Introduction
Multiple factors contribute to the pathogenesis of
benign prostatic hyperplasia (BPH) (1–5). The
biological roles of the extracellular matrix (ECM) in adhesion,
growth and proliferation have been investigated extensively
(6). The ECM not only serves as an
external environment for the physiological activities of cells, but
supports the intercellular signal transduction pathways involved in
multiple physiological and pathological processes (7,8).
Qianliening capsule (QC) is a traditional Chinese medicine
developed at the People’s Hospital of Fujian University of
Traditional Chinese Medicine (cat. no. Z20110009). QC was developed
based on the understanding of BPH in traditional Chinese medicine
of the pathology of BPH. The predominant actions of QC include
heat-clearing and detoxification, promoting blood circulation,
removing blood stasis, toning up the kidney and nourishing vitality
(termed ‘replenishing qi’ in Chinese) (9). Our previous studies revealed that QC
inhibits the proliferation of benign prostatic hyperplastia
epithelial cell line 1 (BPH-1) cells, promotes their apoptosis and
regulates the effects of certain cytokines (10–13)
to exert its therapeutic effects on BPH. However, the specific
mechanisms underlying the therapeutic effectiveness of QC remain to
be elucidated. The present study investigated the effect of QC on
the ECM to evaluate its effects on apoptosis and proliferation in
the BPH-1 cells and examined the association between the ECM and
BPH-1 apoptosis to reveal the potential mechanism underlying the
therapeutic effect of QC in BPH.
Materials and methods
Drugs and reagents
QC (Food and Drug Administration approval no.
Z20110009), a capsule containing five Chinese products, was
provided by the Academy of Pharmacology of Fujian University of
Traditional Chinese Medicine (Fujian, China) (14,15).
The powder inside the capsule was dissolved in 40% DMSO to a
concentration of 50 mg/ml (Sigma-Aldrich, St. Louis, MO, USA) and
stored at 4°C. Human collagen IV (Sigma-Aldrich), fibronectin (FN;
Sigma-Aldrich) and an enzyme-linked immunosorbent assay (ELISA) kit
was obtained from Shanghai XiTang Biological Technology Co., Ltd.
(Shanghai, China). RPMI-1640, trypsin and fetal bovine serum (FBS)
were purchased from HyClone (Logan, UT, USA). MTT was obtained from
Sigma-Aldrich. The reverse transcription (RT), TRIzol and
quantitative polymerase chain reaction (PCR) reagents (5X reaction
buffer, 10 mM deoxyribonucleotide triphosphate mix,
RevertAid™M-MuLV reverse transcriptase) were purchased from
Invitrogen Life Technologies (Carlsbad, CA, USA). Taq polymerase
and the RNase inhibitor, ribonuclease inhibitor, were provided by
Takara Bio, Inc. (Otsu, Japan) and primers were synthesized by
Shanghai Yingjun Biological Technology Co., Ltd. (Shanghai, China).
The Annexin V-fluorescein isothiocyanate (FITC) Apoptosis Detection
kit was provided by BD Biosciences (San Jose, CA, USA). Polyclonal
anti-rabbit primary antibodies against collagen IV (1:1,000;
ab6581) was purchased from Abcam, Cambridge, MA, USA; FN (1:1,000;
610077) was purchased from BD Biosciences and B-cell lymphoma 2
(Bcl-2; 1:1,000, #2876), Bcl-2-associated X protein (Bax; 1:1,000;
#2772) and cyclin D1 (1:1,000; #2922) were obtained from Cell
Signaling Technology, Inc., Danvers, MA, USA). The mouse
anti-rabbit secondary IgG antibody (3,3′-diaminobenzidine) was
obtained from Hebei Bohai Biotechnology Development Co., Ltd.
(Hebei, China).
Cell lines
The BPH-1 cell line was provided by the Institute
for Molecular Biology, College of Life Sciences, Nankai University,
Tianjin, China.
Culture of BPH-1 cells
The BPH-1 cells were cultured in RPMI-1640 medium
containing 10% (v/v) FBS, 100 U/ml penicillin and 100 μg/ml
streptomycin (HyClone, SV30010; GE Healthcare Life Sciences, Little
Chalfont, UK), at 37°C in a humidified incubator with 5%
CO2. The cells were subcultured at 80–90%
confluency.
Culture of BPH-1 cells in an environment
rich in collagen IV and FN
Collagen IV was diluted to 1% in phosphate-buffered
saline (PBS; HyClone, SH30256.01B) according to the manufacturer’s
instructions and 800–1,000 μl/well was added to 6-well plates and
incubated at 37°C for 4 h. The wells were washed three times with
PBS and the cells were seeded into the wells at a density of
2×105 cells/well. FN was diluted to 1% in PBS and
800–1,000 μl/well was added to 6-well plates and incubated at 37°C
for 4 h, following by seeding of the cells into the wells
(2×105 cells/well).
Determination of the concentrations of
collagen IV and FN in the medium by ELISA
BPH-1 cells in the logarithmic growth phase were
digested with trypsin and seeded into 100 μl medium in 96-well
plates (1×104 cells/well), which were coated with either
collagen IV or FN, or with PBS as a control group. Collagen IV was
diluted to 1% in PBS according to the manufacturer’s instructions,
20 μl/well was added to 96-well plates and they were subsequently
incubated at 37°C for 4 h (1×104 cells/well). The wells
were washed three times with PBS and the cells were seeded into the
wells. FN was diluted to 1% in PBS and 20 μl/well was added to
96-well plates (1×104 cells/well) and incubated at 37°C
for 4 h, followed by seeding of the cells into the wells. After 24
h in culture, QC was added to the cells at different concentrations
(0, 1.25, 2.5 or 5 mg/ml) in duplicate for a further 24 h. The cell
culture medium was removed and the concentrations of collagen IV
and FN were measured using an ELISA kit (Shanghai Xitang
Biotechnology Co., Ltd), according to the manufacturer’s
instructions. The absorbance was measured at 490 nm using a
microplate reader (Model ELX800; BioTek Instruments, Inc.,
Winooski, VT, USA) and the concentrations were calculated according
to the standard curve.
Cell morphology observation
BPH-1 cells in the logarithmic growth phase were
digested with trypsin and seeded into 6-well plates at a density of
2×105 cells/ml in 2 ml medium. The cells were treated
with various concentrations of QC for 24 h and cell morphology was
observed using a DP70 phase-contrast microscope (Olympus
Corporation, Tokyo, Japan). Images were captured at a magnification
of ×100.
Determination of cell viability by MTT
assay
Following treatment with various concentrations of
QC, the cells in each well were incubated with 0.5 mg/ml MTT (100
μl) at 37°C for 4 h. The medium in each well was removed and 100 μl
dimethyl sufoxide (Sigma-Aldrich) was added prior to incubation at
room temperature for 10 min to resolve the crystals. The absorbance
(A) was determined using a microplate reader at 570 nm. The
survival rate was calculated as follows: Survival rate (%) =
Aexperiment/Acontrol × 100.
Detection of the mRNA expression levels
of collagen IV, FN, Bcl-2, Bax and cyclin D1 by RT-PCR
BPH-1 cells in the logarithmic growth phase were
digested with trypsin and seeded into 2 ml medium in 6-well plates
(2×105 cells/well), which were coated with either
collagen IV, FN or PBS (control group). After 24 h, the cells were
treated with various concentrations of QC for a further 24 h. The
mRNA expression levels of Bcl-2, Bax and cyclin D1 were then
detected by RT-PCR in the uncoated samples and in the samples
coated with either collagen IV or FN. The total RNA was isolated
from the BPH-1 cells using TRIzol reagent. Oligo (dT)-primed RNA (1
μg) was reverse-transcribed using SuperScript II Reverse
Transcriptase (Promega Corporation, Madison, WI, USA) according to
the manufacturer’s instructions. The cDNA was used to determine the
mRNA expression levels of collagen IV, FN, Bcl-2, Bax and cyclin D1
by PCR using Taq DNA polymerase (Fermentas, Hanover, MD, USA).
GAPDH was used as an internal control. The sequences of the primers
used for amplification were as follows: Collagen IV, forward 5′-ATC
GGC TAC CTC CTG GTG A-3′ and reverse 5′-GCT GAT GTG TGT GCG GAT
GA-3′ [annealing temperature (Tm)=58°C; 648 bp]; FN, forward 5′-TGG
ACC TTC TAC CAG TGC GAC-3′ and reverse 5′-TGT CTT CCC ATC ATC GTA
ACA C-3′ (annealing Tm=58°C; 451 bp); Bcl-2, forward 5′-CAG CTG CAC
CTG ACG CCC TT-3′ and reverse 5′-GCC TCC GTT ATC CTG GAT CC-3′
(annealing Tm=55°C; 362 bp); Bax, forward 5′-TGC TTC AGG GTT TCA
TCC AGG-3′ and reverse 5′-TGG CAA AGT AGA AAA GGG CGA-3′ (annealing
Tm=55°C; 253 bp); cyclin D1, forward: 5′-TGG ATG CTG GAG GTC TGC
GAG GAA-3′ and reverse 5′-GGC TTC GAT CTG CTC CTG GCA GGC-3′
(annealing Tm=55°C; 537 bp) and GAPDH, forward 5′-GTC ATC CAT GAC
AAC TTT GG-3′ and reverse 5′-GAG CTT GAC AAA GTG GTC GT-3′
(annealing Tm=58°C; 450 bp). The samples were analyzed using 1.5%
agarose gel electrophoresis (HyClone). The DNA bands were
visualized using a Gel Documentation system (Model Gel Doc 2000;
Bio-Rad Laboratories, Inc., Hercules, CA, USA).
Detection of the protein expression
levels of collagen IV, FN, Bcl-2, Bax and cyclin D1 by western blot
analysis
The BPH-1 cells (2×105 cells/well) were
seeded into 2 ml medium in 6-well plates coated with either
collagen IV, FN or PBS (control group). The cells were cultured for
24 h and were then treated with various concentrations (0, 1.25,
2.5 or 5 mg/ml) of QC. After 24 h, the cells were lysed in lysis
buffer (50 mM Tris-Cl, pH 6.8; 15 mM NaCl, 5 mM EDTA, 0.5% NP-40
and 1 mM PMSF; Sigma-Aldrich) and the total protein was extracted.
In the collagen IV group and FN group, the proteins were dissolved
in buffer for the concentration of the proteins, Bcl-2, Bax and
cyclin D1, in the lysates to be determined. Following protein
denaturation by boiling at 100°C for 5 min, the lysates were
subjected to polyacrylamide gel electrophoresis (SDS-PAGE) and the
proteins were transferred onto a polyvinylidene fluoride membrane
(EMD Millipore, Billerica, MA, USA). The membrane was blocked using
1% non-fat milk for 2 h, prior to washing with Tris-buffered saline
(TBS) with Tween 20 (Sigma-Aldrich) and incubation overnight at 4°C
with the primary antibodies (1:1,000) against collagen IV, FN,
Bcl-2, Bax, cyclin D1 and β-actin (Cell Signaling Technology,
Inc.). The antibodies were diluted in 5% w/v bovine serum albumin
(Sigma-Aldrich), 1X TBS, 0.1% Tween® 20 at 4°C with
gentle shaking, overnight. The membrane was then incubated with
horseradish-peroxidase-conjugated secondary antibody (1:25,000) at
room temperature for 1 h. The membrane was washed in TBS containing
0.25% Tween-20 and then incubated with enhanced chemilluminescence
solution (1:1; Technology Co., Ltd, Shanghai, China) at 25°C for 5
min, followed by film exposure using a Bio-Rad Chemi Doc XRS+
system (Bio-Rad Laboratories, Inc.).
Detection of cell apoptosis
The cells were trypsinized (0.25% trypsin without
EDTA) and a cell suspension in RPMI-1640 was prepared. The cell
density was adjusted to between 5×105 and
5×106 cells/ml. Aliquots (1 ml) of the cell suspension
were washed three times in PBS with centrifugation at 645 × g for
10 min at 4°C. Following the final wash, the cells were resuspended
in 500 μL binding buffer (Beyotime Institute of Biotechnology,
Shanghai, China). Subsequently, 5 μl annexin V-FITC and 5 μL
propidium iodide (PI) were added and the cells were incubated at
room temperature for 15 min. The cells were then analyzed using a
FACScalibur flow cytometer (BD Biosciences).
Statistical analysis
Statistical analysis was performed using SPSS 12.0
software (SPSS, Inc., Chicago, IL, USA). Data are expressed as the
mean ± standard deviation. Statistical analyses of the data was
performed using Student’s t-test and analysis of variance.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Effect of QC on the expression levels of
collagen IV and FN in BPH-1 cells
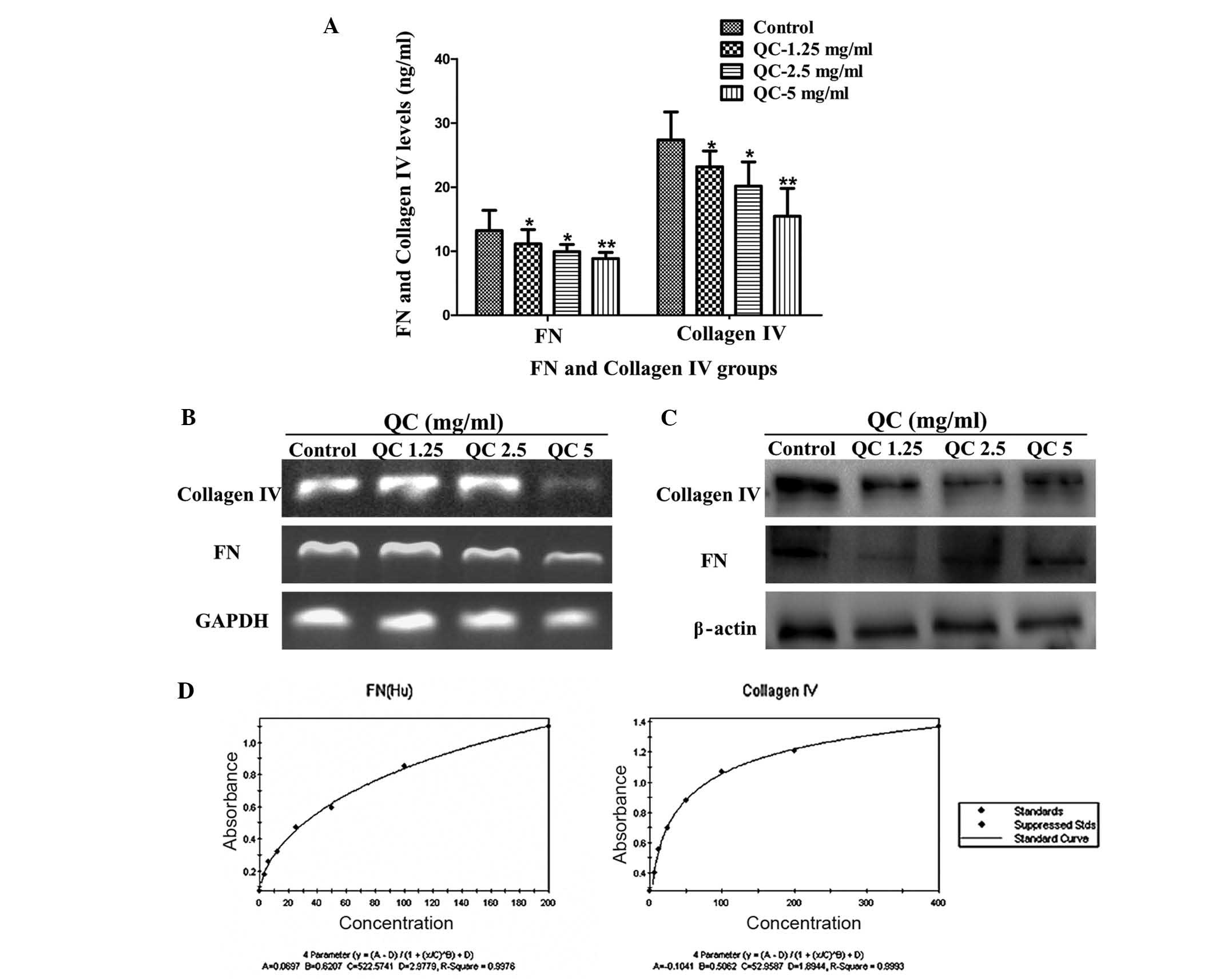
In the control group, QC significantly reduced the
levels of collagen IV and FN in the BPH-1 cell culture medium
(P<0.05 and P<0.01, respectively) and more marked inhibition
was observed with higher concentrations of QC (Fig. 1A). Additionally, treatment with QC
markedly inhibited the mRNA and protein expression levels of
collagen IV and FN in the BPH-1 cells (Fig. 1B and C).
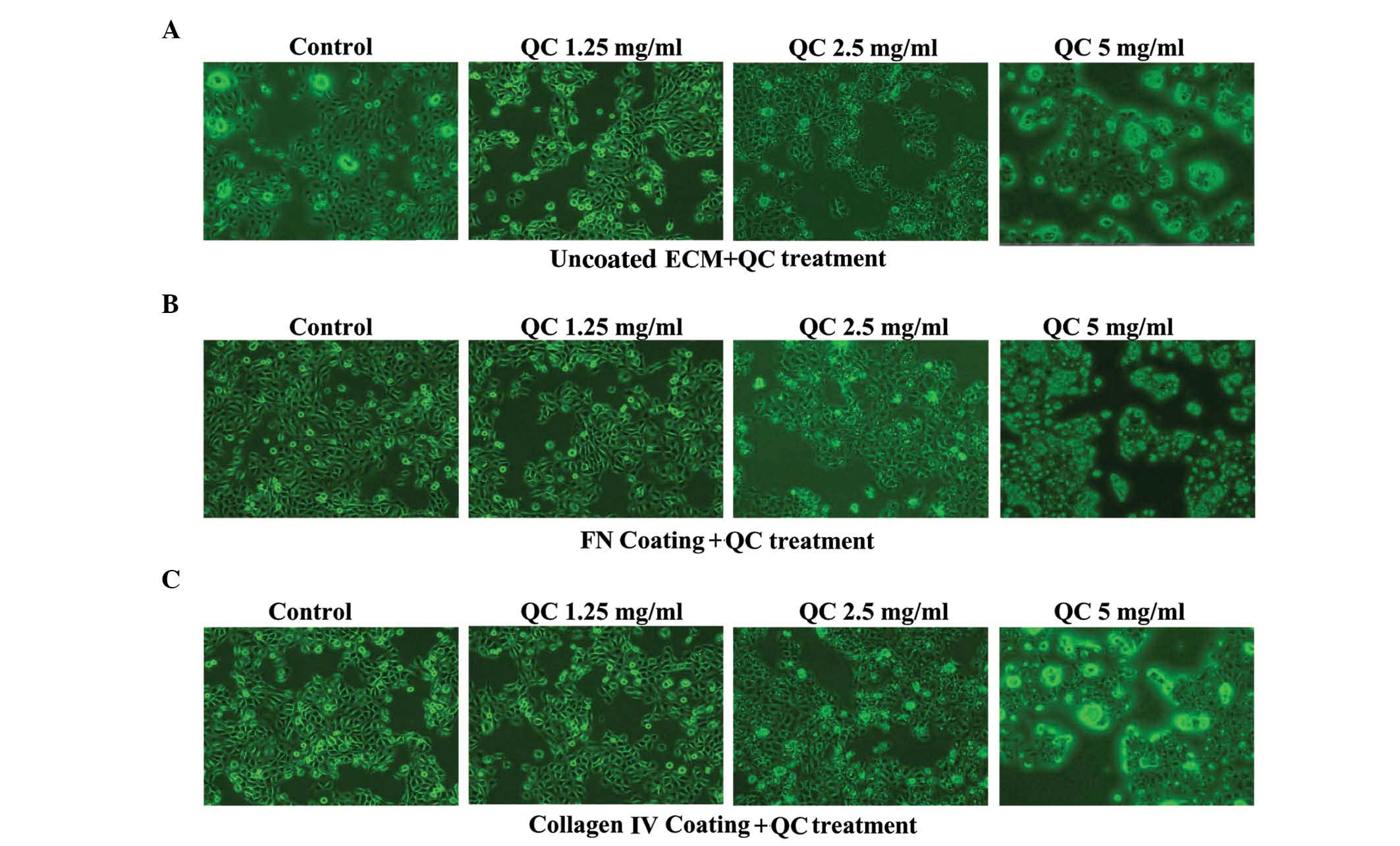
Effect of QC on the morphology and
viability of the BPH-1 cells
On visualization under an inverted microscope, the
BPH-1 cells in the collagen IV and FN groups were rhombic, evenly
dense and had clear nuclei. The cell density and extensibility were
higher and the number of apoptotic cells were lower in the collagen
IV and FN groups compared with the control group. The morphological
integrity and the number of cells decreased with increasing
concentrations of QC (Fig. 2A–C).
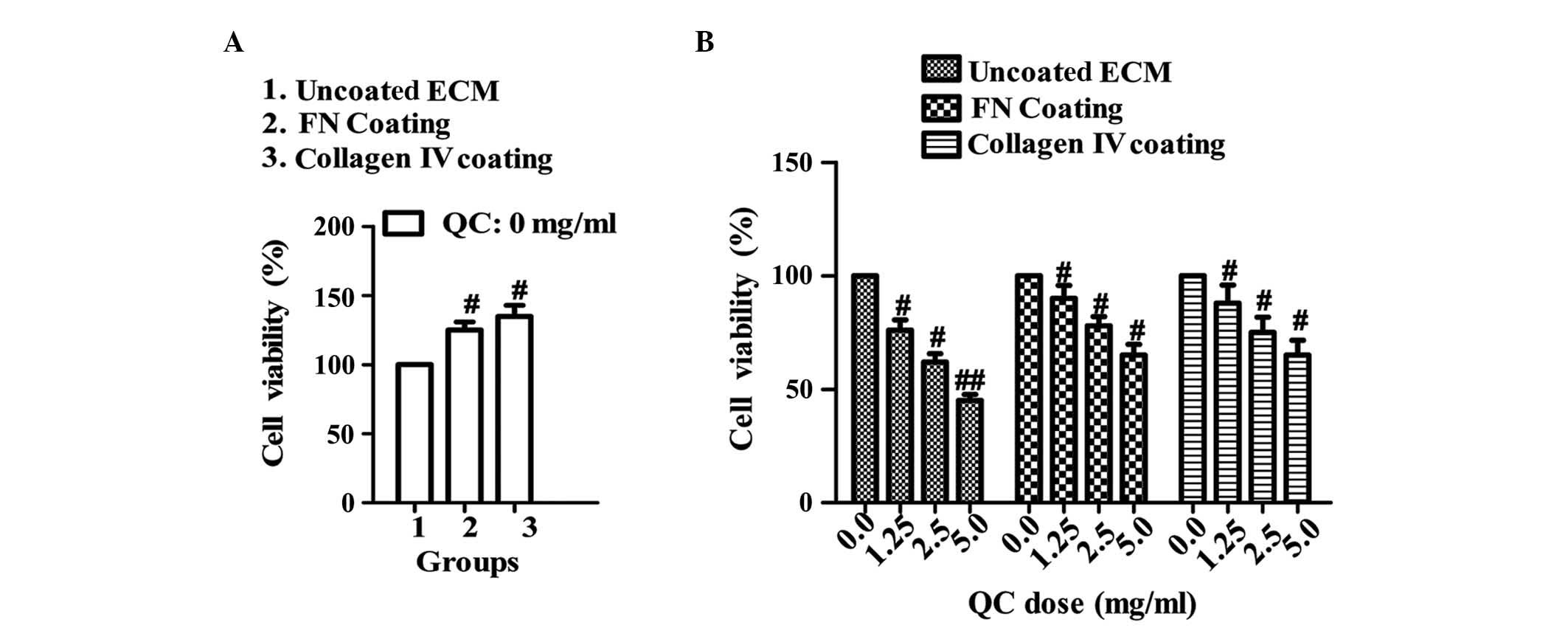
The MTT assay revealed that the viability of the BPH-1 cells coated
with either collagen IV or FN were significantly higher compared
with the uncoated ECM group (P<0.05 and P<0.01, respectively;
Fig. 3A). In addition, increasing
concentrations of QC decreased the viability of the BPH-1 cells in
all three groups, to different extents (P<0.05 or P<0.01,
respectively; Fig. 3B),
particularly in the uncoated ECM group.
Effect of QC on the proliferation and
apoptosis of BPH-1 cells
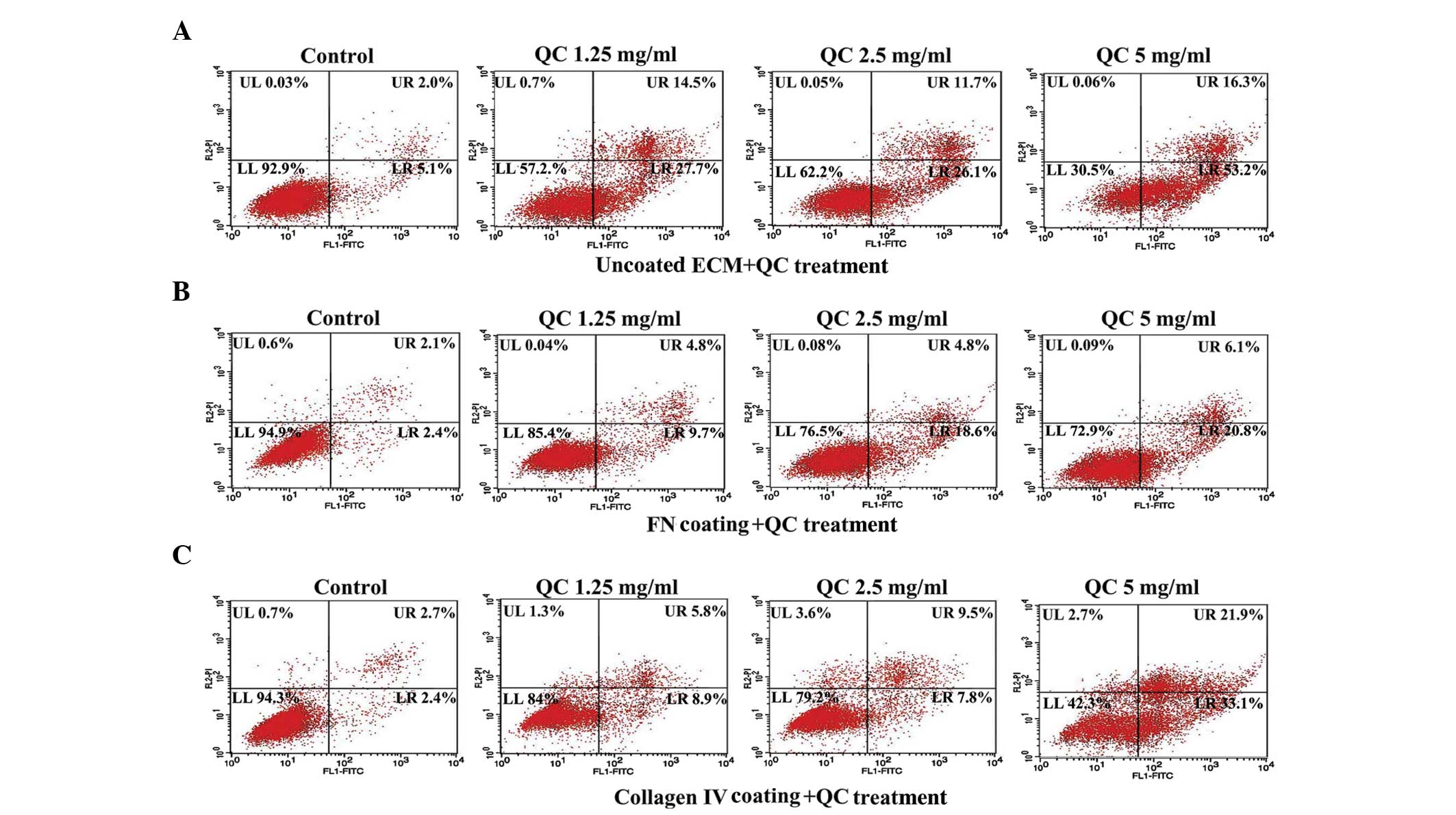
The annexin V-FITC staining indicated that, in the
absence of QC, the apoptotic index of the cells coated with either
collagen IV or FN decreased significantly compared with the
uncoated ECM control group. In each group, the apoptotic index
increased as the concentration of QC increased (Fig. 4A–C). RT-PCR and western blot
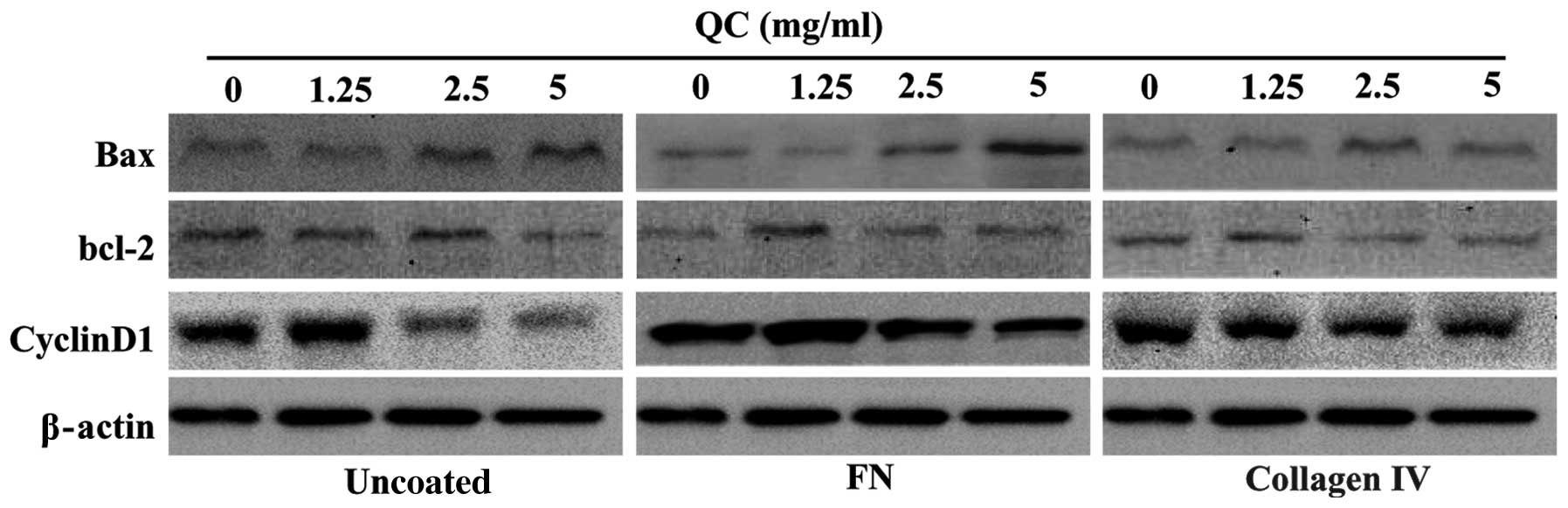
analysis revealed that the mRNA and protein expression levels of
Bax in the cells coated with either collagen IV or FN were
significantly lower compared with those in the uncoated ECM group
and increased with increasing concentrations of QC. However, the
levels of Bcl-2 and cyclin D1 were markedly higher in the cells
coated with either collagen IV or FN compared with those in the
uncoated ECM group. In each group, the mRNA and protein expression
levels of Bcl-2 and cyclin D1 decreased as the concentration of QC
increased (Figs. 5 and 6).
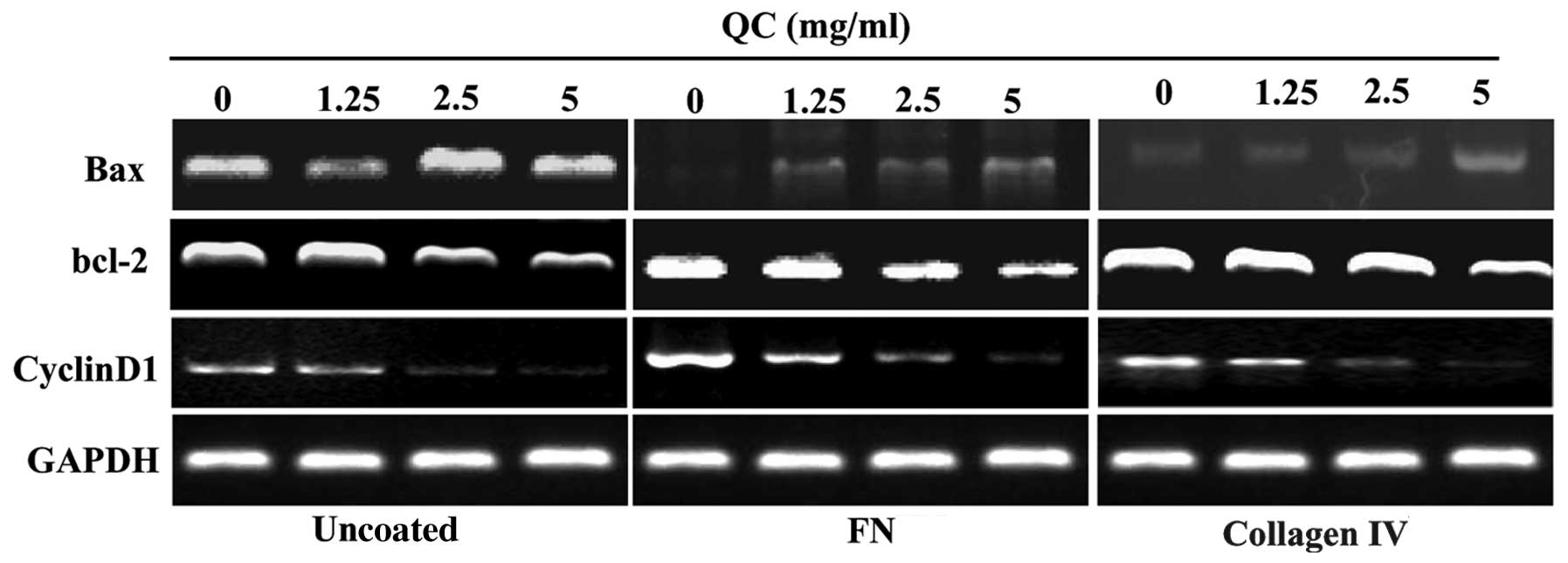 | Figure 5Effect of QC on the mRNA expression
levels of Bcl-2, Bax and cyclin D1 in BPH-1 cells. The mRNA
expression levels of Bcl-2, Bax and cyclin D1 in the BPH-1 cells
were determined by reverse transcription quantitative polymerase
chain reaction and visualized by electrophoresis. GAPDH was used as
an internal control. Uncoated ECM cells and cells coated with
either FN or collagen IV were treated with various concentrations
(0, 1.25, 2.5 or 5 mg/ml) of QC. QC, Qianliening capsule; FN,
fibronectin; ECM, extracellular matrix; Bcl-2, B-cell lymphoma-2;
BAX, Bcl-2 associated X protein; BPH-1, benign prostatic
hyperplasia epithelial cell line 1. |
Discussion
Multiple factors contribute to the pathogenesis of
BPH. An increasing number of studies have focused on the role of
the ECM in the adhesion, growth and proliferation of cells
(6). The ECM not only serves as an
external environment for the physiological activities of cells, but
supports the intercellular signal transduction pathways involved in
multiple pathological and physiological processes (7,8). The
ECM is involved in the pathogenesis of BPH in the following ways:
The ECM, together with proliferating cells, causes changes in the
morphology and physiological functions of the prostate (16,17);
androgen, estrogen and their receptors regulate active peptides in
tissues (18) and stimulate cells
to produce different components of the ECM (19) and the ECM interacts with hormones
and growth factors to increase the sensitivity of prostate cells to
androgen and growth factors, which alters the morphology of
fibroblasts, sensitivity to hormones and gene expression (20). The ECM can also regulate the
secretion of different growth factors, including transforming
growth factor-β, by the prostate cells to regulate prostate cell
growth in a paracrine or autocrine manner (17).
QC is a compound found in Chinese herbs, including
rhubarb, leeches, astragalus and achyranthes (14,15).
Previous studies have demonstrated that QC inhibits the
proliferation of BPH cells, induces apoptosis and regulates the
expression of certain cytokines (11–13,16),
thus exerting a therapeutic effect on BPH. However, the mechanisms
underlying the therapeutic effects of QC on BPH remain to be
elucidated.
The present study provided further evidence that
components of the ECM are involved in the pathology of BPH. It was
observed that BPH-1 cells in the collagen IV and FN groups were
rhombic, exhibited even density and had clear nuclei. The cell
density and extensibility in each of the groups were higher
compared with the controls. In addition, the number of apoptotic
cells in the collagen IV and FN groups were significantly lower
compared with the control group. MTT assays revealed that QC
markedly increased the viability of the BPH-1 cells in the collagen
IV- and FN-coated groups compared with the uncoated ECM group.
These results indicated that collagen IV and FN promoted the growth
of BPH-1 cells, which was consistent with the findings of previous
studies (21–23).
The levels of collagen IV and FN secreted into the
medium and their mRNA and protein expression levels were
significantly reduced in the QC-treated cells compared with the
untreated cells. These results suggested that QC inhibited the
synthesis and secretion of collagen IV and FN in the BPH-1 cells
during the growth process. In addition, the apoptotic index of the
BPH-1 cells increased and the proliferation rate decreased
dose-dependently following treatment with QC Therefore QC inhibited
the proliferation of BPH-1 cells and promoted their apoptosis. The
present study also demonstrated that QC inhibited the mRNA and
protein expression of cyclin D1, suggesting that QC can inhibit the
cell cycle of BPH-1 cells. However, the mechanism underlying this
requires further investigation. As the ECM is important in
regulating the proliferation and apoptosis of BPH cells during the
pathogenesis of BPH (16,17) and QC inhibited the secretion of FN
and collagen IV during the growth of BPH-1 cells, it was
hypothesized that QC alters ECM production in BPH cells. The
resulting effect on the proliferation and apoptosis of BPH cells
may be one of the mechanisms underlying the therapeutic effects of
QC on BPH.
Acknowledgements
This study was supported by the Nature Science
Foundation of China (nos. 81373817 and 81273928), the Natural
Science Foundation of Fujian Province of China (no. 2009J01169) and
the Research Foundation of the Education Bureau of Fujian Province
of China (no. JA09135).
Abbreviations:
|
BPH-1
|
benign prostatic hyperplasia
epithelial-1
|
|
QC
|
Qianliening capsule
|
|
FN
|
fibronectin
|
|
ECM
|
extracellular matrix
|
|
PBS
|
phosphate-buffered saline
|
References
|
1
|
Harman SM, Metter EJ, Tobin JD, Pearson J
and Blackman MR: Longitudinal effects of aging on serum total and
free testosterone levels in healthy men. Baltimore Longitudinal
Study of Aging. J Clin Endocrinol Metab. 86:724–731. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
McNeal J: Pathology of benign prostatic
hyperplasia. Insight into etiology. Urol Clin North Am. 17:477–486.
1990.PubMed/NCBI
|
|
3
|
Untergasser G, Madersbacher S and Berger
P: Benign prostatic hyperplasia: Age-related tissue-remodeling. Exp
Gerontol. 40:121–128. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lucia MS and Lambert JR: Growth factors in
benign prostatic hyperplasia: Basic science implications. Curr Urol
Rep. 9:272–278. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Sciarra A, Mariotti G, Salciccia S, Autran
Gomez A, Monti S, Toscano V and Di Silverio F: Prostate growth and
inflammation. J Steroid Biochem Mol Biol. 108:254–260. 2008.
View Article : Google Scholar
|
|
6
|
Von Der Mark K, Von Der Mark H and Goodman
S: Cellular responses to extracellular matrix. Kidney Int.
41:632–640. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Raghow R: The role of extracellular matrix
in postinflammatory wound healing and fibrosis. FASEB J. 8:823–831.
1994.PubMed/NCBI
|
|
8
|
Shu CX and Cheng TM: Structure and
function of extracellular matrix. Zhong Guo Xi Nan Guo Fang Yi Yao.
11:220–223. 2001.(In Chinese).
|
|
9
|
Cao W and Zhao AG: Prescription rules of
Chinese herbal medicines in treatment of gastric cancer. Zhong Xi
Yi Jie He Xue Bao. 7:1–8. 2009.(In Chinese). View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhou JH, Lin JM, Xu W, Zhong XY, Zheng YQ,
Hong ZF and Peng J: Qianliening capsule treats benign prostatic
hyperplasia through regulating the expression of sex hormones
estrogen receptor and androgen receptor. Afr J Pharm Pharmacol.
6:173–180. 2012. View Article : Google Scholar
|
|
11
|
Zhong XY, Lin JM, Zhou JH, Xu W, Hong ZF
and Peng J: Qianliening capsule treats benign prostatic hyperplasia
(BPH) by down-regulating the expression of PCNA, CyclinD1 and CDK4.
Afr J Biotechnol. 11:7731–7737. 2012.
|
|
12
|
Zhou JH, Lin JM, Xu W, Zhong XY, Zheng YQ,
Peng J, Xie JD and Hong ZF: Effects of Qianliening capsule on the
expression of IL-10 and TNF-α in benign prostate hyperplasia. Chin
Archives Trad Chin Med. 28:2657–2569. 2010.
|
|
13
|
Lin JM, Zhou JH, Zhong XY, Peng J, Xu W,
Zheng HY, Zhao JY and Hong ZF: Effects of Qianliening capsule on
the expression of EGF and EGFR in BPH Rats. Fujian J Trad Chin Med.
41:45–47. 2010.
|
|
14
|
Zheng HY, Xu W, Lin JM, Peng J and Hong
ZF: Qianliening capsule treats benign prostatic hyperplasia via
induction of prostatic cell apoptosis. Mol Med Rep. 7:848–854.
2013.PubMed/NCBI
|
|
15
|
Lin JM, Zhou JH, Xu W, Zhong XY, Hong ZF
and Peng J: Qianliening capsule treats benign prostatic hyperplasia
via suppression of the EGF/STAT3 signaling pathway. Exp Ther Med.
5:1293–1300. 2013.PubMed/NCBI
|
|
16
|
Li YM and Fang YH: Steroid hormones
binding sites and extracellular matrix in the tissue of human
benign prostatic hyperplasia. Chin J Urology. 15:269–271. 1994.
|
|
17
|
Li YM, Li YK, Hou SL, Fang SH, Li DQ and
Liu XX: Extracellular matrix and transforming growth factor-β in
the tissue of benign prostatic hyperplasia. Chin J Urology.
16:421–422. 1995.
|
|
18
|
Lubrano C, Petrangeli E, Catizone A,
Santonati A, Concolino G, Rombolá N, Frati L, Di Silverio F and
Sciarra F: Epidermal growth factor binding and steroid receptor
content in human benign prostatic hyperplasia. J Steroid Biochem.
34:499–504. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Roberts AB, McCune BK and Sporn MB: TGF-β:
Regulation of extrancellular matrix. Kidney Int. 41:577–559. 1992.
View Article : Google Scholar
|
|
20
|
Freeman MR, Song Y, Carson DD, Guthrie PD
and Chung LW: Extrancellular matrix and androgen receptor
expression associated with spontaneous transformation of rat
prostate fibroblast. Cancer Res. 51:1910–1916. 1991.PubMed/NCBI
|
|
21
|
Janković MM and Kosanović MM: Fibronectin
pattern in benign hyperplasia and cancer of the prostate. Dis
Markers. 25:49–58. 2008. View Article : Google Scholar
|
|
22
|
Djavan B, Lin V, Seitz C, Kramer G, Kaplan
P, Richier J, Marberger M and McConnell: Elastin gene expression in
benign prostatic hyperplasia. Prostate. 40:242–247. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Elliot SJ, Zorn BH, Mcleod DG, Moul JW,
Nyberg L, Striker LJ and Striker GE: Pentosan polysulfate decreases
prostate smooth muscle proliferation and extracellular matrix
turnover. Prostate Cancer Prostatic Dis. 6:138–142. 2003.
View Article : Google Scholar : PubMed/NCBI
|