Introduction
Acute myeloid leukemia (AML) is a common
hematological malignancy, which is characterized by the arrest of
myeloid cells at various differentiation stages, resulting in their
accumulation as they are unable to differentiate into mature,
functional blood cells (1,2). Numerous genetic abnormalities have
been identified in AML. Among these, FMS-like tyrosine kinase 3
(FLT3) abnormality is common and a previous study has
demonstrated that FLT3 was abnormally activated in 70–90% of
patients with AML, including overexpression of wild-type
FLT3 and FLT3 mutations (3).
AML patients with FLT3 abnormalities present
unfavorable prognoses (4),
including a high risk of relapse and lower long-term survival rates
compared with patients with wild-type FLT3 (5,6). The
use of cytarabine- and anthracycline-based intensive chemotherapy,
in combination with advanced supportive care and the introduction
of allogeneic stem cell transplantation has been demonstrated to
initially improve the outcome of patient responses (7,8).
However, these responses were found to be transient, lasting for
weeks to months, followed by progressive disease development and
subsequent drug-resistance (9).
The median survival of FLT3-mutated patients with AML has
been reported to be ≤5 months, following the first disease relapse
(10,11). To date, multiple small molecule
FLT3-tyrosine kinase inhibitors (FLT3-TKIs) have been developed,
and their effect in AML patients as single agents or in combination
with chemotherapy have been evaluated (12). Protein kinase C (PKC)412
(N-benzoyl-staurosporine) is a FLT3-TKI that was originally
developed as a PKC and vascular growth factor receptor inhibitor.
PKC412 has been used in phase I clinical trials for the treatment
of solid tumors, which demonstrated a tolerable dose (13,14).
Subsequently, PKC412 was found to specifically and potently inhibit
the growth of leukemic cell lines expressing FLT3-internal
tandem duplication (ITD)-induced-myeloproliferative-like syndrome
in the nanomolar range (15). The
results of phase II and IIB trials, where patients with FLT3
mutations were treated with PKC412, indicated that PKC412 was
generally well-tolerated. These results suggested a potentially
effective strategy comprising a combination of PKC412 and other
agents, including chemotherapy, in AML patients with
FLT3-mutations (16,17).
Individuals with a subtype of AML known as acute
promyelocytic leukaemia (APL) are frequently treated with all-trans
retinoic acid (ATRA). ATRA is a derivative of vitamin A
(retinoids), also known as tretinoin (Vesanoid®). ATRA
induces leukemia cell maturation and differentiation, and is
therefore able to rapidly reduce leukemia symptoms by preventing
myeloid cell accumulation (18).
In addition, ATRA has been demonstrated to induce cell growth
arrest, differentiation and cell death of various types of cancer
cells in vitro (19,20).
However, the clinical applications of ATRA are limited by
side-effects, which include acute retinoid resistance,
hypertriglyceridemia, mucocutaneous dryness, headaches, disease
relapse following a brief remission period and drug resistance
(21–23). In addition, its clinical
applications are further limited due to its low plasma
concentrations. Therefore, in order to circumvent these
limitations, combinations of ATRA and other anticancer drugs have
been investigated (24,25). Previous studies have indicated that
ATRA was able to enhance the cytotoxic effect of chemotherapeutic
drugs (26,27). Furthermore, certain preclinical
trials have demonstrated the efficacy of using a combination of
retinoids and cytotoxic drugs (27–29).
In the present study, an in vitro
investigation was performed to evaluate the effect of the
combination of ATRA and PKC412 in FLT3-mutated cell lines.
The results of the present study may establish whether a clinical
trial on patients with FLT3 mutations should be
conducted.
Materials and methods
Cell lines and culture conditions
Four human leukemia cell lines were used in this
study and were obtained as described previously (30). Briefly, the two sister cell lines,
MOLM13 and MOLM14, were obtained from a patient with acute
monocytic leukemia (M5a) presenting the t(9;11) mutation (31), MV4-11 cell line was acquired from
an AML patient carrying the t(4;11) mutation (32) and KOCL-48 cell line was obtained
from an infant leukemic patient carrying the t(4;11) mutation
(33).
Two mutations within FLT3 exon 14 were
detected in MOLM13 and MOLM14 cells, including an ITD (21 bp)
corresponding to codons Phe594-Asp600 and a novel missense
nucleotide substitution at codon 599 (Tyr599Phe) (34,35).
These mutations were located on the same allele (35). In MV4-11 cells, an ITD (30 bp)
within FLT3 exon 14, corresponding to codons Tyr591-Asp600,
and a Tyr591His mutation were detected (34,35).
In the KOCL-48 cell line, only the FLT3-Asp835Glu mutation
was detected (34).
All the cell lines were cultured in RPMI-1640 medium
(Sigma-Aldrich Japan K.K., Tokyo, Japan) supplemented with 10%
heat-inactivated fetal bovine serum (FBS; JRH Biosciences, Lenexa,
KS, USA), 100 IU/ml penicillin and 0.1 mg/ml streptomycin (Nakalai
Tesque, Kyoto, Japan) in a humidified atmosphere containing 5%
CO2 at 37°C.
Reagents
PKC412 was provided by Novartis Pharma AG (Basel,
Switzerland) and ATRA was purchased from Wako Pure Chemical
Industries, Ltd. (Osaka, Japan). All the drugs were dissolved in
dimethylsulfoxide (DMSO; Merck-Millipore, Darmstadt,
Germany). Control cells were cultured with the same DMSO
concentration as that used for the highest drug dose (1:1000
dilution). To avoid cytotoxicity, the concentration of DMSO was
maintained at <0.1%.
Cell proliferation assays
Cell proliferation was determined using the trypan
blue dye exclusion test as described previously (30). Briefly, the cells were seeded in
six-well plates (1×105 cells/ml) in the presence of
various ATRA or PKC412 concentrations for 72 h. Following
treatment, 10 μl cell suspension was mixed with 10 μl 0.4% trypan
blue (Nacalai Tesque, Tokyo, Japan), and live cells were counted
manually using a hemacytometer (Erma, Tokyo, Japan). The results
were expressed as the percentage of the values obtained when the
cells were grown in the absence of reagents.
Morphological assessment for the
detection of apoptotic cells
In order to detect fragmented nuclei and condensed
chromatin, the cells (1×105 cells/ml) were treated with
1 μM ATRA. Following incubation for 8 h, the cells were harvested
and fixed onto slides using a Cytospin 2 (Shandon Southern Products
Ltd., Cheshire, UK). Subsequently, the cells were stained with a
Wright-Giemsa solution (Merck Co., Ltd, Tokyo, Japan) and the cell
morphology was observed under an inverted microscope (IX70; Olympus
Corp. Tokyo, Japan).
Western blot analysis
Cells were plated onto 10-cm dishes at a density of
1×105 cells/ml in the presence of 1 μM ATRA. Following
incubation for the indicated time periods, the cells were collected
and washed twice with PBS. Next, the cells were dissolved in
protein lysis buffer (Wako Pure Chemical Industries, Ltd),
consisting of 5 mM EDTA, 50 mM NaF, 10 mM
Na2H2P2O7, 0.01% Triton
X-100, 5 mM HEPES, 150 mM NaCl, 1 mM Na3VO4,
1 mM phenylmethylsulfonyl fluoride and 75 μg/ml aprotinin, on ice
for 30 min with a brief vortex of four times every 10 min. The
samples were centrifuged at 16,000 × g at 4°C for 10 min and the
total cell lysates were collected for western blot analysis.
Subsequently, the protein samples were subjected to 12%
polyacrylamide gel electrophoresis and transferred to Hypond-P
membranes (GE Healthcare Life Sciences, Little Chalfont, UK) using
electroblotting. Following washing with PBS, the membranes were
incubated with various antibodies and antibody-binding was detected
using enhanced chemiluminescence system (Amersham Pharmacia
Biotech, Tokyo, Japan). Rabbit polyclonal anti-actin (A2066; 1:500
in 5% skimmed milk) was obtained from Sigma-Aldrich, rabbit
polyclonal anti-caspase-3 (#9662; 1:1,000 in 5% BSA) and mouse
anti-caspase-9 (#9508 1:1,000 in 5% BSA) antibodies were purchased
from Cell Signaling Technology (Tokyo, Japan) and
rabbit-anti-poly-adenosine diphosphate ribose polymerase (PARP)
antibody (#9542; 1:1,000 in 5% BSA) was purchased from Wako Pure
Chemical Industries, Ltd. Secondary antibodies horseradish
peroxidase (HRP)-conjugated anti-rabbit immunoglobulin (Ig)G
(sc-2317) and anti-mouse IgG-HRP (sc-2031) were purchased from
Santa Cruz Biotechnology, Inc. (Dallas, TX, USA)
Isobologram analysis
The isobologram method described by Steel and
Peckham (36) was used to evaluate
the dose-response interactions between ATRA and PKC412 in the
MOLM13, MOLM14, MV4-11 and KOCL-48 cells at the IC50
level. IC50 was defined as the concentration of the
reagent that produced 50% cell-growth inhibition. The isobologram
method used in this study was selected since it can be used to
investigate anticancer agents with unclear cytotoxic mechanisms and
various dose-response curves (36).
Statistical analysis
Data obtained from the isobolograms were analyzed as
described previously (37). The
drug combinations were considered to have a synergistic effect when
the majority of the observed data points were found in the area of
supra-additivity (mean value of observed data less than predicted
minimum values). By contrast, an antagonistic effect was considered
between the drug combinations when the majority of data points were
located in the areas of subadditivity and protection (mean value of
observed data more than predicted maximum value). Statistical
analyses were performed to analyze the significance of the detected
synergism (or antagonism). The Wilcoxon signed-rank test was used
to compare the observed data with the predicted minimum or maximum
values for additive effects, which were closest to the observed
data. Values are expressed as the mean ± standard deviation of
three independent experiments. P<0.05 was considered to indicate
a statistically significant difference. P≥0.05 was considered to
indicate additive to synergistic (or additive to antagonistic)
effect. Additional data were analyzed using Student’s t-test.
Statistical analyses were performed using StatView 4.01 software
program (Abacus Concepts, Berkeley, CA, USA).
Results
ATRA and PKC412 inhibit proliferation of
FLT3-mutated AML cells
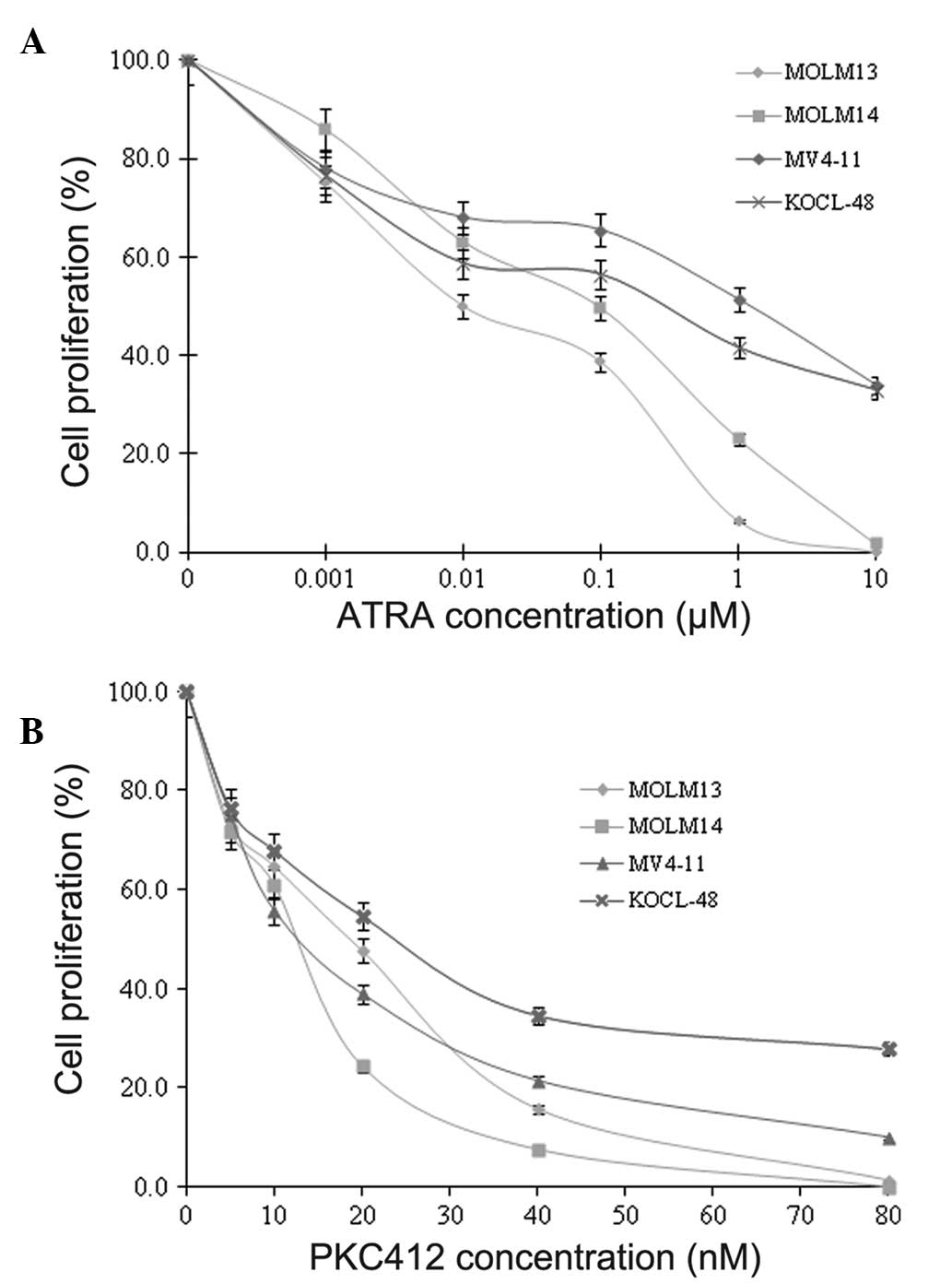
In order to evaluate the effect of ATRA and PKC412
on cell growth, the cell lines (MOLM13, MOLM14, MV4-11 and KOCL-48)
were incubated with DMSO alone (0 μM reagents), with various
concentrations of ATRA (0.001–10 μM) or with various concentrations
of PKC412 (5–80 nM) for 72 h. Cell proliferation was evaluated
using the trypan blue exclusion test. The results indicated that
ATRA and PKC412 inhibited the cellular proliferation of MOLM13,
MOLM14, MV4-11 and KOCL-48 cells in a dose-dependent manner
(Fig. 1).
ATRA induces apoptosis in FLT3-mutated
AML cells
Previous studies demonstrated that ATRA was able to
induce the differentiation of immature leukemic blasts into
terminally differentiated granulocytic cells (38,39),
or the apoptosis of specific tumor cells (340,41). To elucidate the
mechanism underlying the ATRA-induced suppression of cellular
proliferation in MOLM13, MOLM14, MV4-11 and KOCL-48 cells, the cell
morphology and expression levels of apoptotic markers in these
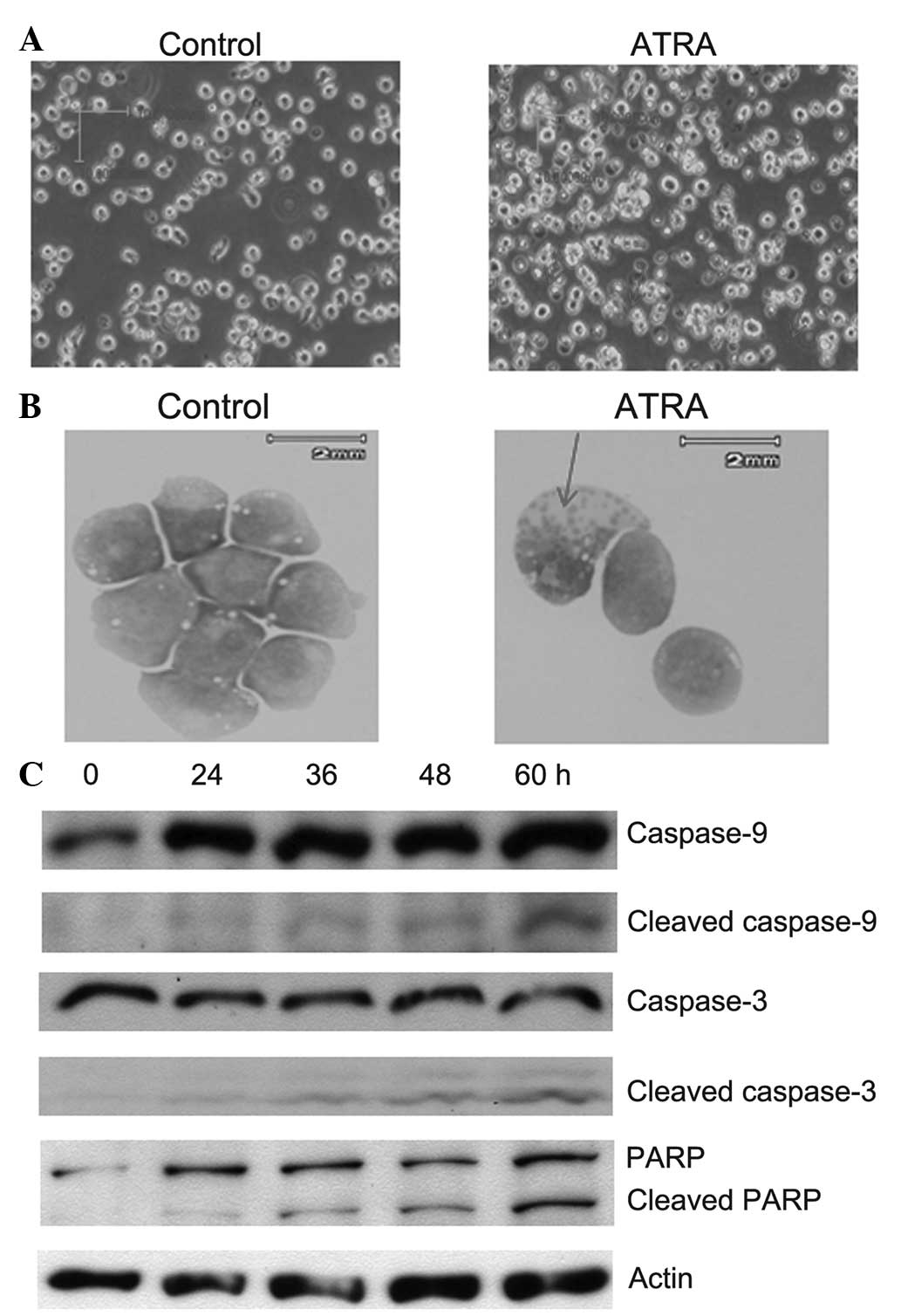
cells following treatment with 1 μM ATRA were evaluated. After 8 h
of treatment with 1 μM ATRA, higher apoptosis was observed in the
cell membranes of MOLM13 cells compared with the non-treated cells
(0-h treatment; Fig. 2A and B).
Furthermore, bands of cleaved caspase-9 were detected after 8 h of
incubation with 1 μM ATRA. Cleaved caspase-9 induced the activation
of caspase 3 (indicated by the presence of the cleaved caspase-3
band), which subsequently inactived an enzymes involved in DNA
repair, known as PARP (Fig. 2C).
The caspase 3-mediated proteolytic cleavage of PARP is a key event
in apoptosis. In addition, apoptotic bodies were also observed
after 24 h of treatment with 1 μM ATRA in MOLM13 cells (Fig. 2B), which indicated that ATRA
induced apoptosis in cell lines exhibiting FLT3 mutations.
Analogous results were observed following evaluation of apoptosis
in the remainder cell lines (data not shown).
Synergistic effect of combined ATRA and
PKC412 treatment
ATRA was demonstrated to suppress cellular
proliferation by inducing apoptosis, whereas PKC412 is known to be
a specific FLT3-inhibitor that suppresses cell proliferation
by inhibiting FLT3 expression in FLT3-mutated cell
lines (15). The present study
aimed to elucidate whether a combination of ATRA and PKC412 was
able to enhance the effect of these drugs on the suppression of
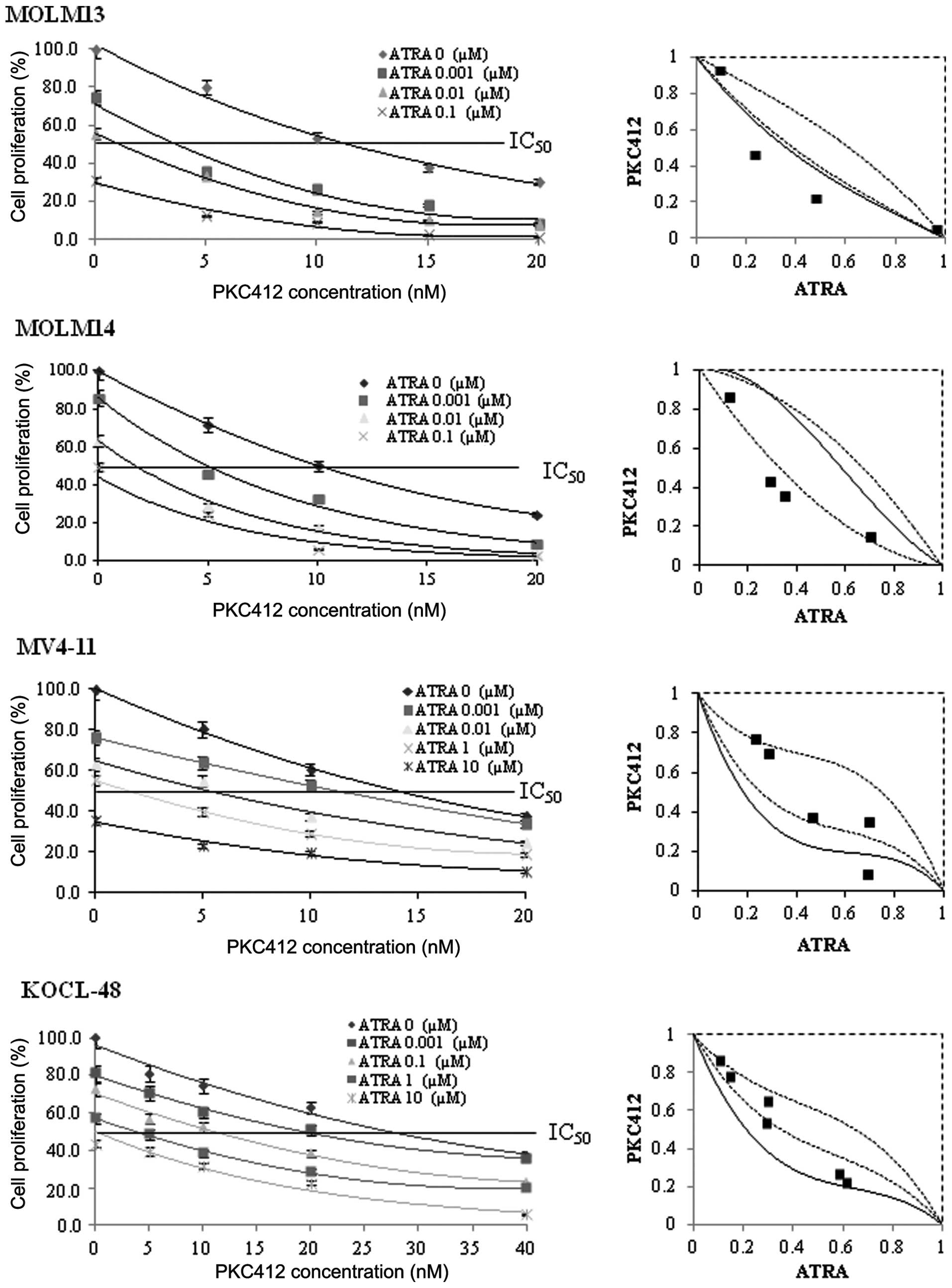
cell proliferation in FLT3-mutated cell lines. The results
revealed that combined treatment with ATRA and PKC412 had a
synergistic effect on MOLM13 and MOLM14 cells and an additive
effect on KOCL-48 and MV4-11 cells (Fig. 3). In the MOLM13 and MOLM14 cells,
the combined data points were detected in the areas of
supra-additivity and additivity. The mean values obtained were
lower compared with the predicted minimum values (Table I), and the differences were found
to be significant (P<0.01), indicating synergistic effects.
However, in the KOCL-48 and MV4-11 cells, the combined data points
were detected within the envelope of additivity, indicating an
additive effect. In addition, the mean values detected for the
KOCL-48 and MV4-11 cells were lower compared with the predicted
maximum valies and higher than the predicted minimum values
(Table I), confirming the presence
of additive effects.
 | Table IMean values of observed data and
predicted minimum and maximum values of the combination of
all-trans retinoic acid and protein kinase C 412. |
Table I
Mean values of observed data and
predicted minimum and maximum values of the combination of
all-trans retinoic acid and protein kinase C 412.
| | | Predicted values
for an additive effect | |
|---|
| | |
| |
|---|
| Cell line | n | Observed data | Minimum | Maximum | Effect |
|---|
| MOLM13 | 4 | 0.150 | 0.771 | 0.895 | Synergistic
(<0.01) |
| MOLM14 | 4 | 0.231 | 0.265 | 0.584 | Synergistic
(<0.01) |
| MV4-11 | 5 | 0.442 | 0.245 | 0.653 | Additive |
| KOCL-48 | 6 | 0.432 | 0.335 | 0.632 | Additive |
Discussion
Despite the positive response to certain therapeutic
strategies, the decreased ability of cancer cells to undergo
apoptosis by malignant evolution represents a major challenge in
the development of effective therapeutic approaches (19). Currently, novel selective
strategies aiming to manipulate cancer cells, but not healthy
cells, towards apoptosis are under development as potential
therapies (42). Therefore,
apoptosis-inducing agents that do not induce cytotoxicity in normal
cells represent a potential anticancer treatment. The results of
the present study demonstrated that ATRA inhibited cell
proliferation and induced apoptosis in FLT3-mutated cell
lines, indicating that ATRA may be a potentially useful drug for
the treatment of AML patients with FLT3 mutations.
ATRA has been demonstrated to inhibit vascular
endothelial growth factor, which is crucial for the process of
angiogenesis (29), and represents
a major development in the treatment of APL with differentiation
therapy. However, the duration of remission induced and maintained
by ATRA therapy alone has been found to be short-lived, and ATRA
alone failed to induce a second remission in the majority of
patients following relapse (43,44).
To circumvent these limitations, improving the effectiveness of
ATRA on first drug application is required. Notably, AML results
from by a combination of at least two pathophysiological problems.
Therefore, the application of a therapy targeting only one
pathophysiological pathway may not be sufficient to induce a major
response, unless using a therapeutic combination. Furthermore,
anticancer drugs may induce severe cytotoxic side-effects, which
limit the doses that can be administered during treatment, thereby
limiting the potential effectiveness of these therapeutic
approaches (45,46). The use of differential combinations
of anticancer drugs may circumvent these limitations by improving
the effectiveness of cancer chemotherapy (45–47).
The majority of anticancer drugs have distinct mechanisms of action
and are associated with specific cytotoxic side-effects. In
addition, an upper concentration limit exists for each drug in
order to achieve effective inhibition of tumor-cell proliferation,
whilst minimizing the extent of damage to healthy cells. Therefore,
an ideal anticancer drug combination should maximize the
therapeutic efficacy and minimize the associated cytotoxic
side-effects (48–50).
Based on the aforementioned principals, the present
study aimed to evaluate the effect of a combination of ATRA and
PKC412 on FLT3-mutated AML cell lines. Preliminary
preclinical data obtained in the present study demonstrated that
the combination of ATRA and PKC412 had a synergistic/additive
cytotoxic effect on FLT3-mutated cell lines as compared to
each agent alone. For instance, the IC50 of ATRA alone
in MOLM13 cells was found to be 0.01 μM; however, upon combined
treatment with PKC412 (3.5 nM), the IC50 concentration
of ATRA was significantly reduced to 0.001 μM (P<0.01).
Similarly, the IC50 of PKC412 alone in MOLM13 cells was
found to be 20 nM; however, upon combined treatment with ATRA
(0.001 μM), the IC50 concentration of PKC412 was reduced
to 3.5 nM (P<0.01).
A previous study indicated that the side-effects
associated with PKC412 treatment were associated with the dosage
administered (14). Therefore, in
the treatment combination, ATRA and PKC412 may maximize the
therapeutic efficacy and minimize cytotoxic side-effects. In
conclusion, the results of the present study provided experimental
evidence for the effect of a combined ATRA and PKC412 therapeutic
strategy in the prevention and treatment of AML patients with
FLT3-mutations.
References
|
1
|
Breitenbuecher F, Schnittger S, Grundler
R, Markova B, Carius B, Brecht A, Duyster J, Haferlach T, Huber C
and Fischer T: Identification of a novel type of ITD mutations
located in nonjuxtamembrane domains of the FLT3 tyrosine kinase
receptor. Blood. 113:4074–4077. 2009. View Article : Google Scholar
|
|
2
|
O’Donnell MR, Appelbaum FR, Coutre SE,
Damon LE, Erba HP, Foran J, et al: Acute myeloid leukemia. J Natl
Compr Canc Netw. 6:962–993. 2008.
|
|
3
|
Foran JM: New prognostic markers in acute
myeloid leukemia: perspective from the clinic. Hematology Am Soc
Hematol Educ Program. 2010:47–55. 2010. View Article : Google Scholar
|
|
4
|
Scholl C, Gilliland DG and Frohling S:
Deregulation of signaling pathways in acute myeloid leukemia. Semin
Oncol. 35:336–345. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kuchenbauer F, Kern W, Schoch C, Kohlmann
A, Hiddemann W, Haferlach T, et al: Detailed analysis of FLT3
expression levels in acute myeloid leukemia. Haematologica.
90:1617–1625. 2005.PubMed/NCBI
|
|
6
|
Kang HJ, Lee JW, Kho SH, Kim MJ, Seo YJ,
Kim H, et al: High transcript level of FLT3 associated with high
risk of relapse in pediatric acute myeloid leukemia. J Korean Med
Sci. 25:841–845. 2005. View Article : Google Scholar
|
|
7
|
Burnett AK: Acute myeloid leukemia:
treatment of adults under 60 years. Rev Clin Exp Hematol. 6:26–45.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Löwenberg B, Downing JR and Burnett A:
Acute myeloid leukemia. N Engl J Med. 341:1051–1062. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Chu SH and Small D: Mechanisms of
resistance to FLT3 inhibitors. Drug Resist Updat. 12:8–16. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ravandi F, Kantarjian H, Faderl S,
Garcia-Manero G, O’Brien S, Koller C, Pierce S, Brandt M, Kennedy
D, Cortes J and Beran M: Outcome of patients with FLT3-mutated
acute myeloid leukemia in first relapse. Leuk Res. 34:752–756.
2010. View Article : Google Scholar
|
|
11
|
Levis M, Ravandi F, Wang ES, Baer MR, Perl
A, Coutre S, Erba H, Stuart RK, Baccarani M, Cripe LD, et al:
Results from a randomized trial of salvage chemotherapy followed by
lestaurtinib for patients with FLT3 mutant AML in first relapse.
Blood. 117:3294–3301. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kindler T, Lipka DB and Fischer T: FLT3 as
a therapeutic target in AML: still challenging after all these
years. Blood. 116:5089–5102. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Monnerat C, Henriksson R, Le Chevalier T,
Novello S, Berthaud P, Faivre S, et al: Phase I study of PKC412
(N-benzoyl-staurosporine), a novel oral protein kinase C inhibitor,
combined with gemcitabine and cisplatin in patients with
non-small-cell lung cancer. Ann Oncol. 15:316–323. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Propper DJ, McDonald AC, Man A, Thavasu P,
Balkwill F, Braybrooke JP, et al: Phase I and pharmacokinetic study
of PKC412, an inhibitor of protein kinase C. J Clin Oncol.
19:1485–1492. 2001.PubMed/NCBI
|
|
15
|
Weisberg E, Boulton C, Kelly LM, Manley P,
Fabbro D, Meyer T, Gilliland DG and Griffin JD: Inhibition of
mutant FLT3 receptors in leukemia cells by the small molecule
tyrosine kinase inhibitor PKC412. Cancer Cell. 1:433–443. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Stone RM, DeAngelo DJ, Klimek V, Galinsky
I, Estey E, Nimer SD, Grandin W, Lebwohl D, Wang Y, Cohen P, et al:
Patients with acute myeloid leukemia and an activating mutation in
FLT3 respond to a small-molecule FLT3 tyrosine kinase inhibitor,
PKC412. Blood. 105:54–60. 2005. View Article : Google Scholar
|
|
17
|
Fischer T, Stone RM, Deangelo DJ, Galinsky
I, Estey E, Lanza C, et al: Phase IIB trial of oral Midostaurin
(PKC412), the FMS-like tyrosine kinase 3 receptor (FLT3) and
multi-targeted kinase inhibitor, in patients with acute myeloid
leukemia and high-risk myelodysplastic syndrome with either
wild-type or mutated FLT3. J Clin Oncol. 28:4339–4345. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Lotan R: Suppression of squamous cell
carcinoma growth and differentiation by retinoids. Cancer Res.
54(Suppl 7): 1987s–1990s. 1994.PubMed/NCBI
|
|
19
|
Caliaro MJ, Marmouget C, Guichard S,
Mazars P, Valette A, Moisand A, Bugat R and Jozan S: Response of
four human ovarian carcinoma cell lines to all-trans retinoic acid:
relationship with induction of differentiation and retinoic acid
receptor expression. Int J Cancer. 56:743–748. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Amos B and Lotan R: Retinoid-sensitive
cells and cell lines. Methods Enzymol. 190:217–225. 1990.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Frankel SR, Eardley A, Lauwers G, Weiss M
and Warrell RP Jr: The “retinoic acid syndrome” in acute
promyelocytic leukemia. Ann Intern Med. 117:292–296. 1992.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Muindi J, Frankel SR, Miller WH Jr,
Jakubowski A, Scheinberg DA, Young CW, et al: Continuous treatment
with all-trans retinoic acid causes a progressive reduction in
plasma drug concentrations: implications for relapse and retinoid
“resistance” in patients with acute promyelocytic leukemia. Blood.
79:299–303. 1992.PubMed/NCBI
|
|
23
|
Conley BA, Egorin MJ, Sridhara R, Finley
R, Hemady R, Wu S, et al: Phase I clinical trial of
all-trans-retinoic acid with correlation of its pharmacokinetics
and pharmacodynamics. Cancer Chemother Pharmacol. 39:291–299. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Karmakar S, Banik NL and Ray SK:
Combination of all-trans retinoic acid and paclitaxel-induced
differentiation and apoptosis in human glioblastoma U87MG
xenografts in nude mice. Cancer. 112:596–607. 2008. View Article : Google Scholar
|
|
25
|
Ortiz MA, Bayon Y, Lopez-Hernandez FJ and
Piedrafita FJ: Retinoids in combination therapies for the treatment
of cancer: mechanisms and perspectives. Drug Resist Updat.
5:162–175. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Arrieta O, González-De la Rosa CH,
Aréchaga-Ocampo E, Villanueva-Rodríguez G, Cerón-Lizárraga TL,
Martínez-Barrera L, Vázquez-Manríquez ME, et al: Randomized phase
II trial of All-trans-retinoic acid with chemotherapy based on
paclitaxel and cisplatin as first-line treatment in patients with
advanced non-small-cell lung cancer. J Clin Oncol. 28:3463–3471.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Boorjian SA, Milowsky MI, Kaplan J, Albert
M, Cobham MV, Coll DM, Mongan NP, Shelton G, Petrylak D, Gudas LJ
and Nanus DM: Phase 1/2 clinical trial of interferon alpha2b and
weekly liposome-encapsulated all-trans retinoic acid in patients
with advanced renal cell carcinoma. J Immunother. 30:655–662. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Bryan M, Pulte ED, Toomey KC, Pliner L,
Pavlick AC, Saunders T and Wieder R: A pilot phase II trial of
all-trans retinoic acid (Vesanoid) and paclitaxel (Taxol) in
patients with recurrent or metastatic breast cancer. Invest New
Drugs. 29:1482–1487. 2011. View Article : Google Scholar
|
|
29
|
Kini AR, Peterson LA, Tallman MS and
Lingen MW: Angiogenesis in acute promyelocytic leukemia: induction
by vascular endothelial growth factor and inhibition by all-trans
retinoic acid. Blood. 97:3919–3924. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Ly BT, Chi HT, Yamagishi M, et al:
Inhibition of FLT3 expression by green tea catechins in FLT3
mutated-AML cells. PLoS One. 8:e663782013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Matsuo Y, MacLeod RA, Uphoff CC, Drexler
HG, Nishizaki C, Katayama Y, Kimura G, Fujii N, Omoto E, Harada M
and Orita K: Two acute monocytic leukemia (AML-M5a) cell lines
(MOLM-13 and MOLM-14) with interclonal phenotypic heterogeneity
showing MLL-AF9 fusion resulting from an occult chromosome
insertion, ins (11;9) (q23;p22p23). Leukemia. 11:1469–1477. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Lange B, Valtieri M, Santoli D, Caracciolo
D, Mavilio F, Gemperlein I, et al: Growth factor requirements of
childhood acute leukemia: establishment of GM-CSF-dependent cell
lines. Blood. 70:192–199. 1987.PubMed/NCBI
|
|
33
|
Iida S, Saito M, Okazaki T, Seto M,
Yamamoto K, Akao Y, et al: Phenotypic and genotypic
characterization of 14 leukemia and lymphoma cell lines with 11q23
translocations. Leuk Res. 16:1155–1163. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Taketani T, Taki T, Sugita K, Furuichi Y,
Ishii E, Hanada R, Tsuchida M, Ida K and Hayashi Y: FLT3 mutations
in the activation loop of tyrosine kinase domain are frequently
found in infant ALL with MLL rearrangements and pediatric ALL with
hyperdiploidy. Blood. 103:1085–1088. 2004. View Article : Google Scholar
|
|
35
|
Furukawa Y, Vu HA, Akutsu M, Odgerel T,
Izumi T, Tsunoda S, et al: Divergent cytotoxic effects of PKC412 in
combination with conventional antileukemic agents in FLT3
mutation-positive versus -negative leukemia cell lines. Leukemia.
21:1005–1014. 2007.PubMed/NCBI
|
|
36
|
Steel GG and Peckham MJ: Exploitable
mechanisms in combined radiotherapy-chemotherapy: the concept of
additivity. Int J Radiat Oncol Biol Phys. 5:85–91. 1979. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Kano Y, Akutsu M, Tsunoda S, Mori K,
Suzuki K and Adachi KI: In vitro schedule-dependent interaction
between paclitaxel and SN-38 (the active metabolite of irinotecan)
in human carcinoma cell lines. Cancer Chemother Pharmacol.
42:91–98. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Breitman TR, Collins SJ and Keene BR:
Terminal differentiation of human promyelocytic leukemic cells in
primary culture in response to retinoic acid. Blood. 57:1000–10004.
1981.PubMed/NCBI
|
|
39
|
Warrell RP Jr, Frankel SR, Miller WH Jr,
Scheinberg DA, Itri LM, Hittelman WN, Vyas R, And-reeff M, Tafuri
A, Jakubowski A, et al: Differentiation therapy of acute
promyelocytic leukemia with tretinoin (all-trans-retinoic acid). N
Engl J Med. 324:1385–1393. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Lotan R: Retinoids as modulators of tumor
cells invasion and metastasis. Semin Cancer Biol. 2:197–208.
1991.PubMed/NCBI
|
|
41
|
Hofmann SL: Retinoids--”differentiation
agents” for cancer treatment and prevention. Am J Med Sci.
304:202–213. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Ashkenazi A, Holland P and Eckhardt SG:
Ligand-based targeting of apoptosis in cancer: the potential of
recombinant human apoptosis ligand 2/Tumor necrosis factor-related
apoptosis-inducing ligand (rhApo2L/TRAIL). J Clin Oncol.
26:3621–3630. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Warrell RP Jr, de Thé H, Wang ZY and Degos
L: Acute promyelocytic leukemia. N Engl J Med. 329:177–189. 1993.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Degos L, Dombret H, Chomienne C, Daniel
MT, Micléa JM, Chastang C, Castaigne S and Fenaux P:
All-trans-retinoic acid as a differentiating agent in the treatment
of acute promyelocytic leukemia. Blood. 85:2643–2653.
1995.PubMed/NCBI
|
|
45
|
Das A, Banik NL and Ray SK: Retinoids
induced astrocytic differentiation with down regulation of
telomerase activity and enhanced sensitivity to taxol for apoptosis
in human glioblastoma T98G and U87MG cells. J Neurooncol. 87:9–22.
2008. View Article : Google Scholar
|
|
46
|
Nagai S, Takenaka K, Sonobe M, Wada H and
Tanaka F: Schedule-dependent synergistic effect of pemetrexed
combined with gemcitabine against malignant pleural mesothelioma
and non-small cell lung cancer cell lines. Chemotherapy.
54:166–175. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Ridwelski K, Gebauer T, Fahlke J, Kröning
H, Kettner E, Meyer F, et al: Combination chemotherapy with
docetaxel and cisplatin for locally advanced and metastatic gastric
cancer. Ann Oncol. 12:47–51. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Sarkar K and Yang H: Encapsulation and
extended release of anti-cancer anastrozole by stealth
nanoparticles. Drug Deliv. 15:343–346. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Sofou S: Radionuclide carriers for
targeting of cancer. Int J Nanomedicine. 3:181–199. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Torchilin V: Antibody-modified liposomes
for cancer chemotherapy. Expert Opin Drug Deliv. 5:1003–1025. 2008.
View Article : Google Scholar : PubMed/NCBI
|