Introduction
Corneal disease is the second most frequent cause of
blindness amongst ocular diseases (1). A number of these cornea conditions,
including inflammation or infection, are treated with medication.
For more severe cases which are not treatable with these
strategies, or in cases where there is scarring or cloudiness of
the cornea resulting in visual loss, corneal transplantation may be
required to improve vision. Corneal transplantation, which is also
known as penetrating keratoplasty, is one of the most common and
successful types of solid organ transplantation in humans (2). However, Williams et al
(3) reported that the probability
of survival of patients following penetrating corneal graft
survival in an Australian cohort was 87, 73, 60 and 46% at 1, 5, 10
and 15 years. In addition, in high-risk grafts which have either
received a previous corneal transplant or which have
prevascularized graft beds, the success rate is markedly reduced,
often as low as 20–40% (4).
Irreversible immune rejection of the transplanted cornea is the
major cause of human allograft failure in the intermediate and late
postoperative period (5). The
corneal graft rejection is a complex immune process consisting of a
sequence of events. Corneal allograft rejection requires T cells,
amongst which CD4+ T cells are the most important T-cell
population, and two ocular antigen-presenting cell populations -
corneal Langerhans cells and conjunctival macrophages - are
required (6). Previously, a number
of studies have identified that corneal allograft survival is
associated with the CD4+CD25+Foxp3+ T-regulatory cells (Treg cells)
(7,8).
Currently available immunosuppressive drugs,
including corticosteroids and cyclosporin A (CsA), are used to
prevent or treat corneal graft rejection in humans. However, the
long-term survival of corneal grafts, particularly in high-risk
recipients, was not achieved with satisfaction (9,10).
Therefore, novel strategies to achieve long-term survival of
corneal grafts, particularly in high-risk recipients, are required.
As a synthetic structural analog of myriocin,
sphingosine-1-phosphate (S1P)1 is a potent immunosuppressant which
prolongs allograft survival (11).
Once it is phosphorylated in vivo by sphingosine kinase 2
(SphK2), FTY720-P acts as an agonist on four of the five known S1P
receptors (S1P1, S1P3, S1P4, and S1P5) (12). By contrast to classical
immunosuppressants, it has been proved that S1P1 does not interfere
with T-cell proliferation, but induces a severe deprivation in
lymphocytes in the blood due to modification of S1P signaling
(13). Oral administration of a
selective agonist of the S1P receptor type 1 (S1P1) induced a
profound and reversible reduction in circulating lymphocytes and
prolonged the survival of cardiac allografts in stringent rat
transplantation (14). Sedláková
et al (15) found that
intraperitoneal injections of FTY720 prolonged the graft survival
in rat-to-mouse corneal xenografts. Another two studies also found
that oral immunosuppression with FTY720 significantly prolonged the
survival of corneal allografts (16,17).
However, systemic FTY720 treatment was reported to cause non-fatal
herpesvirus infections, bradycardia and atrioventricular block,
hypertension, macular edema, skin cancer and elevated liver-enzyme
levels (18). Therefore, in order
to avoid the side effects of systemically administered
immunosuppressants, the present study topically administered S1P1
to mice following allogeneic corneal transplantation.
Materials and methods
Animals
Orthotopic corneal transplantation was performed
using inbred BALB/c and C57BL/6 male six- to eight-week-old mice.
The mice used in grafting experiments weighed 18–22 g. C57BL/6 mice
served as donors and BALB/c mice were recipients of the corneal
allografts. These were fully mismatched for major
histocompatibility complex (MHC) and multiple minor
histocompatibility antigens between the two inbred mouse strains.
The mice were obtained from Beijing HFK Bio-Technology Co., Ltd.
(Beijing, China). The present study was conducted in strict
accordance with the recommendations in the Guide for the Care and
Use of Laboratory Animals of the National Institutes of Health. The
animal use procedure was reviewed and approved by the Institutional
Animal Care and Use Committee of the PLA General Hospital (Beijing,
China).
Orthotopic allogeneic corneal
transplantation
A total of 45 BALB/c mice received corneal grafts
from C57BL/6 donors. Prior to all surgical procedures, the mice
were deeply anesthetized by intraperitoneal injection of 3%
pentobarbital sodium (50 mg/kg, Nembutal; Beijing Chemical Co.,
Beijing, China). Tropicamide-phenylephrine ophthalmic solution
(Santen Pharmaceutical Co., Ltd., Osaka, Japan) was topically
applied to dilate the pupils of both the donors (C57BL/6) and the
recipients (BALB/c). The donors’ cornea was prepared according to
the ‘underwater technique’ originally described by Zhang et
al (19). The central 2.0 mm
of a C57BL/6 cornea were marked with a 2.0-mm trephine, excised
with Vannas scissors and placed into balanced salt solution (BSS™,
Alcon Co., USA), prior to grafting. The donor cornea graft was
sutured into a 1.5-mm BALB/c recipient corneal bed with 8–10
interrupted 11–0 nylon sutures (Sharpoint™, Reading, PA, USA). The
anterior eye chamber was restored at the end of surgery by
injecting air. Corneal sutures were removed on day 10 following
transplantation. The recipient mice were treated in situ
with ophthalmic gel containing S1P1 (provided by the Department of
Molecular Drug Design, Institute of Pharmacology and Toxicology
Sciences, Beijing, China) at a concentration of 5 mg/ml (0.5%)
following transplantation, which was performed successively from
days 0–30. The recipient mice which received CsA eye drops (10
mg/ml, 1%, North China Pharmaceutical Group Co., Ltd., Hebei,
China) served as positive controls. The recipient mice which
received in situ ophthalmic gels without any drugs served as
negative controls.
Clinical evaluation of grafted
corneas
The degree of opacity as well as the degree of
neovascularization was evaluated daily within the first
postoperative two weeks and then three times weekly for up to 30
days. Briefly, donor corneal opacity score (0–4), edema score (0–2)
and neovascularization score (0–4) were graded according to
criteria previously described (20). Rejection was defined as the day on
which indices of opacity, edema and neovascularization reached
moderate or severe levels, with an opacity score ≥3 and a total ≥5,
in grafts which had been transparent following operation (21). The grafts with technical
difficulties, including intraocular hemorrhage, cataract or
infection, were excluded.
ELISA
Standard ELISA assays were used to measure the serum
autoantibody production on day 30 following transplantation. To
prepare the serum samples, peripheral blood (PBL) samples from the
mice were maintained on ice for 2 h, centrifuged at 1,000 × g for
20 min at 4°C, and its supernatant was immediately separated from
the pellet. The serum levels of IL-2, IL-10, TGF-β1 and IFN-γ were
measured using a corresponding ELISA kit (Dakewe Biotech Co., Ltd.,
Shenzhen China). The plates were read using an ELISA microplate
reader (DNM-9606; Nanjing Huadong Electronics Group Medical
Equipment Co., Ltd., Nanjing, China) at an optical density (OD) of
450 nm.
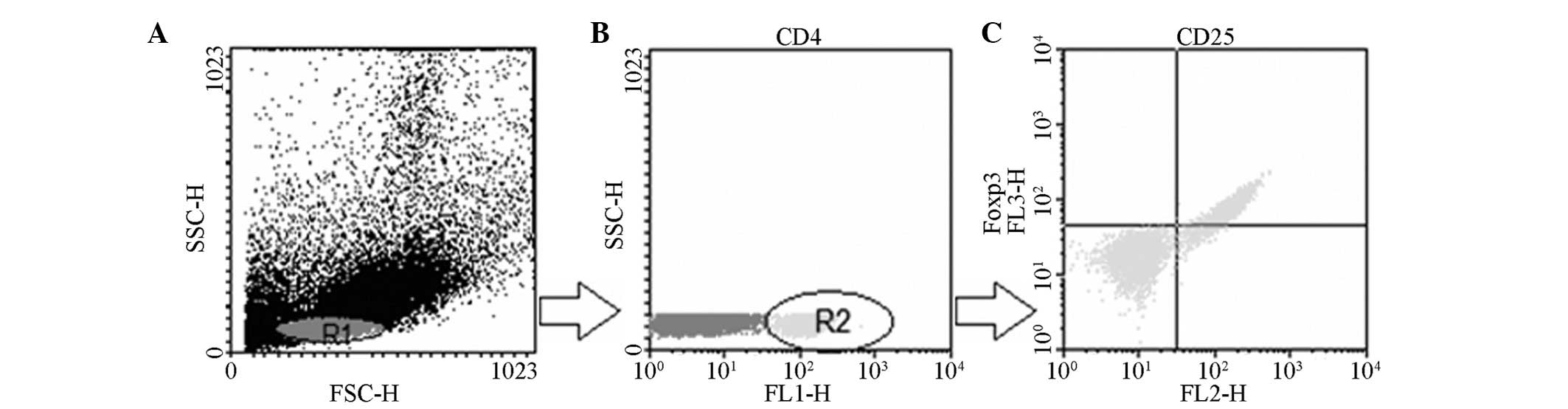
Flow cytometric analysis
The T-cell phenotype in the right cervical lymph
node, the blood and the spleen from the experimental mice was
analyzed by flow cytometry on day 30 following transplantation. The
Treg cells were detected by a Mouse Regulatory T-cell Staining kit
(phycoerythrin Foxp3 FJK-16s, fluorescein isothiocyanate CD4 and
allophycocyanine CD25) from eBioscience (San Diego, CA, USA),
according to the manufacturer’s instructions (eBioscience). The
data were acquired using a FACSCalibur flow cytometer (BD
Biosciences, San Jose, CA, USA) and analyzed by using Winmid 2.9
software (http://scripps.edu/software.html; Scripps Institute,
La Jolla, CA, USA). The cells were gated for CD4+ and the
percentage of CD4+CD25+FoxP3+ cells was calculated.
Quantitative polymerase chain reaction
(qPCR)
Corneas were excised (2.5 mm in diameter), frozen in
liquid nitrogen and stored at −80°C on day 30 following
transplantation. For intragraft gene expression analysis, the total
cellular RNA was isolated using the TRIzol® (Invitrogen
Life Technologies, Carlsbad, CA, USA) and liquid nitrogen. The
reverse transcription of mRNA to cDNA was performed in 20 μl
reaction volumes with random priming and EasyScript RT using an
Easy RT-PCR kit (Beijing TransGen Biotech Co., Ltd., Beijing,
China). Gene expression was examined using an iCycler IQ Real-time
PCR Detection System (Bio-Rad, Hercules, CA, USA) using the SYBR
Green Realtime PCR Master Mix (Toyobo Co., Ltd., Osaka, Japan) with
the respective qPCR primers for IL-2, IL-10, TGF-β1, IFN-γ, Foxp3
and GAPDH (22,23). The primer sequences are used are
provided in Table I. The cycle
number at which the reporter fluorescence reached the threshold (CT
value) was used for quantitative measurement. The relative
expression data was determined by normalizing to GAPDH expression
measured contemporaneously from the same sample to calculate a
fold-change in value using the 2−ΔΔCT method.
 | Table IPrimer sequences used for
quantitative polymerase chain reaction. |
Table I
Primer sequences used for
quantitative polymerase chain reaction.
| Genes | Primer 1 (forward,
5′→3′) | Primer 2 (reverse,
5′→3′) |
|---|
| GAPDH |
TGAAGGTCGGTGTGAACGGATTTG |
GTTGAATTTGCCGTGAGTGGAGTC |
| IL-2 |
GCACCCACTTCAAGCTCCA |
AAATTTGAAGGTGAGCATCCTG |
| IL-10 |
TGCCTTCAGCCAGGTGAAGACTTTC |
CTTGATTTCTGGGCCATGCTTCTCTG |
| TGF-β1 |
ATACCAACTATTGCTTCAGCTCCACAG |
GTACTGTGTGTCCAGGCTCCAAATAT |
| IFN-γ |
GCACAGTCATTGAAAGCCTAGAAAGTC |
GGTAGAAAGAGATAATCTGGCTCTG |
| Foxp3 |
ATGCCCAACCCTAGGCCAGCCAAG |
TGGGCCCCACTTCGCAGGTCCCGAC |
Statistical analysis
Actuarial graft survival was analyzed using the
Kaplan-Meier survival method, and the log-rank test was used to
examine for statistical differences among the groups. One-way
analysis of variance followed by multiple comparisons with the
Bonferroni test was used in all other cases. Statistical analyses
were performed using SPSS 13.0 (SPSS, Inc., Chicago, IL, USA) In
each case, P<0.05 was considered to indicate a statistically
significant difference and only significant probabilities are
shown.
Results
Orthotopic allogeneic corneal
transplantation
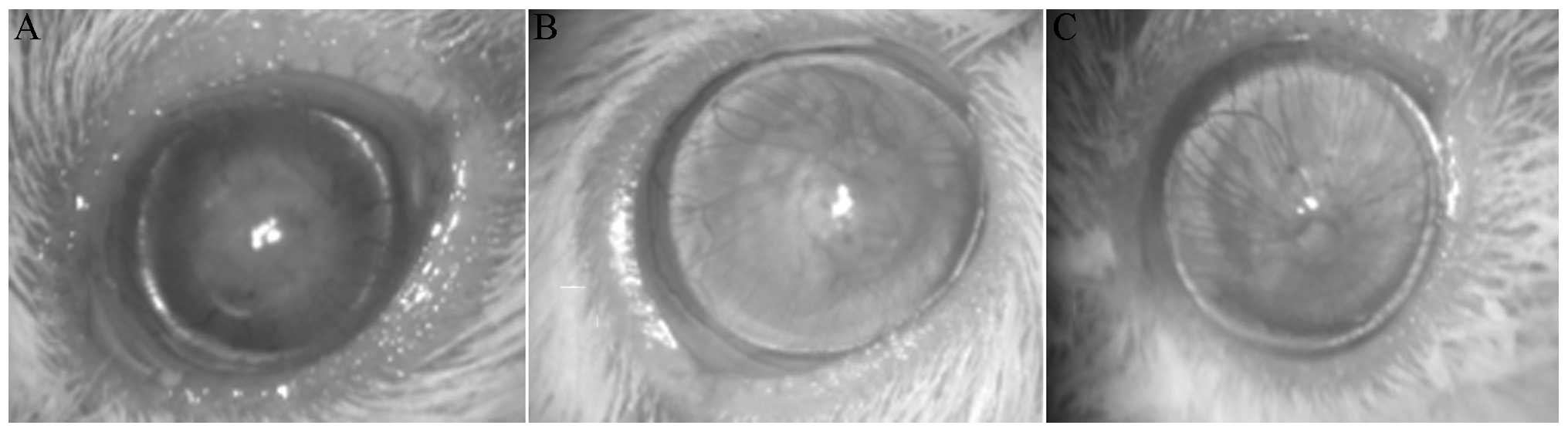
Transplantation of C57BL/6 corneal grafts to BALB/c
recipients resulted in a rejection rate of 100% in the control
group [mean survival time (MST), 13.44±0.48 days]. Compared with
the control group, there were statistical differences both in the
0.5% S1P1 ophthalmic gel group (MST, 24.11±1.58 days) and in the 1%
CsA eye drop group (MST, 25.00±1.91 days) (both P<0.001). The
corneal allografts that were being rejected exhibited pronounced
opacity, edema and neovascularization at postoperative day 30
(Fig. 1A). As demonstrated in
Fig. 1B and C, the corneal
allografts in the 0.5% S1P1 and 1% CsA groups were clear.
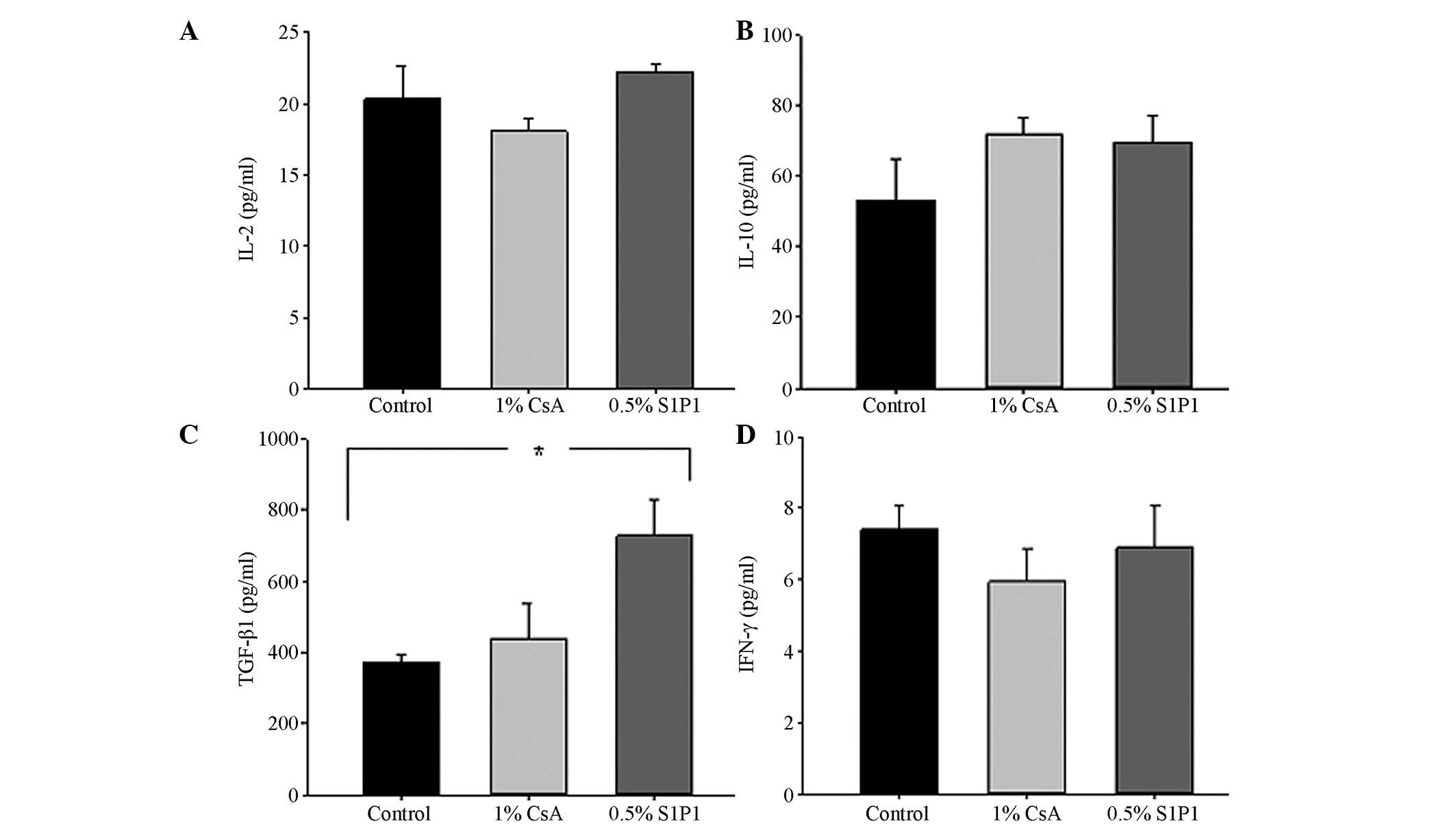
ELISA
The serum levels of IL-2, IL-10, TGF-β1 and IFN-γ in
the different groups are demonstrated in Fig. 2. Although the levels of IL-2 in the
1% CsA eye-drop group were lower than those in the other groups,
there were no statistically significant differences among these
three groups (P>0.05; Fig. 2A).
The levels of IL-10 in the 1% CsA group and the 0.5% S1P1 group
appeared higher than those in the control group (Fig. 2B). Compared with the control group,
a strong secretion of TGF-β1 in the 0.5% S1P1 group was observed
(P<0.001; Fig. 2C). Although
the levels of IFN-γ in the 1% CsA group and the 0.5% S1P1 group
were lower than those in the control group, there was no
statistically significant difference between the groups (P>0.05,
Fig. 2D).
Flow cytometric analysis
Fig. 3 is an
illustrative example of how the determination was performed by flow
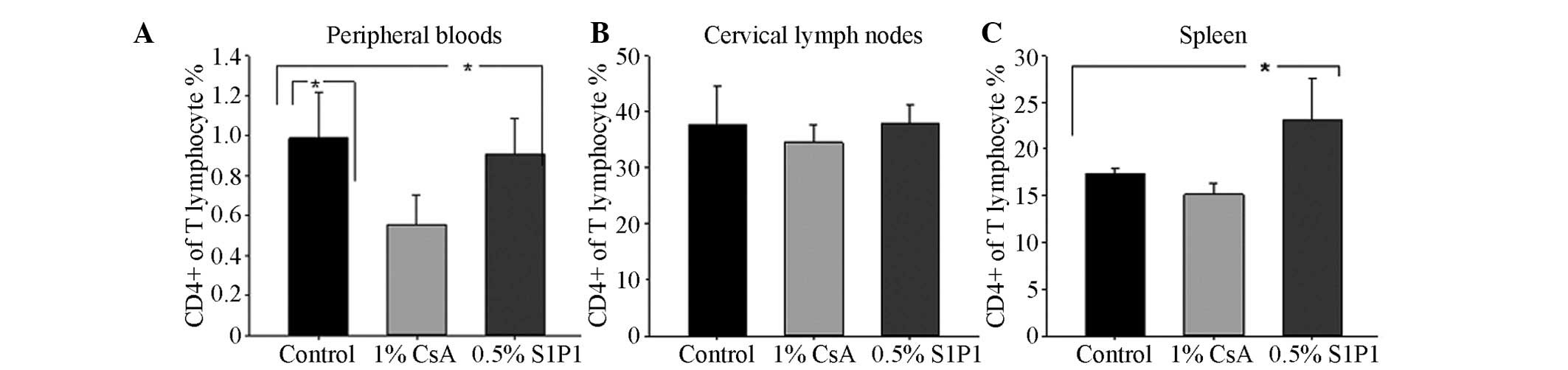
cytometry. A decrease in the mean percentage of CD4+ T cells in
peripheral blood lymphocytes (PBLs) in the 1% CsA eye drop group
and in 0.5% S1P1 ophthalmic gel group were observed, compared with
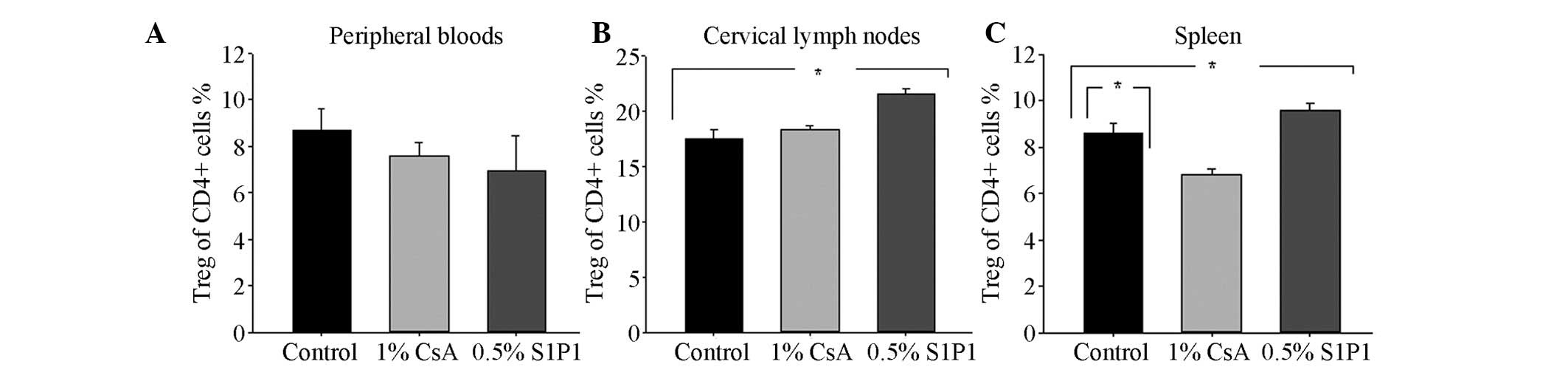
that in the control group (P=0.022 and P=0.046; Fig. 4A). There were no significant
differences in the mean percentage of CD4+ T cells in the cervical
lymph nodes among the three groups (P>0.05; Fig. 4B). Compared with the percentage of
CD4+ T cells in the control group, an increase in the mean
percentage of CD4+ T cells in the spleen in the 0.5% S1P1
ophthalmic gel group was observed (P=0.041; Fig. 4C). With regard to the mean
percentage of CD4+CD25+Foxp3+T cells in CD4+ T cells in PBLs, there
was no statistically significant difference among the three groups
(P>0.05; Fig. 5A). However,
there was a significantly higher percentage of CD4+CD25+ Foxp3+T
cells in CD4+ T cells in cervical lymph nodes in the 0.5% S1P1
ophthalmic gel group when compared with that in the control group
(P<0.001; Fig. 5B). Consistent
with the cervical lymph nodes, the mean percentage of
CD4+CD25+Foxp3+ cells in CD4+ T cells in the spleen in the 0.5%
S1P1 ophthalmic gel group was significantly higher than that in the
control group (P=0.046; Fig. 5C).
In addition, a decrease in the percentage of CD4+CD25+Foxp3+ cells
in CD4+ T cells in the spleen in the 1% CsA group was observed
(P=0.022; Fig. 5C).
qPCR
The results of mRNA expression in the corneal grafts
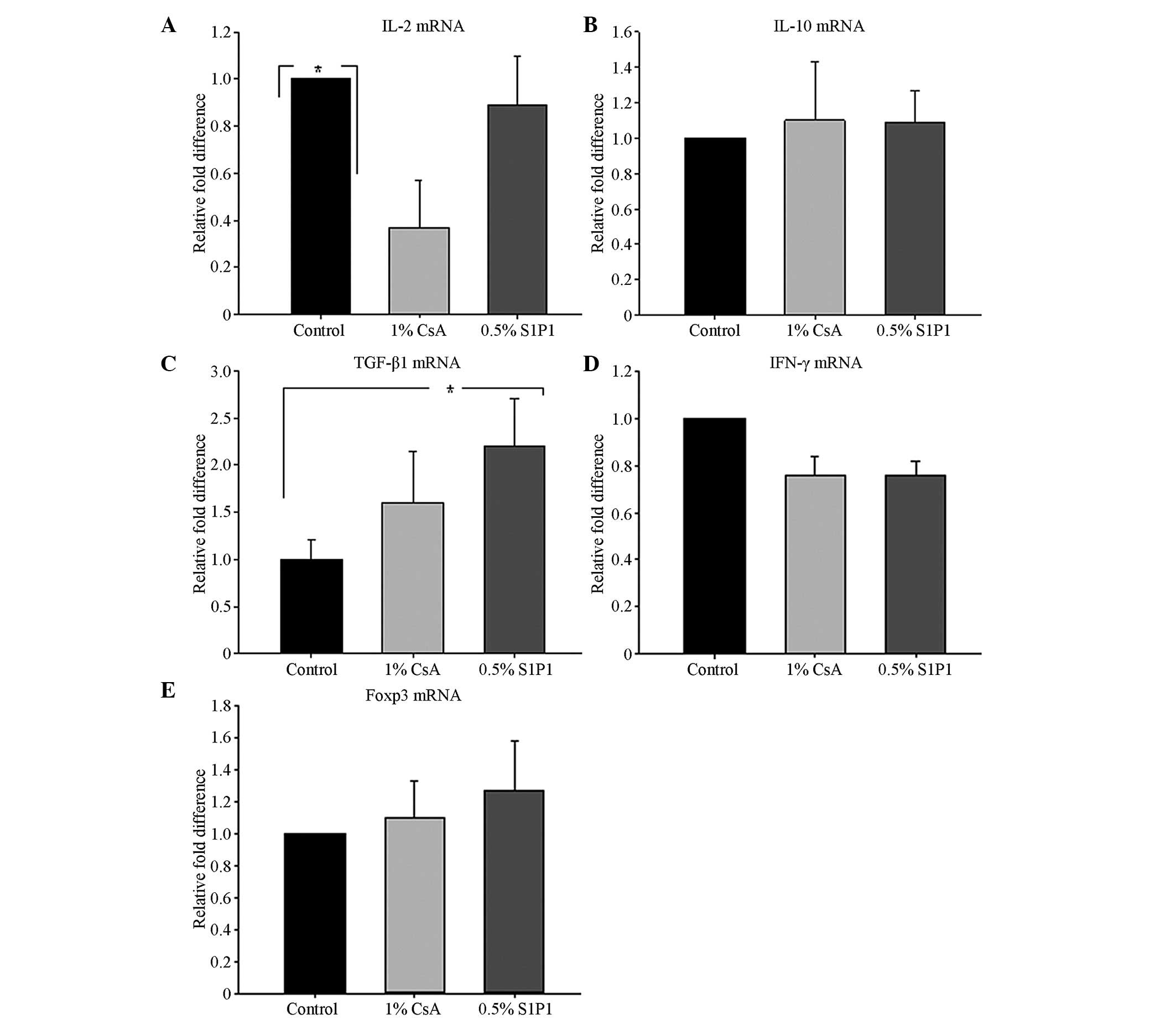
are revealed in Fig. 6. Compared
with the control group, the qPCR analysis demonstrated a reduced
mRNA expression pattern of IL-2 in the topical 1% CsA group
(P=0.036; Fig. 6A). The levels of
IL-10 mRNA expression in the corneal graft in the topical 1% CsA
group and the 0.5% S1P1 group were marginally higher than those in
the control group, but there was no statistically significant
difference among the five groups (P>0.05; Fig. 6B). Compared with the control group,
TGF-β1 mRNA transcription in the corneal grafts in the topical 0.5%
S1P1 group increased significantly (P=0.017; Fig. 6C). Although the levels of
expression of IFN-γ mRNA in the corneal grafts in the 1% CsA group
and the 0.5% S1P1 group were lower than those in the other three
groups, there was no statistically significant difference among the
three groups (P>0.05; Fig. 6D).
The increase in Foxp3 mRNA expression in the corneal grafts in the
topical 0.3% and 0.5% S1P1 groups was observed, but there was also
no statistically significant difference among the three groups
(P>0.05; Fig. 6E).
Discussion
Corneal allotransplantation is the most common type
of solid tissue transplantation in humans and is characterized by a
high success in graft survival, but immunological rejection remains
a serious risk factor for corneal graft failure (4–8,24).
Immunosuppressants, including CsA and FK506, are currently used for
the prevention of allograft rejection in clinical corneal
transplantation. Although systemic CsA has clear therapeutic
efficacy, its use is limited by the potential for systemic side
effects (25). Topical CsA has
been used and studied extensively with regard to the management of
corneal graft rejection in recent years, but the results of a
number of studies remain contradictory (26,27).
As one of the novel immunosuppressants, systemic treatment with
FTY720 has prolonged the corneal graft survival in a number of
studies, but there are also certain associated side effects
(16–18). The immunomodulator S1P1 as an
agonist of G-protein-coupled receptors does not suppress lymphocyte
activation and clonal expansion but rather modulates lymphocyte
circulation (13). Therefore, the
present study investigated the topical application of S1P1 on
allografts following corneal transplantation in mice.
In the present study, it was identified that 0.5%
S1P1 ophthalmic gel prolonged the survival of mouse corneal
allografts effectively. Although Unal et al (28) and Poon A et al (29) demonstrated that topical application
of 0.05% CsA had no significant effect on corneal graft survival,
Alalwani et al (30) found
that eye drops containing 2% CsA inhibited corneal rejection
effectively. From the results of the present study, it was
identified that the application of eye drops containing 1% CsA also
prolonged corneal graft survival. Therefore, an opthalmic gel
containing 0.5% S1P1 and eye drops containing 1% CsA inhibited the
rejection of mouse allogeneic corneal grafts effectively.
Although the systemic absorption of topically
administered drugs is limited, the present study identified that
ophthalmic gel containing 0.5% S1P1 significantly increases the
serum levels of TGF-β1. TGF-β1 is a major type of cytokine
expressed in the immune system in mammals (43). It is produced by every leukocyte
lineage, including lymphocytes, macrophages and dendritic cells,
and its expression serves in both autocrine and paracrine modes to
control the differentiation, proliferation and state of activation
of these immune cells. TGF-β is synthesized as a precursor protein
and released in an inactive form as either a small or large latent.
It stimulates as well as inhibits cell growth and proliferation
(31). Therefore, TGF-β1 has been
observed as a regulatory molecule, acting to restore balance
following deviations from normal levels, and subsequently induce
lymphocyte apoptosis (32).
Numerous studies have reported that TGF-β was produced by Treg
cells, controlled T-cell tolerance, and had an important role in
the induction of Treg and the maintenance of immunological
tolerance (33,34). It was identified that topical
application of 0.5% S1P1 enhanced serum levels of TGF-β1.
Considering its evident inhibitory effect, it was hypothesized that
topical application of 0.5% S1P1 may enhance the immune function of
Treg cells with high levels of TGF-β1. The present study did not
identify any statistically significant difference in the serum
levels of IL-2, IL-10 and IFN-γ among all of the groups.
Flow cytometric analysis demonstrated that there was
a statistically significant difference in the percentage of CD4+ T
cells in the PBLs in the 0.5% S1P1 group. These results coincided
with the results of numerous studies, which had proved that S1P1
induced a severe deprivation in lymphocytes in the blood and
induced inhibition of T-cell egress from lymphoid organs due to
modification of S1P (13,15,35).
A number of studies had proved that CD4+CD25+Foxp3+
Treg cells were a functionally distinct subset of T cells with
suppressive ability, and that they prevented allograft rejection
(36). Matsuoka et al
(35) found that CD4+ lymphopenia
was a critical factor in Treg-cell homeostasis, and that prolonged
imbalance of Treg-cell homeostasis resulted in the loss of
tolerance and significant clinical disease manifestations.
Furthermore, a number of studies have demonstrated that homing of
Treg cells into the draining lymph nodes was required for the
suppressive function of these cells (37). For the percentage of
CD4+CD25+Foxp3+ T cells in the present study, it was identified
that topical application of 0.5% S1P1 enhanced the percentage of
CD4+CD25+Foxp3+ T cells in the lymphoid organs followed by a change
in CD4+ T-cell distribution. It has been reported that CD4+CD25+
Treg expressed lower levels of mRNA for S1P1 and S1P4 receptors and
demonstrated a reduced chemotactic response to S1P (38). Therefore, it is hypothesized that
the immunosuppressive effect of topical 0.5% S1P1 is correlated
with a high percentage of CD4+CD25+Foxp3+ T cells in the lymphoid
organs by means of the S1P receptor. The result is in line with a
number of studies, which had proved that S1P1 significantly
increased the percentage of Treg cells (13,14).
Flow cytometric analysis identified that the
percentage of CD4+ T cells in lymphocytes in PBLs in the topical 1%
CsA group was lower than that in the control group (P=0.022). This
reduction may be explained by the immunosuppressive effect of CsA
(39). It was also identified that
topical application of 1% CsA reduced the percentage of
CD4+CD25+Foxp3+ T cells in the spleen (P<0.001). This result was
in accordance with several studies, which proved that CsA may also
inhibit the function of CD4+CD25+ Treg cells (40).
In order to further investigate the mechanisms by
which topical application of 0.5% S1P1 prolonged corneal allograft
survival, the intra-graft mRNA gene expression of cytokines was
examined. These cytokines, including IL-12, IL-10, TGF-β1, IFN-γ
and Foxp3 mRNA, were quantified by qPCR. A significant increase in
TGF-β1 mRNA expression in the topical 0.5% S1P1 group was observed.
Several studies had previously found that Treg cells produced
TGF-β1 and the suppression of Treg cells was associated with TGF-β1
(33,34). Furthermore, Yamagami et al
(41) found that draining cervical
lymph nodes (CLN) had a critical role in alloimmunity and rejection
of high-risk corneal grafts. Therefore, the increase in TGF-β1 mRNA
expression in corneal grafts may be associated with the higher
percentage of CD4+CD25+Foxp3+ T cells in CLN. Therefore, the
present study further indicated that topical 0.5% S1P1 prolonged
the survival of corneal grafts by enhancing the functions of Treg
cells. It was identified that the IL-2 mRNA expression in the
topical 1% CsA group was lower than that in other groups, which is
concordant with the results of other studies, which proved that CsA
inhibited the synthesis of IL-2 mRNA (42). In regard to IL-10, IFN-γ and Foxp3
mRNA expression, there was no statistically significant difference
among the five groups. However, the levels of IL-10 and Foxp3 mRNA
in the topical 0.5% S1P1 group were higher than those in the other
groups.
In conclusion, the present study identified that
topical application of 0.5% S1P1 effectively prolonged the survival
of mouse allogeneic corneal grafts as well as topical application
of 1% CsA. However, topical 0.5% S1P1 and topical 1% CsA suppress
corneal graft rejection via different pathways. Topical application
of 0.5% S1P1 may increase the percentage of CD4+CD25+Foxp3+ T cells
in the lymphoid organs followed by a change in the CD4+ T-cell
distribution. It may therefore reduce the infiltration of
inflammatory cells, including CD4+ T lymphocytes, in the corneal
graft. It may also enhance the serum levels of TGF-β1 and increase
the expression of TGF-β1 mRNA in corneal grafts.
Acknowledgements
The present study was supported by National Science
Fund of China (grant no. 81170830). The authors are grateful to Dr
Zhao-Shan Liu (Institute of Medicine, Academy of Military Medical
Sciences, Beijing, China) and Dr Jing-Xiang Huang (Laboratory of
Molecular Biology, Institute of Orthopaedics, The General Hospital
of People’s Liberation Army, Beijing, China) for their excellent
technical assistance.
References
|
1
|
Oliva MS, Schottman T and Gulati M:
Turning the tide of corneal blindness. Indian J Ophthalmo.
160:423–427. 2012. View Article : Google Scholar
|
|
2
|
Streilein JW: New thoughts on the
immunology of corneal transplantation. Eye. 17:943–948. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Williams KA, Lowe M, Bartlett C, Kelly TL
and Coster DJ: Risk factors for human corneal graft failure within
the Australian corneal graft registry. Transplantation.
86:1720–1724. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Maguire MG, Stark WJ, Gottsch JD, et al:
Risk factors for corneal graft failure and rejection in the
collaborative corneal transplantation studies. Collaborative
Corneal Transplantation Studies Research Group. Ophthalmology.
101:1536–1547. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Panda A, Vanathi M, Kumar A, Dash Y and
Priya S: Corneal graft rejection. Surv Ophthalmol. 52:375–396.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Niederkorn JY: Immune mechanisms of
corneal allograft rejection. Curr Eye Res. 32:1005–1016. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Chauhan SK, Saban DR, Lee HK and Dana R:
Levels of Foxp3 in regulatory T cells reflect their functional
status in transplantation. J Immunol. 182:148–153. 2009. View Article : Google Scholar :
|
|
8
|
Cunnusamy K, Paunicka K, Reyes N, et al:
Two different regulatory T cell populations that promote corneal
allograft survival. Invest Ophthalmol Vis Sci. 51:6566–6574. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Inoue K, Kimura C, Amano S, et al:
Long-term outcome of systemic cyclosporine treatment following
penetrating keratoplasty. Jpn J Ophthalmol. 45:378–382. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Shimazaki J, Den S, Omoto M, et al:
Prospective, randomized study of the efficacy of systemic
cyclosporine in high-risk corneal transplantation. Am J Ophthalmol.
152:33–39. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhang ZY, Zhang Z, Zug C, et al: AUY954, a
selective S1P1 modulator, prevents experimental autoimmune
neuritis. J Neuroimmunol. 216:59–65. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Hanessian S, Charron G, Billich A and
Guerini D: Constrained azacyclic analogues of the immunomodulatory
agent FTY720 as molecular probes for sphingosine 1-phosphate
receptors. Bioorg Med Chem Lett. 17:491–494. 2007. View Article : Google Scholar
|
|
13
|
Brinkmann V, Davis MD, Heise CE, et al:
The immune modulator FTY720 targets sphingosine 1-phosphate
receptors. J Biol Chem. 277:21453–21457. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Pan S, Mi Y, Pally C, et al: A
monoselective sphingosine-1-phosphate receptor-1 agonist prevents
allograft rejection in a stringent rat heart transplantation model.
Chem Biol. 13:1227–1234. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Sedláková K, Muckersie E, Robertson M,
Filipec M and Forrester JV: FTY720 in corneal concordant
xenotransplantation. Transplantation. 79:297–303. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Mayer K, Birnbaum F, Reinhard T, et al:
FTY720 prolongs clear corneal allograft survival with a
differential effect on different lymphocyte populations. Br J
Ophthalmol. 88:915–919. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zhang EP, Müller A, Ignatius R and
Hoffmann F: Significant prolongation of orthotopic corneal-graft
survival in FTY720-treated mice. Transplantation. 76:1511–1513.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Cohen JA, Barkhof F, Comi G, et al: Oral
fingolimod or intramuscular interferon for relapsing multiple
sclerosis. N Engl J Med. 362:402–415. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhang EP, Schrunder S and Hoffmann F:
Orthotopic corneal transplantation in the mouse: a new surgical
technique with minimal endothelial cell loss. Graefes Arch Clin Exp
Ophthalmol. 234:714–719. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Brinkmann V, Cyster JG and Hla T: FYT720:
sphingosine 1-phosphate receptor-1 in the control of lymphocyte
egress and endothelial barrier function. Am J Transplant.
4:1019–1025. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhang JL, Sun DJ, Hou CM, et al: CD3 mAb
treatment ameliorated the severity of the cGVHD-induced lupus
nephritis in mice by up-regulation of Foxp3+ regulatory T cells in
the target tissue: kidney. Transpl Immunol. 24:17–25. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Xie Y, Sun HX and Li D: Platycodin D is a
potent adjuvant of specific cellular and humoral immune responses
against recombinant hepatitis B antigen. Vaccine. 27:757–764. 2009.
View Article : Google Scholar
|
|
23
|
Küchle M, Cursiefen C, Nguyen NX, et al:
Risk factors for corneal allograft rejection: intermediate results
of a prospective normal-risk keratoplasty study. Graefes Arch Clin
Exp Ophthalmol. 240:580–584. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Xie L, Shi W, Wang Z, Bei J and Wang S:
Prolongation of corneal allograft survival using cyclosporine in a
polylactide-co-glycolide polymer. Cornea. 20:748–752. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Bourges JL, Lallemand F, Agla E, et al:
Evaluation of a topical cyclosporine A prodrug on corneal graft
rejection in rats. Mol Vis. 12:1461–1466. 2006.PubMed/NCBI
|
|
26
|
Sinha R, Jhanji V, Verma K, et al:
Efficacy of topical cyclosporine A 2% in prevention of graft
rejection in high-risk keratoplasty: a randomized controlled trial.
Graefes Arch Clin Exp Ophthalmol. 248:1167–1172. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Ziaei M and Manzouri B: Topical
cyclosporine in corneal transplantation. Cornea. 29:Oct
29–2014.(Epub ahead of print).
|
|
28
|
Unal M and Yucel I: Evaluation of topical
ciclosporin 0.05% for prevention of rejection in high-risk corneal
grafts. Br J Ophthalmol. 92:1411–1414. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Poon A, Constantinou M, Lamoureux E and
Taylor HR: Topical Cyclosporin A in the treatment of acute graft
rejection: a randomized controlled trial. Clin Experiment
Ophthalmol. 36:415–421. 2008.PubMed/NCBI
|
|
30
|
Alalwani H, Omer Saleh B, Rocher N, et al:
Advantages and limits of multiple grafts (third keratoplasty) under
local cyclosporin 2%. J Fr Ophtalmol. 33:710–714. 2010.(In French).
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Wang GY, Yang Y, Li H, et al: Rapamycin
combined with donor immature dendritic cells promotes liver
allograft survival in association with CD4(+) CD25(+) Foxp3(+)
regulatory T cell expansion. Hepatol Res. 42:192–202. 2012.
View Article : Google Scholar
|
|
32
|
Lu L, Ma J, Wang X, et al: Synergistic
effect of TGF-beta superfamily members on the induction of
Foxp3+ Treg. Eur J Immunol. 40:142–152. 2010. View Article : Google Scholar :
|
|
33
|
Hofmann U, Hu K, Walter F, et al:
Pharmacological pre- and post-conditioning with the
sphingosine-1-phosphate receptor modulator FTY720 after myocardial
ischaemia-reperfusion. Br J Pharmacol. 160:1243–1251. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Stanojlovic S, Schlickeiser S, Appelt C,
et al: Influence of combined treatment of low dose rapamycin and
cyclosporin A on corneal allograft survival. Graefes Arch Clin Exp
Ophthalmol. 248:1447–1456. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Matsuoka K, Kim HT, McDonough S, et al:
Altered regulatory T cell homeostasis in patients with
CD4+ lymphopenia following allogeneic hematopoietic stem
cell transplantation. J Clin Invest. 120:1479–1493. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Schneider MA, Meingassner JG, Lipp M,
Moore HD and Rot A: CCR7 is required for the in vivo function of
CD4+CD25+ regulatory T cells. J Exp Med.
204:735–745. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Commodaro AG, Peron JP, Lopes CT, et al:
Evaluation of experimental autoimmune uveitis in mice treated with
FTY720. Invest Ophthalmol Vis Sci. 51:2568–74. 2010. View Article : Google Scholar
|
|
38
|
Sehrawat S and Rouse BT: Anti-inflammatory
effects of FTY720 against viral-induced immunopathology: role of
drug-induced conversion of T cells to become Foxp3+
regulators. J Immunol. 180:7636–7647. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Bocian K, Borysowski J, Wierzbicki P, et
al: Rapamycin, unlike cyclosporine A, enhances suppressive
functions of in vitro-induced CD4+CD25+
Tregs. Nephrol Dial Transplant. 25:710–717. 2010. View Article : Google Scholar
|
|
40
|
Zeiser R, Nguyen VH, Beilhack A, et al:
Inhibition of CD4+CD25+ regulatory T-cell
function by calcineurin-dependent interleukin-2 production. Blood.
108:390–399. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Yamagami S, Dana MR and Tsuru T: Draining
lymph nodes play an essential role in alloimmunity generated in
response to high-risk corneal transplantation. Cornea. 21:405–409.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Kobayashi T, Momoi Y and Iwasaki T:
Cyclosporine A inhibits the mRNA expressions of IL-2, IL-4 and
IFN-gamma, but not TNF-alpha, in canine mononuclear cells. J Vet
Med Sci. 69:887–892. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Li J, Ballim D, Rodríguez M, et al: The
anti-proliferative function of the TGF-β1 signalling
pathwayinvolves the repression of the oncogenic TBX2 by its
homologue TBX3. J Biol Chem. Nov 4–2014.(Epub ahead of print).
|