol Sci. 12:2994–3005. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Song L, Xiong H, Li J, Liao W, Wang L, Wu
J and Li M: Sphingosine kinase-1 enhances resistance to apoptosis
through activation of PI3K/Akt/NF-kappaB pathway in human non-small
cell lung cancer. Clin Cancer Res. 17:1839–1849. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Zhu Z, Sun H, Ma G, Wang Z, Li E and Liu Y
and Liu Y: Bufalin induces lung cancer cell apoptosis via the
inhibition of pi3k/akt pathway. Int J Mol Sci. 13:2025–2035. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Liu Q, Dong HW, Sun WG, Liu M, Ibla JC,
Liu LX, Parry JW, Han XH, Li MS and Liu JR: Apoptosis initiation of
β-ionone in SGC-7901 gastric carcinoma cancer cells via a PI3K-AKT
pathway. Arch Toxicol. 87:481–490. 2013. View Article : Google Scholar
|
|
44
|
Creagh EM, Conroy H and Martin SJ:
Caspase-activation pathways in apoptosis and immunity. Immunol Rev.
193:10–21. 2003. View Article : Google Scholar : PubMed/NCBI
|