Introduction
Drug dependence, generally defined as compulsive
drug use and loss of control over drug intake, is induced by
repeated exposure to substances, including opioids and
psychostimulants (1,2). A previous study demonstrated that
there is a genetic component for the risk of substance dependence,
particularly opioid dependence (3). Consistently, family and twin studies
have demonstrated a substantial genetic effect on the development
of drug dependence, with inherited risk estimates in the range of
40–60% (4,5). A previous study revealed that the
regulator of G protein signaling 9 (RGS9) gene may be associated
with drug dependence, including opiate dependence (6).
Human and animal studies revealed that the D2-like
dopamine receptors are critical in the development of substance
dependence (7,8). The heterotrimeric G protein system
regulates the activity of the dopamine receptors. RGS regulates the
function of heterotrimeric G protein in part by stimulating the
GTPase activity of the G protein subunits (9,10).
RGS9, a member of the RGS family, has two splice isoforms (9,10).
The short splice isoform RGS9-1, which contains 18 amino acid
residues at the C-terminus is expressed in the photoreceptors of
the retina. In the long splice isoform, RGS9-2, the short
C-terminus is replaced by a longer region of 209 amino acids
(11). RGS9-2 is specifically
expressed in the striatum important for opiate reward, but is lowly
expressed in the periaqueductal gray and spinal cord important for
opiate analgesia (12). Therefore,
RGS9-2 has been regarded as a potential partner for G
protein-coupled receptors involved in drug dependence, including
morphine (12–14).
The RGS9 gene (17q21–25, 1.1 Mb) has 18 exons with
intron-exon junctions conforming to splice-site consensus sequences
(15). Linkage studies
demonstrated that the region was implicated in susceptibility to
major mental illness (16–19). A single nucleotide polymorphism
(SNP) rs4790953 in the 3′-flanking region of the RGS9 gene was
reported to be weakly associated with antipsychotic-induced tardive
dyskinesia in a Chinese population in Taiwan (17). However, to the best of our
knowledge, the association between the RGS9 gene and heroin
dependence has not yet been investigated. In view of the crucial
role of RGS9 in heroin dependence, the present study investigated
10 SNPs (rs8077696, rs8070231, rs2292593, rs2292592, rs9916525,
rs1122079, rs4790953, rs1530351, rs4791230 and rs2869577) in a
Chinese population to verify the putative association between RGS9
polymorphisms and heroin dependence.
Subjects and methods
Subjects
A total of 425 unrelated heroin-dependent patients
(mean age: 35.76±6.16 years, 345 males, 80 females) were recruited
from the Methadone Maintenance Treatment program of Xi’an Mental
Health Center (Xi’an, China). Participants were daily or almost
daily users of heroin for a minimum of one year prior to
assessment. Their dependence status, major central nervous system
(CNS) diseases and psychoses were evaluated by a senior
psychiatrist at the beginning of the methadone management program.
The diagnosis of opioid dependence was based on the Diagnostic and
Statistical Manual of Mental Disorders (DSM)-IV criteria, medical
history, urine test results and interview responses. The exclusion
criteria included: DSM-IV criteria for an additional Axis I
disorder; history of alcohol, cigarette, amphetamine, barbiturate,
benzodiazepine or marijuana dependence according to DSM-IV; taking
other prescribed medications that may affect the CNS; history of
seizures, hematological diseases or severe liver or kidney
impairment or pregnancy. A total of 205 healthy blood donors (mean
age: 36.13±6.23 years, 166 males, 39 females) were recruited at the
First Hospital Affiliated to the Medical College of Xi’an Jiaotong
University (Xi’an, China). Subjects who had substance abuse,
participated in other studies or suffered from chronic brain
diseases were excluded.
All participants were Han Chinese from Shaanxi
(China) and not genetically related. Written informed consent was
obtained from all participants. The study protocol was approved by
the Ethical Committee of Xi’an Mental Health Center (Xi’an,
China).
SNP selection and genotyping
SNPs in the promoter region, untranslated regions,
exons, introns and 3′ end of the RGS9 gene were systematically
screened. The RGS9 gene with a genomic length of 1.1 Mb and 10
selected SNPs selected for genotyping are shown in Table I. Preliminary analysis was
performed using the HapMap data (http://www.ncbi.nlm.nih.gov/SNP/snp). Tag SNPs were
examined with Haploview software version 4.2 (Broad Institute,
Cambridge, MA, USA) using the Chinese Han in Beijing population and
a minor allele frequency cut-off ≥5% (HapMap Data Release 27). The
linkage disequilibrium (LD) pattern of this gene was determined in
the Chinese population using the preliminary data from the HapMap.
These SNPs were further analyzed in an association study.
 | Table IGenotype and allele frequencies of the
RGS9 gene polymorphisms in controls (n=205) and cases (n=425) and
their associated risk. |
Table I
Genotype and allele frequencies of the
RGS9 gene polymorphisms in controls (n=205) and cases (n=425) and
their associated risk.
| ID/bp | Location | MAF | Group | | Genotype (n, %) | Allele (n, %) |
χ2/Pa |
χ2/Pb | OR, 95% CIc |
|---|
| | | | CC | CT | TT | C | T | | | |
|
rs1530351/63131609 | Promoter | 0.046 | Controls (n=205) | 0 (0.000) | 19 (9.268) | 186 (90.732) | 19 (4.634) | 391 (95.366) | 7.419/0.024 | 8.031/0.005* | 2.079,
1.241–3.483 |
| | Cases (n=425) | 4 (0.941) | 68 (16.000) | 353 (83.059) | 76 (8.941) | 774 (91.059 |
| | | | GG | GA | AA | G | A | | | |
|
rs4791230/63132328 | Promoter | 0.046 | Controls (n=205) | 0 (0.000) | 19 (9.268) | 186 (90.732) | 19 (4.634) | 391 (95.366) | 7.789/0.020 | 7.360/0.007* | 2.021,
1.205–3.389 |
| | Cases (n=425) | 7 (1.647) | 64 (15.059) | 354 (83.294) | 78 (9.176) | 772 (90.824) |
| | | | CC | CG | GG | C | G | | | |
|
rs2869577/63195592 | Intron 1 | 0.300 | Controls (n=404) | 103 (53.093) | 79 (35.567) | 22 (11.340) | 275 (70.876) | 113 (29.124) | 1.537/0.464 | 0.628/0.428 | 1.114,
0.853–1.454 |
| | Cases (n=423) | 229 (54.137) | 160 (37.825) | 34 (8.038) | 618 (73.050) | 228 (26.950) |
| | | | AA | AC | CC | A | C | | | |
|
rs8077696/63192561 | Intron 12 | 0.336 | Controls (n=204) | 28 (13.725) | 81 (39.706) | 95 (46.569) | 137 (33.578) | 271 (66.422) | 2.629/0.269 | 1.733/0.188 | 0.844,
0.655–1.087 |
| | Cases (n=423) | 40 (9.456) | 173 (40.898) | 210 (49.645) | 253 (29.905) | 593 (70.095) |
| | | | GG | GA | AA | G | A | | | |
|
rs8070231/63194945 | Intron 13 | 0.334 | Controls (n=205) | 28 (13.659) | 81 (39.512) | 96 (46.829) | 137 (33.415) | 273 (66.585) | 2.575/0.276 | 1.147/0.284 | 0.871,
0.677–1.121 |
| | Cases (n=424) | 40 (9.434) | 178 (41.981) | 206 (48.585) | 258 (30.425) | 590 (69.575) |
| | | | AA | AG | GG | A | G | | | |
|
rs2292593/63197920 | Intron 13 | 0.333 | Controls (n=204) | 96 (47.059) | 80 (39.216) | 28 (13.725) | 272 (66.667) | 136 (33.333) | 3.711/0.156 | 1.512/0.219 | 1.172,
0.910–1.509 |
| | Cases (n=423) | 207 (48.936) | 179 (42.317) | 37 (8.747) | 593 (70.095) | 253 (29.905) |
| | | | TT | TC | CC | T | C | | | |
|
rs2292592/63204209 | Intron 16 | 0.273 | Controls
(n=205) | 112 (54.634) | 74 (36.098) | 19 (9.268) | 298 (72.683) | 112 (27.317) | 1.127/0.569 | 0.622/0.430 | 1.113,
0.852–1.454 |
| | Cases (n=422) | 238 (56.398) | 155 (36.730) | 29 (6.872) | 631 (74.763) | 213 (25.237) |
| | | | TT | TC | CC | T | C | | | |
|
rs9916525/63211437 | Intron 17 | 0.298 | Controls
(n=205) | 23 (11.220) | 76 (37.073) | 106 (51.707) | 122 (29.756) | 288 (70.244) | 1.577/0.454 | 0.072/0.789 | 1.036,
0.801–1.340 |
| | Cases (n=423) | 40 (9.456) | 178 (42.080) | 205 (48.463) | 258 (30.496) | 588 (69.504) |
| | | | TT | TA | AA | T | A | | | |
|
rs1122079/63214503 | Intron 17 | 0.251 | Controls
(n=205) | 119 (58.049) | 69 (33.659) | 17 (8.293) | 307 (74.878) | 103 (25.122) | 1.230/0.541 | 0.061/0.805 | 0.967,
0.737–1.268 |
| | Cases (n=423) | 234 (55.319) | 160 (37.825) | 29 (6.856) | 628 (74.232) | 218 (25.768) |
| | | | GG | GT | TT | G | T | | | |
|
rs4790953/63227030 | 3′near | 0.251 | Controls
(n=205) | 18 (8.780) | 67 (32.683) | 120 (58.537) | 103 (25.122) | 307 (74.878) | 2.439/0.295 | 0.288/0.592 | 1.077,
0.822–1.411 |
| | Cases (n=422) | 30 (7.109) | 164 (38.863) | 228 (54.028) | 224 (26.540) | 620 (73.460) |
Between 3–5 ml of peripheral blood were collected in
tubes coated with EDTA. Genomic DNA was extracted from blood
leukocytes using the EZNA™ Blood DNA Midi kit (Omega Bio-Tek,
Norcross, GA, USA), according to the manufacturer’s instructions.
DNA was stored at −80°C for SNP analysis. Cases and controls were
mixed on the same plates and a double-blind procedure was
performed. Probes and primers were designed using the Assay Design
software version 3.4 (Sequenom Inc., San Diego, CA, USA) (Table II). SNP genotyping was performed
using matrix assisted laser desorption ionization-time of flight
mass spectrometry (MassARRAY system; Sequenom Inc.). Genotype
calling was performed in real time using the MassARRAY RT software
version 3.0.0.4 and data analysis was performed using the MassARRAY
Typer software (version 3.4; Sequenom Inc.).
 | Table IIPrimer sequences used for genotyping
of RGS9 gene SNPs with the MALDI-TOF Sequenom platform. |
Table II
Primer sequences used for genotyping
of RGS9 gene SNPs with the MALDI-TOF Sequenom platform.
| SNPs | Forward
primers | Reverse
primers | Extension
primers |
|---|
| rs1530351 |
ACGTTGGATGCTTCTGCTTGGATGGATCTC |
ACGTTGGATGTGTGCCTTTTCAGGAATCCC |
taTCTGCAGGGTCCAGC |
| rs4791230 |
ACGTTGGATGCCATGATCAACATCCTCCAC |
ACGTTGGATGCGCTAACATAATGAGCTGTC |
TGGCCCATTTGTTACAATTGAT |
| rs2869577 |
ACGTTGGATGTCAAGGCTTGTCTGTGTTTC |
ACGTTGGATGGGCGACAGAGTGAGAATTTG |
gaaGCTTGTCTGTGTTTCAGCATATAA |
| rs8077696 |
ACGTTGGATGCCAGCTAGCAGAGATCTAAC |
ACGTTGGATGGGAGAATTCCATAAGGGATAG |
tgcGAATCAGAAAAAGACTCAGT |
| rs8070231 |
ACGTTGGATGTAGACTTGATTAGGGAGATG |
ACGTTGGATGTTTAGCCTGGCTGACTGCCG |
CAATCCATATTATCATGTAATGTT |
| rs2292593 |
ACGTTGGATGAATGTTCTTCCAGGCCGTAG |
ACGTTGGATGTGCACATTGTGATGCTAGAC |
gggcaAGGCCGTAGAAGTGAAGATG |
| rs2292592 |
ACGTTGGATGAAGTGAGGGTCTCTTTCAGC |
ACGTTGGATGAGCAATACTTCCCAGTCCCC |
aaTCAGCCAAATGGCTATT |
| rs9916525 |
ACGTTGGATGTCCAGTCCTCTTTCATAATC |
ACGTTGGATGAGACCCAGAGATTTTGATCC |
CCTTTCTTAACATCACCCTT |
| rs1122079 |
ACGTTGGATGCTGGAACCACAAGACAGAGA |
ACGTTGGATGTCCAGTCAGAACAGCACTTG |
AGAGATGTGTTCATTTAGCGA |
| rs4790953 |
ACGTTGGATGCTTGCAGCATAGGTTTAAAG |
ACGTTGGATGCTGCTCAGAACCTTATGTAG |
gaAGCATAGGTTTAAAGGAGCATC |
Statistical analysis
All statistical tests were conducted with SPSS 16.0
for Windows (SPSS, Inc., Chicago, IL, USA). The Hardy-Weinberg
equilibrium (HWE) for each SNP was assessed using the Genepop v4.0
(19). Associations between the
case-control status and each polymorphism were assessed by Fisher’s
exact test or Pearson’s χ2 test. The odds ratio (OR) and
95% confidence interval (CI) were used to measure the strength of
the association between allele frequencies and the RGS9 gene. The
Bonferroni correction was used to adjust the test level when
multiple comparisons were conducted and the P-value was divided by
the total number of loci or haplotypes. Pair-wise LD statistics (D′
and r2) and haplotype frequencies were computed using
Haploview 4.0 to construct haplotype blocks. All P values presented
were two sided, and P<0.05 was considered to indicate a
statistically significant difference.
Results
The distribution frequencies of the 10 genotyped
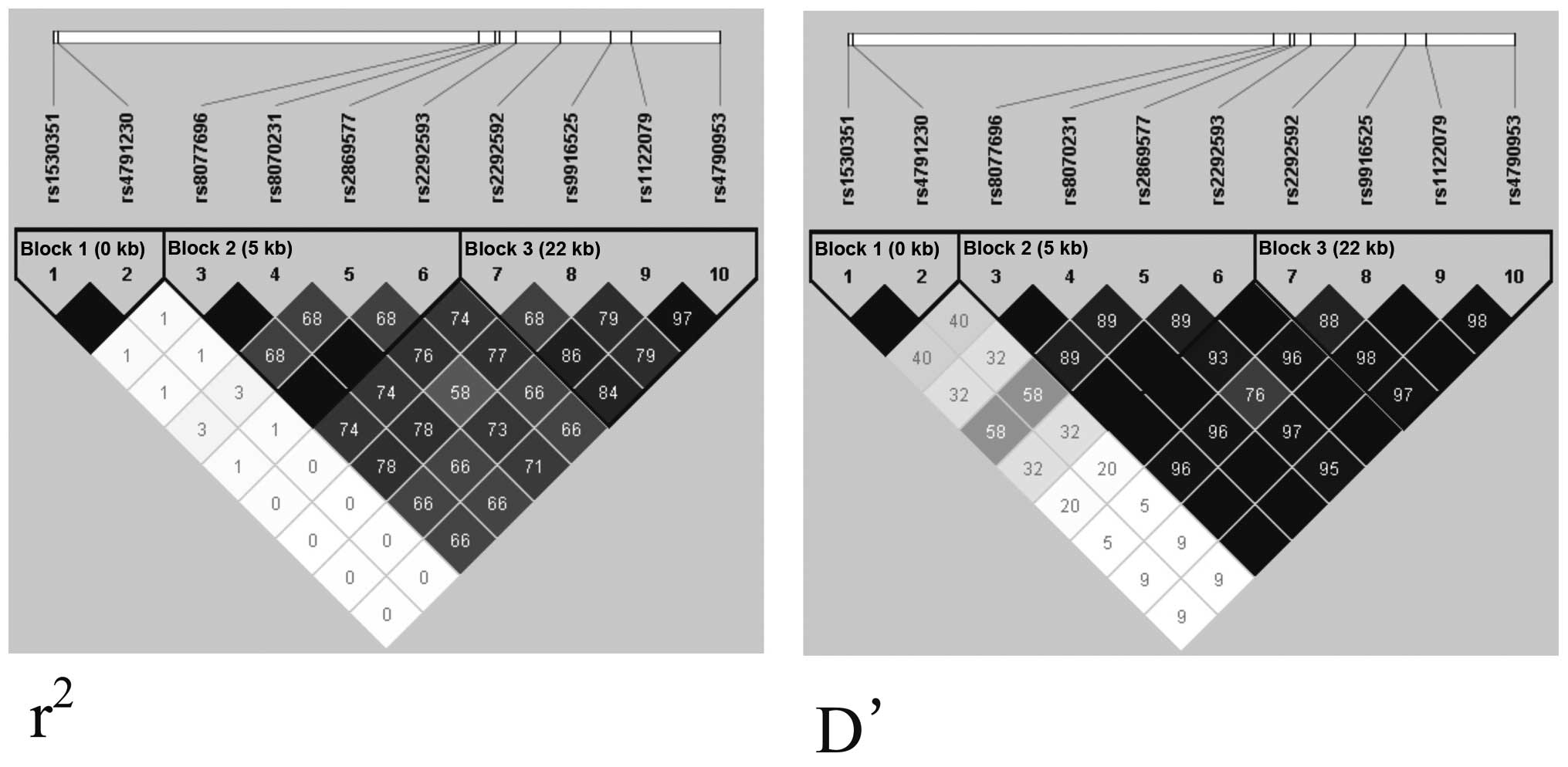
SNPs were in agreement with the HWE. The analysis of linkage
disequilibrium in the heroin-dependent patients and the healthy
controls revealed that two SNPs were located in haplotype block 1
(rs1530351 and rs4791230), four SNPs were located in haplotype
block 2 (rs8077696, rs8070231, rs2869577 and rs2292593) and four
SNPs were located in haplotype block 3 (rs2292592, rs9916525,
rs1122079 and rs4790953; D′>0.9; Fig. 1). The genotype distribution,
allelic frequencies and haplotypes in the heroin-dependent patients
and the healthy controls are shown in Tables I and III–V.
 | Table IIIRGS9 haplotypes in block 1
frequencies and the results of their associations with the risk of
heroin dependence. |
Table III
RGS9 haplotypes in block 1
frequencies and the results of their associations with the risk of
heroin dependence.
| | | Statistics |
|---|
| | |
|
|---|
| Haplotypea | Cases (n, %) | Controls (n,
%) | χ2 | Pb | OR | 95% CI |
|---|
| T-A | 385 (90.59) | 196 (95.61) | 4.867 | 0.027 | 0.442 | 0.210–0.929 |
| C-G | 37 (8.71) | 10 (4.88) | 2.935 | 0.087 | 1.860 | 0.906–3.818 |
 | Table VRGS9 haplotypes in block 3
frequencies and the results of their associations with the risk of
heroin dependence. |
Table V
RGS9 haplotypes in block 3
frequencies and the results of their associations with the risk of
heroin dependence.
| | | Statistics |
|---|
| | |
|
|---|
| Haplotypea | Cases (n, %) | Controls (n,
%) | χ2 | Pb | OR | 95% CI |
|---|
| T-C-T-T | 288 (67.76) | 139 (67.80) | 0.000 | 0.992 | 0.998 | 0.699–1.426 |
| C-T-A-G | 102 (24.00) | 50 (24.39) | 0.012 | 0.915 | 0.979 | 0.664–1.444 |
| T-T-T-T | 20 (4.71) | 9 (4.39) | 0.096 | 0.764 | 1.131 | 0.506–2.531 |
The results demonstrated that there were significant
differences in the genotype frequency and allele frequency
distribution of two SNPs (rs1530351 and rs4791230) between the
heroin-dependent patients and the healthy controls. The frequency
of the rs1530351 C allele was significantly higher in the
heroin-dependent patients than in the healthy controls
(χ2=8.031, P=0.005, OR=2.079, 95% CI=1.241–3.483).
Compared with the healthy controls, the heroin-dependent patients
carried a higher frequency of the rs4791230G allele
(χ2=7.360, P=0.007, OR=2.021, 95%CI=1.205–3.389),
whereas the remaining eight RGS9 SNPs exhibited negative results
(Table I).
Furthermore, LD was observed in three blocks
(D′>0.9) and significantly less T-A haplotypes
(χ2=4.867, P=0.027, OR=0.442, 95% CI=0.210–0.929) were
identified in the heroin-dependent patients, suggesting that they
may exhibit protective effects against heroin dependence, however,
it did not pass the threshold value (P=0.025).
Discussion
RGS9 is a member of the RGS family of proteins and
is specifically expressed in the striatum, which is involved in
movement, motivation, mood and dependence (14,20).
Previous studies have demonstrated that acute morphine
administration increases the expression of RGS9-2 in the nucleus
accumbens and other CNS regions, whereas chronic administration
decreases its expression (12–14).
Mice lacking RGS9 exhibit enhanced behavioral responses to acute
and chronic morphine administration, including a significant
increase in morphine reward, increased morphine analgesia with
delayed tolerance and exacerbated morphine physical dependence and
withdrawal (6). This suggests that
RGS9 acts upon the reward system of the brain, which is important
in opiate dependence. Accumulated evidence indicates that RGS9
receptors are pivotal in the development of tolerance and physical
dependence to opiates (6). These
findings establish RGS9 as a potent negative modulator of opiate
action in vivo and suggest that opiate-induced alterations
in the level of RGS9 contribute to the behavioral and neural
plasticity associated with chronic opiate administration (6). To the best of our knowledge, the
present results provide the first direct evidence that a genetic
change in the RGS9 gene is associated with heroin dependence in
humans.
In the present study, significant differences were
identified in the genotype frequency and allele frequency
distribution of two SNPs (rs1530351 and rs4791230) in the promoter
region between the heroin-dependent patients and the healthy
controls. It was found that one SNP (rs1530351) in the RGS9 gene
was significantly associated with increased risk of heroin
dependence and another SNP (rs4791230) was also associated with
increased risk of heroin dependence. To the best of our knowledge,
this is the first study demonstrating a significant association
between the RGS9 rs1530351 and rs4791230 SNPs with heroin
dependence. A study confirmed that SNPs in the promoter region
could alter microRNA binding to the promoter, resulting in the
regulation of mRNA expression (21). These studies suggested that the
RGS9 gene may contribute to the susceptibility to psychotic
diseases. The SNPs in the promoter of the RGS9 gene affected mRNA
stability and regulated mRNA expression (21), thereby affecting the genetic
susceptibility to heroin dependence. Nevertheless, the role of the
two SNPs in the genetic susceptibility to heroin dependence
requires further investigation.
The genetic interactions among polymorphisms was
further investigated and strong LD was observed. Haplotype analysis
revealed that significantly less T-A haplotypes were identified in
the heroin-dependent patients, although it did not pass the
threshold value (P=0.025). There were significant point-wise
associations of these variants with heroin dependence. These
results indicated that the patients with T-A haplotypes of the RGS9
gene were less prone to heroin dependence, suggesting that they may
exhibit protective effects against heroin dependence. Further
studies are required to examine the protective effect of the T-A
haplotypes of the RGS9 gene on the risk of heroin dependence.
In conclusion, the present study identified a strong
association between two SNPs (rs1530351 and rs4791230) in the RGS9
gene and heroin dependence. These findings encourage future efforts
in searching for functional polymorphisms within and close to the
RGS9 gene using a systemic approach in a larger sample
population.
Acknowledgements
This study was supported by the Research and
Development Foundation of Science and Technology of Shaanxi
Province grant no. 2012K16-03-01) and the National Natural Science
Foundation of China (grant no. NSFC31100900).
References
|
1
|
Koob GF: The neurobiology of addiction: a
neuroadaptational view relevant for diagnosis. Addiction. 101(Suppl
1): 23–30. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kreek MJ, Nielsen DA, Butelman ER and
LaForge KS: Genetic influences on impulsivity, risk taking, stress
responsivity and vulnerability to drug abuse and addiction. Nat
Neurosci. 8:1450–1457. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
3
|
Cadoret RJ, Yates WR, Troughton E,
Woodworth G and Stewart MA: Adoption study demonstrating two
genetic pathways to drug abuse. Arch Gen Psychiatry. 52:42–52.
1995. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Uhl GR: Molecular genetics of substance
abuse vulnerability: remarkable recent convergence of genome scan
results. Ann NY Acad Sci. 1025:1–13. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Uhl GR, Drgon T, Johnson C, et al: ‘Higher
order’ addiction molecular genetics: convergent data from
genome-wide association in humans and mice. Biochem Pharmacol.
75:98–111. 2008. View Article : Google Scholar
|
|
6
|
Zachariou V, Georgescu D, Sanchez N, et
al: Essential role for RGS9 in opiate action. Proc Nal Acad Sci
USA. 100:13656–13661. 2003. View Article : Google Scholar
|
|
7
|
Badgaiyan RD: A novel perspective on
dopaminergic processing of human addiction. J Alcohol Drug Depend.
1:1000e1012013.PubMed/NCBI
|
|
8
|
Volkow ND, Fowler JS, Wang GJ, Swanson JM
and Telang F: Dopamine in drug abuse and addiction: results of
imaging studies and treatment implications. Arch Neurol.
64:1575–1579. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Dohlman HG and Thorner J: RGS proteins and
signaling by heterotrimeric G proteins. J Biol Chem. 272:3871–3874.
1997. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Rahman Z, Gold SJ, Potenza MN, et al:
Cloning and characterization of RGS9-2: a striatal-enriched
alternatively spliced product of the RGS9 gene. J Neurosci.
19:2016–2026. 1999.PubMed/NCBI
|
|
11
|
Birnbaumer L: Expansion of signal
transduction by G proteins. The second 15 years or so: from 3 to 16
alpha subunits plus betagamma dimers. Biochim Biophys Acta.
1768:772–793. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Traynor JR and Neubig RR: Regulators of G
protein signaling and drugs of abuse. Mol Interv. 5:30–41. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Hooks SB, Martemyanov K and Zachariou V: A
role of RGS proteins in drug addiction. Biochem Pharmacol.
75:76–84. 2008. View Article : Google Scholar
|
|
14
|
Traynor JR, Terzi D, Caldarone BJ and
Zachariou V: RGS9-2: probing an intracellular modulator of behavior
as a drug target. Trends Pharmacol Sci. 30:105–111. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhang K, Howes KA, He W, et al: Structure,
alternative splicing and expression of the human RGS9 gene. Gene.
240:23–34. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Escamilla M, Hare E, Dassori AM, et al: A
schizophrenia gene locus on chromosome 17q21 in a new set of
families of Mexican and central american ancestry: evidence from
the NIMH genetics of schizophrenia in latino populations study. Am
J Psychiatry. 166:442–449. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Greenbaum L, Pelov I, Teltsh O, Lerer B
and Kohn Y: No association between regulator of G-protein signaling
9 (RGS9) and schizophrenia in a Jewish population. Psychiatr Genet.
20:47–48. 2010. View Article : Google Scholar
|
|
18
|
Clarke GM, Anderson CA, Pettersson FH,
Cardon LR, Morris AP and Zondervan KT: Basic statistical analysis
in genetic case-control studies. Nat Protoc. 6:121–133. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Rousset F and Raymond M: Testing
heterozygote excess and deficiency. Genetics. 140:1413–1419.
1995.PubMed/NCBI
|
|
20
|
Anderson GR, Posokhova E and Martemyanov
KA: The R7 RGS protein family: multi-subunit regulators of neuronal
G protein signaling. Cell Biochem Biophys. 54:33–46. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhou Y, Shi T, Mozola MA, et al: Evidence
that the promoter can influence assembly of antitermination
complexes at downstream RNA sites. J Bacteriol. 188:2222–2232.
2006. View Article : Google Scholar : PubMed/NCBI
|